Virus
World
Less developed
More developed
HPV
600,000
520,000
80,000
HBV
380,000
330,000
44,000
HCV
220,000
190,000
37,000
EBV
110,000
96,000
16,000
KSHV
43,000
39,000
4,000
HTLV
2,100
660
1,500
1 Viruses that Cause Human Cancer
The types of cancers induced by the various viruses and the fraction of these cancers attributed to the virus infection also varies widely (de Martel et al. 2012) (Table 2). In addition, viral prevalence in cases can vary depending on geographical location. HPVs, which normally infect stratified squamous epithelium, are causally associated with a number of anogenital cancers, from almost 100 % of cervical cancers to less than 50 % of vulvar cancers. They have more recently been implicated in oropharyngeal cancers, with prevalence estimates varying quite widely by region (Gillison 2008). Rates of HPV-associated oropharyngeal cancers appear to be substantially increasing, with current prevalences of over 50 % in the U.S. and several other (Arora et al. 2012) industrialized countries (Chaturvedi et al. 2011; Chaturvedi 2012) (Table 2). HBV and HCV have a strict tropism for hepatocytes and together are the major cause of liver cancer (El-Serag 2012). EBV normally infects epithelial cells and lymphocytes, especially B cells, and is the cause of most cases of Hodgkin’s and Burkitt’s lymphomas (Saha and Robertson 2011). It is also an etiological agent in most cases of an epithelial cancer, nasopharyngeal carcinoma (Kutok and Wang 2006). KSHV is detected in virtually all Kaposi’s sarcomas and is also strongly associated with two relatively rare B-cell neoplasias, multicentric Castleman’s disease and primary effusion lymphoma (Gantt and Casper 2011). HTLV-1 also targets lymphocytes and is a primary cause of adult T-cell leukemia and lymphoma (Gallo 2011). MCPyV appears to be part of the normal flora of the skin and is causally related to approximately three-quarters of a relatively rare skin cancer, Merkel cell carcinoma (Arora et al. 2012).
Virus | Cancer | Geographical area | Prevalence in cases (%) |
|---|---|---|---|
HPV | Cervix | World | 100 |
HPV | Penile | World | 50 |
HPV | Anal | World | 88 |
HPV | Vulvar | World | 43 |
HPV | Vaginal | World | 70 |
HPV | Oropharynx | North America | 56 |
HPV | Oropharynx | Southern Europe | 17 |
HPV | Oropharynx | Japan | 52 |
HBV | Liver | Developing | 59 |
HBV | Liver | Developed | 23 |
HCV | Liver | Developing | 33 |
HCV | Liver | Developed | 20 |
EBV | Hodgkin’s lymphoma | Developing-children | 90 |
EBV | Hodgkin’s lymphoma | Developing-adults | 60 |
EBV | Hodgkin’s lymphoma | Developed | 40 |
EBV | Burkitt’s lymphoma | Sub-saharan Africa | 100 |
EBV | Burkitt’s lymphoma | Other regions | 20–30 |
EBV | Nasopharyngeal carcinoma | High-incidence areas | 100 |
EBV | Nasopharyngeal carcinoma | Low-incidence areas | 80 |
KSHV | Kaposi’s sarcoma | World | 100 |
HTLV-1 | Adult T-cell leukaemia and lymphoma | World | 100 |
MCPyV | Merkel cell carcinoma | World | 74 |
Human tumor viruses encompass several distinct viral groups, including those with small DNA genomes (HPV, HBV, and MCPyV), large DNA genomes (EBV and KSHV), positive sense RNA genomes (HCV), and retroviruses (HTLV-1) (Table 3) (Butel and Fan 2012). Their specific mechanisms of carcinogenesis also vary widely. However, a common feature of human tumor viruses is that oncogenesis is an aberration of their normal viral life cycle and an uncommon outcome of infection. With some viruses, e.g. HPV and MCPyV, the viral genomes in cancer cells are usually altered by mutation and/or insertion into the host DNA, such that they can no longer produce infectious virions (Vinokurova et al. 2008; Arora et al. 2012). Virally associated cancers almost always arise as monoclonal events from chronic infections, usually after an interval of many years, indicating that the infections are just one component in a multi-step process of carcinogenesis. A notable exception is KSHV-induced Kaposi’s sarcoma, which can arise as a polyclonal tumor within months of infection in immunosuppressed individuals (Mesri et al. 2010) (also see Chap. 13).
Table 3
Basic features of human oncoviruses
Virus | Genome | Virion structure | Normal tropism | Year isolated (reference) |
|---|---|---|---|---|
HPV16 | Circular 7.9 kb DS DNA | 55 nm naked Icosahedron | Stratified squamous epithelium | 1983 (Dürst et al. 1983) |
HBV | Circular 3.2 kb partial DS DNA | 42 nm enveloped | Hepatocytes | 1970 (Dane et al. 1970) |
HCV | Linear 9.6 k nt positive sense RNA | Enveloped | Hepatocytes | 1989 (Choo et al. 1989) |
EBV | Linear 172 kb DS DNA | Enveloped | Epithelium and B cells | 1964 (Epstein et al. 1964) |
KSHV | Linear 165 kb DS DNA | Enveloped | Oropharyngeal epithelium | 1994 (Chang et al. 1994) |
HTLV-1 | Linear 9.0 k nt positive sense RNA | Enveloped | T and B cells | 1980 (Poiesz et al. 1980) |
MCPyV | Circular 5.4 kb DS DNA | 40 nm naked icosahedron | Skin | 2008 (Feng et al. 2008) |
2 Oncogenic Mechanisms
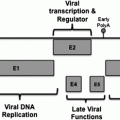
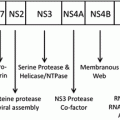
As discussed in detail in later chapters of this book, the oncogenic mechanisms of most tumor viruses involve the continued expression of specific viral gene (oncogene) products that regulate proliferative or anti-apoptotic activities through an interaction with cellular gene products. Examples of oncoproteins include E6 and E7 of HPVs, LMP1 of EBV, and Tax of HTLV-1 (Chaps. 8, 10 and 11, respectively). Virally encoded microRNAs, for instance those of EBV, may also play a role in carcinogenesis by decreasing the expression of negative regulators of cell growth (Raab-Traub 2012). KSHV may act primarily by altering complex cytokine/chemokine networks (Mesri et al. 2010) (Chap. 13). In contrast, some tumor viruses, such as HCV and HBV, may induce cancer more indirectly, as a result of continued tissue injury and regeneration and the chronic inflammatory response of the host to persistent infection (Alison et al. 2011) (Chaps. 3, 5 and 6).
Some viruses, particularly retroviruses, can induce cancers by insertional mutagenesis in animal models (Fan and Johnson 2011). However, this mechanism has not been convincingly documented in humans, except in a few patients in experimental gene transfer trials involving delivery of high doses of recombinant retroviral vectors (Romano et al. 2009). HIV could also be considered a tumor virus in that HIV infection is a strong risk factor for several cancers, including most cancers that are associated with infections by other viruses (Parkin 2006). However, the effect of HIV infection on oncogenesis is thought to be indirect, by inhibiting normal host immune functions that would otherwise control or eliminate oncovirus infections and/or provide immunosurveillance of nascent tumors (Clifford and Franceschi 2009). Consistent with this conjecture, increases in many of the same cancers are seen in patients with other forms of immunosuppression (Rama and Grinyo 2010).
2.1 Causal Association of Viral Infection and Cancer
The causal associations between the seven viruses and specific cancers noted above are well established. They fulfill most, if not all, of the causality criteria proposed by Sir A. Bradford Hill in the early 1970s (Hill 1971). The strength and consistency of association between infection and cancer are high based upon multiple epidemiological studies in varying settings. For instance, the relative risk of HPV and KSHV infection for the development of cervical carcinoma and Kaposi’s sarcoma, respectively, is over 100 in most studies. In some instances, establishing a strong association required identification of especially oncogenic types, e.g., HPV16 and 18 among mucosotropic HPVs, and a specific subset tumors, e.g., oropharyngeal among head and neck cancers. Temporality was established by demonstrating that infection proceeded cancer, usually by many years. In some cases, the viruses are consistently detected in well-established cancer precursor lesions, as is the case for HPV and high-grade cervical intraepithelial neoplasia (Chap. 8). Dose–response relationships were established by demonstrating that, for the most part, populations with higher prevalences of virus infection also had higher incidences of the associated tumor, e.g., HBV and liver cancer (El-Serag 2012) (Chap. 5). However, these associations were sometimes confounded by high prevalence of the oncovirus in the general population and variability in the prevalence of additional risk factors. An example is the high frequency of EBV infection in the general population and the strongly associated cofactor of malaria infection in the induction of EBV-positive Burkitt’s lymphoma (Magrath 2012) (Chap. 10). Biological plausibility as oncogenic agents was established in numerous laboratory studies that identified the interaction of viral proteins with key regulators of proliferation and apoptosis, their immortalizing and transforming activity in vitro, and their oncogenic activity in animal models (Chaps. 4, 6, 8, 10, 11, and 13). These studies also support the criterion that the associations be in agreement with current understanding of disease pathogenesis, in this case, the process of tumorigenic progression. The last criterion, that removing the exposure prevents the disease, has been most convincing demonstrated for HBV, as discussed below and in Chap. 5.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree