Fig. 1
The figure illustrates different stages of the development of inflammation-associated cancer, the corresponding host’s immune defense mechanisms involved in tumor prevention and applicable interventions to prevent or treat cancer
4.1 Antiviral Vaccinations and Therapies
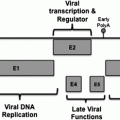
HBV, HCV, or HPV are causative for the vast majority of solid cancers that are associated with chronic virus infections and infection-related inflammation.
The options to prevent HBV-related HCC is to ward off acquisition of chronic HBV infection for risk groups like intravenous drug-addicts or recipients of blood-infusions. Furthermore, effective vaccines against HBV exist since more than 20 years. Vaccination programs, including passive immunizations as part of a post-exposition-prophylaxis for children of HBV infected women, have led to a dramatic reduction of HBV carriers, and to a reduction of HCC during childhood (Chang et al. 1997). HBV vaccination is now part of the National Infant Immunization Schedule in 162 countries and represents the first example of cancer preventive vaccination. However, the actual clinical benefit of the ongoing vaccination programs on mortality due to HBV-related HCC will become apparent after further decades (Zanetti et al. 2008). Additionally, routine HBsAg-screening of blood donors will help to reduce blood transfusion-associated transmission of HBV (Schreiber et al. 1996). For the multitude of chronically HBV-infected persons, HBV vaccinations would not be effective in preventing HCC. Here, control of HBV viremia is the major therapeutic goal to reduce hepatic inflammation and disease progression to cirrhosis and HCC. If liver histologic diagnosis in chronic HBV carriers indicates the requirement of a therapeutic intervention, interferon-α and/or nucleoside analoga are applied to control the viral burden, a management that additionally has the beneficial effect to avoid HCC development. However, resistance development to nucleoside analoga remains a problem (Colombo and Donato 2005).
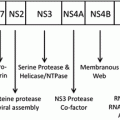
Unfortunately, no functional vaccines against HCV are available to prevent HCV-associated HCC. Only a few vaccine candidates have proceeded to clinical phase I/II trials but it is not yet clear whether these will reach clinical applicability (Torresi et al. 2011). Whereas effective vaccination strategies are still under investigation, significant advances have been made in the treatment of both acute and chronic HCV infection. With current medical therapy, based on pegylated IFN-α and ribavirin, approximately 50 % of patients can be cured. However, the recently developed ‘directly acting viral agents’ (DAAs) such as inhibitors of the NS3/4A protease or cyclophilin B inhibitors, promise further improvements to achieve sustained virologic responses in treatment of HCV infection (Patel and Heathcote 2011; Kronenberger and Zeuzem 2012; McHutchison et al. 2009).
In case of HPV, the availability of an effective vaccine represents a milestone for prevention of cervical cancer worldwide. Bivalent and quadrivalent vaccines prepared from empty virus shells (virus-like-particles) effectively prevent infection by high-risk HPV genotypes 16 and 18 that account for 70 % of cervical cancer (Garland et al. 2007; Paavonen et al. 2007). Some protection against other genotypes related to 16/18 has been reported (Joura et al. 2007).
4.2 Antifibrotic Therapy
Transient fibrogenesis and later reversal of fibrotic scar tissue is a hallmark of wound healing. Though not fully understood, the mechanisms of perpetuating fibrogenesis in case of chronic tissue damage and their fatal consequences have been intensively studied in the liver. Liver cirrhosis, the end stage of fibrotic reorganization of liver tissue, is a frequent complication of chronic HBV or HCV infections. Liver cirrhosis is associated with portal hypertension, reduced liver functions, and with an increased risk at developing HCC. A heterogenous cell population of profibrogenic myofibroblasts (MFB), originating from hepatic stellate cells (HSC) is known to orchestrate liver fibrogenesis (Lee and Friedman 2011). Upon liver injury and inflammation, a key event in the onset of fibrosis is the activation of quiescent HSCs, that can be triggered by several factors including reactive oxygen species, TLR4 ligands, uptake of apoptotic bodies and paracrine stimulation by adjacent hepatocytes, Kupffer cells and liver sinusoidal endothelial cells (LSEC). The perpetuation of the fibrogenic phenotype of MFB mainly results from a microenvironment wherein profibrogenic cytokines and growth factors such as TGF-β and PDGF are dominating. Activated cholangiocytes have been identified as an important source of these profibrogenic mediators. Phenotypic markers of activated MFBs are expression of α-smooth muscle actin (SMA), excessive collagen production, enhanced proliferation and reduced lipid content contributing to tissue stiffness. With regard to specific gene regulation, it has been shown that angiotensin 2 activates the transcription factor NF-κB thus rendering MFBs less sensitive against induction of apoptosis and promoting cell survival (Oakley et al. 2009). Recent data demonstrated that activation of the NF-κB pathway in hepatocytes induces liver fibrosis in mice (Sunami et al. 2012). Accumulation of extracellular matrix (ECM) is a further characteristic of fibrogenesis which contributes to tissue stiffening. Major regulators of ECM are matrix metalloproteinases (MMP), enzymes that are responsible for matrix degradation and removal of scar tissue. Net production of ECM results from a dysbalance of MMP expression, and secretion of their specific inhibitors (tissue inhibitors of metalloproteinases or TIMPs) by MFBs. During physiological regeneration and repair of liver damage, mechanisms exist that can mediate regression of fibrotic scar tissue once the initial cause of tissue damage has been resolved. In this process, MFBs finally disappear from the hepatic scar by phenotypic reversion, senescence, and deletion by natural killer cells. Thereby, fibrotic tissue is removed by enhanced MMP activity and reduced TIMP levels. Hepatic macrophages have been demonstrated to be involved in this regulation and scar tissue remodeling.
The ideal anti-fibrotic therapy consists of the withdrawal of the underlying disease trigger. Virus elimination in chronic HBV or HCV infection can lead to regression of fibrosis and improved liver function even in cirrhotic patients. For patients that do not sufficiently respond to antiviral treatments, specific antifibrotic therapies are urgently needed to slow down the progress of fibrosis and to reduce the risk of late stage complications and HCC development. Patients with increased risk at developing fibrosis should be determined by targeted screenings. As example, a ‘seven gene signature’ with specific polymorphisms in genes with relevance to fibrosis risk such as e.g. TGF-β, TNF-α, or IL-10, has a significant predictive value (cirrhosis risk score) for fibrosis progression in patients with chronic HCV (Huang et al. 2007). Consequent monitoring of those patients to determine progression of fibrosis should be performed. Non-invasive methods like Fibroscan, a sonographic evaluation of liver stiffness, could be valuable alternatives to liver biopsy in the future. Combined with serologic markers like α-fetoprotein this allows for detecting HCC at an early, potentially curable stage (Mok et al. 2005). For antifibrotic therapies, all molecular factors or mechanisms that contribute to progression or regression of fibrosis can be considered suitable targets (Fallowfield 2011). Liver damage due to inflammation and oxidative stress can be addressed by so-called hepatoprotectants. The hepatoprotective activity of some of these substances can be attributed to their ability to suppress the inflammatory activation of NFκB. A number of natural substances that can be long-term consumed in significant amounts have been investigated for their antioxidant and hepatoprotective effect such as resveratrol (from red wine), silymarin (from milk thistle), coffee and vitamin E.
Hepatocyte growth factor (HGF) has been identified to stimulate hepatocyte generation and demonstrated promising results in inhibition of experimental fibrosis in animal models (Xia et al. 2006). However, since HGF is a potent mitogen for hepatocytes, concerns about potential oncogenesis remain. A further potential target is the reduction of apoptotic cell turnover during liver inflammation. Apoptosis of hepatocytes activates HSC directly or via Kupffer cells. Consequently, the pan-caspase inhibitor VX-166 demonstrated antifibrotic activity in animal models (Witek et al. 2009). In contrast, pro-apoptotic strategies could also be an alternative if cell death can be selectively induced in activated HSC. To this end, the proapoptotic IkB inhibitor gliotoxin has been coupled to a single-chain antibody against synaptophysin which is selectively expressed on these cells. This approach reduced fibrosis in a rat model of CCl4 intoxication (Douglass et al. 2008). As an alternative strategy to induce apoptosis of activated HSC, interference with cannabinoid receptor signaling has led to promising results in fibrotic animal models (Teixeira-Clerc et al. 2006), but showed psychomimetic side effects in clinical trials. Further strategies to prevent HSC activation like ligands (“glitazones”) for the peroxisome proliferator-activator receptor-γ (PPAR-γ), Farnesoid-X-receptor-agonists, or HMG-CoA reductase inhibitors (“statins”) have shown antifibrotic activity in experimental models that awaits confirmation in clinical trials. Angiotensin system inhibitors are on the cusp to clinical application. Both angiotensin-converting enzyme inhibitors and angiotensin-1 receptor antagonists (“sartans”) can inhibit fibrosis in animals. Importantly, the AT1R antagonist losartan slowed down progression of fibrosis in patients with chronic HCV. Further future strategies could be targeting of the TGF-β pathway. Neutralizing antibodies, decoy receptors and siRNA have been studied but are not yet ready for clinical application. Pirfenidone, an inhibitor of TGF-β production has shown promising results in a non-controlled pilot study (Armendariz-Borunda et al. 2006). ανβ6 integrin activates matrix-bound, latent TGF-β in the local microenvironment. The specific inhibition of this integrin could therefore be an intriguing approach to avoid side effects of systemic TGF-β inhibition. Neutralizing antibodies against ανβ6 integrin inhibited cholangiocyte activation and collagen deposition in biliary and non-biliary fibrosis models (Popov et al. 2008). Agents, that stimulate the collagenolytic activity of MMP, such as the oral alkaloid Halofuginone, showed promise in animal models but still needs investigations in patients. Notably, the clinical success achieved so far does not correlate with the antifibrotic effects in experimental animal models suggesting that the available animal fibrosis models may not fully reflect the influence of ECM maturation by crosslinking and the potential existence of a “point of no return” in fibrosis (Rockey 2008). Nevertheless, functional antifibrotic therapies are urgently needed, and first progress towards clinical application becomes visible.
4.3 Anti-Inflammatory Treatment
Since chronic inflammation contributes to carcinogenesis and dissemination, antiphlogistic drugs appear to be a promising preventive regimen for infected patients upon diagnosis of virus-mediated tissue damage. Several factors that fuel cancer-associated inflammatory processes have been identified and are therefore reasonable molecular targets to prevent cancer in risk-associated patients. Several antiphlogistic drugs have been shown to reduce the incidence of cancer when used as prophylactics or to slow down tumor growth and improve survival when given as therapeutics, e.g. in colon cancer (Gupta and DuBois 2001). At first, preventive use of classical non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin could be a method of choice (Cuzick et al. 2009; Langley et al. 2011). NSAIDs are cheap, off-patent, well established and long-term clinical experience exists for low-dose usage as anti-thrombotic prophylaxis. It has been demonstrated that preventive Aspirin treatment can significantly reduce the long-term risk to develop colorectal cancer (Flossmann and Rothwell 2007; Rothwell et al. 2010). A large meta analysis showed a reduced cancer risk in several solid, mainly gastrointestinal tumors, such as gastric, oesophageal, colorectal and pancreatic cancer, but also in lung cancer (Rothwell et al. 2011). Benefit correlated with duration of aspirin uptake and with increasing age of the individual. Notably, no benefit was seen in hematological malignancies consistent with the hypothesis that the anti-inflammatory treatment is mainly directed against the chronic inflammatory microinvironment of a solid tumor. Regarding the risk and benefit balance, the Rothwell study suggests that the additional risk of aspirin-associated gastrointestinal bleedings (including fatal bleedings) is outweighed by a gain of 10 % reduction in all-cause mortality after 5–10 years daily aspirin consumption. It has to be pointed out, that the patients in these studies were not stratified whether they actually had any inflammatory burden or not. It has been shown that aspirin reduces the risk of prostate cancers, but only in individuals carrying a particular polymorphic allele of the lymphotoxin α gene which leads to increased lymphotoxin expression (Liu et al. 2006). This observation illustrates the need to early identify those patients with elevated cancer risk by genetic screenings and/or by monitoring inflammatory parameters, which would likely profit most from anti-inflammatory treatment. To reduce gastrointestinal complications in long-term treatments, selective COX-2 inhibitors could be an alternative as preventive cancer treatment (Kawamori et al. 1998; Reddy et al. 2000). In studies of several preclinical models of inherited or carcinogen-induced cancers of colon, intestine, skin and bladder it could be observed that celecoxib could significantly reduce cancer incidence (Fischer et al. 2011). Also in studies in human patients, celecoxib showed promise in prevention of spontaneous colorectal adenomas and reduced the number of polyps in familial adenomatous polyposis (Bertagnolli et al. 2006; Arber et al. 2006; Steinbach et al. 2000). However, it has to be considered that celecoxib has been associated with adverse cardiovascular events (Mukherjee et al. 2001).
Drugs that address specific molecular targets in cytokine or chemokine signaling involved in inflammatory processes also represent promising alternatives. However, most of these agents are already in use for the treatment of chronic inflammatory diseases such as rheumatoid arthritis, psoriasis, or inflammatory bowel diseases (IBD) or are in clinical development for cancer therapy. It is yet unclear whether these agents are suitable for long-term treatments. The structural analogue of thalidomide, lenalidomide, is known to suppress the production of several inflammation-associated cytokines and has shown to be active against melanoma in combination with dexamethasone (Weber et al. 2007).
As already mentioned, NFκB and STAT3 pathways play a critical role in both carcinogenesis and resistance to therapy suggesting that targeted inhibitors could have significant preventive or therapeutic potential. However, pharmacologic long-term inhibition of NFκB can lead to severe immunodeficiency, neutrophilia and enhanced acute inflammation due to increased IL-1β levels (Greten et al. 2007). Alternatively, it appears to be more promising to address defined upstream targets of NFκB since multiple extrinsic and intrinsic pathways converge to activate this central transcription factor. Inhibitors for STAT3 and JAK2, which is upstream of STAT3, have been developed and showed oncostatic activity in solid tumors in mouse xenograft models (Hedvat et al. 2009). Furthermore, receptor antagonists and blocking antibodies addressing IL-6, IL-6 receptor, CCR2, CCR4, and CXCR4 are in clinical development for the treatment of several tumor entities. TNFα-antibodies such as infliximab has been a breakthrough for the treatment of IBD and, importantly, long term application appears to be safe (Fidder et al. 2009). First clinical studies of TNFα antagonists in patients with advanced cancer have resulted in stable disease or partial responses (Brown et al. 2008; Harrison et al. 2007). It is further known that infliximab also reduced colitis-associated cancer in mice (Kim et al. 2010) suggesting a cancer preventive benefit in patients. On the other hand, TNFα has been implicated in the clearance of virus infections (Trevejo et al. 2001) and has been shown to be critical for induction of antitumoral T cell responses in animals (Calzascia et al. 2007). Due to this complex and sometimes contradictory biology of TNFα it is currently difficult to estimate whether TNFα antagonists could be promising as preventive mean for cancer that is caused by virus-mediated inflammation. From the clinical point of view, metastases are the much more challenging manifestation of a malignant disease compared to the primary nodule. Since inflammation is a central driving force of dissemination, application of anti-inflammatory drugs could be an effective intervention to prevent the development of metastases. Recently, the antagonistic RANKL antibody denosumab, initially developed for the treatment of osteoporosis, delayed the development of bone metastases in advanced clinical studies in prostate and breast cancer (Stopeck et al. 2010; Smith et al. 2012). For prevention of metastases, inhibition of TNFα could also be promising, since mouse experiments have shown that blocking TNFα can convert inflammation-promoted metastatic tumor growth to TRAIL-mediated tumor regression (Luo et al. 2004). Altogether, these examples show that molecular mechanisms and signaling pathways involved in tumor- and metastases-associated inflammation are promising targets for preventive and therapeutic interventions.
5 Outlook: Perspectives in Cancer Immunotherapy
Not only for prevention but also in therapy of cancer, antitumoral immune responses are coming more and more into focus. Antitumoral immune responses can even be triggered by conventional chemotherapeutic treatments and may significantly contribute to the therapeutic outcome (Casares et al. 2005; Apetoh et al. 2008). Our current knowledge about tumor-immune responses basically suggests two different strategies for tumor immunotherapy. One strategy is to support or reactivate innate or preexisting adaptive immune responses, the other is de novo induction of adaptive responses by therapeutic interventions. Both are about equally challenging, since tumors have developed numerous ways to escape immune attacks. Even if we are able to elucidate the underlying mechanisms and have many therapies at hand, we still cannot be sure, whether a certain therapy could be undermined by the tumor’s mechanisms of immune suppression in the individual case. Therefore, the ideal immunotherapeutic strategy aims at the achilles heel of the tumor and would be additionally applicable for all solid tumor entities.
Recent findings on the importance of the chronic inflammatory tumor-environment for tumor-maintainance and –progression have lead to the idea to use anti-inflammatory drugs for cancer therapy. The fundamental advantage of this approach is, that immune cells are not prone to develop drug resistance. However, it is likely, that anti-inflammatory therapy does not exhibit sufficient cytotoxic effects on cancer cells and thus must be combined with additional therapies to manifest effective therapeutic effects. As already mentioned above, some phase I/II clinical trials investigate efficacy of anti-IL6 and anti-TNF-α drugs in various cancers (Balkwill 2009).
Among adaptive immune responses, cytotoxic T cells, which are able to mediate direct lysis of transformed cells, are commonly regarded as the most promising cell type for cancer immunotherapy. However, effective tumor-specific CD8 T cell responses are difficult to induce in tumor-bearing hosts. Paracrine mediators, like adenosine, prostaglandin E2, VEGF-A, and TGF-ß mediate direct and indirect immunosuppressive activities and act on different levels of the immune system. It has been shown that immunosuppressive activities lead to blunted spontaneous adaptive responses by T cell exhaustion (Fourcade et al. 2010; Sakuishi et al. 2010; Baitsch et al. 2011) or take effect on dendritic cells, inducing defective maturation by inhibiting costimulatory signals, which in turn fails to prime a tumor-directed CD8 T cell response (Sharma et al. 2010). Therefore, a careful design of a vaccine to elicit an effective tumor-directed CD8 T cell response must encompass a strategy to block the tumors counteractions as well. In a melanoma mouse model inhibition of IDO (indoleamine 2,3-dioxygenase)-mediated immune suppression by use of 1-methyl-tryptophan allowed for induction of potent cytotoxic responses with a CD8 T cell-vaccine by protecting DCs in TDLN from malfunction (Sharma et al. 2010). Another approach to circumvent tumor-mediated immunosuppression is an infection mediated by a lytic virus in a tumor nodule. The infection disrupts tumor architecture and leads to an inflammation, which is accompanied by leukocytic infiltration and abundant tumor-cell death. When a tumor-directed DC-vaccine is applied at this time point of virus-mediated inflammation, it triggers a strong cytotoxic CD8 T cell response, whereas other timepoints of vaccination in combination with virotherapy does not exhibit a significant therapeutic effect. Interestingly, compared to a true virus infection, inflammations mediated by toll-like receptor ligands failed to support effective induction of CD8 T cell response by DC-vaccination, most likely due to lacking cross-presentation of tumor-associated antigens by dendritic cells within the infected tumor (Woller et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree