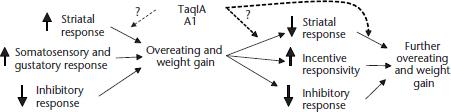
Figure 1 Decreased activation in the caudate in response to milkshake receipt (contrasted with tasteless receipt) by weight change group over a 6-month period. Those that gained weight (solid line) showed decreases in activation, whereas those that lost weight (dashed line) or were weight stable (dotted line) showed slight increases in activation in this region (Stice et al., 2010a).
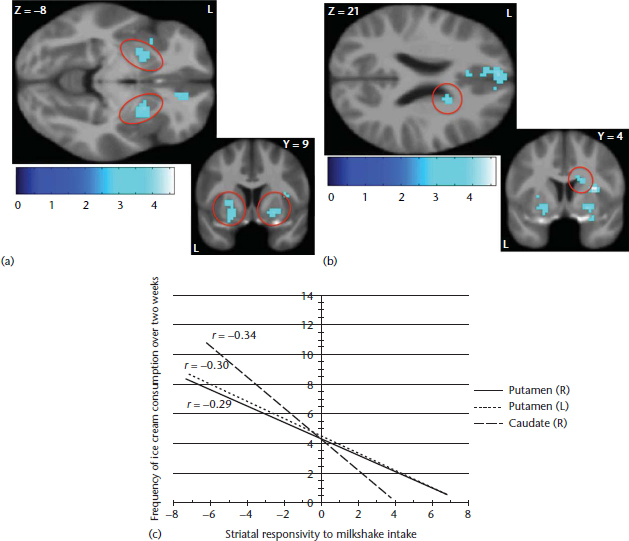
Figure 2 Reduced striatal responsivity to milkshake receipt as a function of frequency of ice cream consumption (tasteless solution receipt>milkshake receipt contrast). Participant’s frequency of ice cream intake was associated with reduced responsivity to milkshake receipt in the (a) bilateral putamen, (b) right caudate and (c) the graph of activation (Burger and Stice, 2012).
Collectively, these results suggest that regular intake of energy dense foods results in down-regulation of DA circuitry, but further intimate that overconsumption of a particularly energy dense food results in attenuated activation of DA-based reward circuitry in response to that particular type of food, reminiscent of the opponent-process theory (Solomon and Corbit, 1974). The opponent-process theory suggests that a salient affective state (either positive or negative) is inherently opposed by a centrally mediated process that attempts to counter the effects of that state and retain homoeostasis. For example, as seen in drug addiction, regular intake of a particular psychoactive drug (e.g. cocaine) causes the body to enter a biological state that opposes the effects of cocaine (i.e. lethargy) that occurs in response to cues that become associated with cocaine administration (e.g. razor blades). It is this opponent process that is thought to result in tolerance, which requires escalating amounts of cocaine for the same degree of psychoactive effects and pleasure as originally experienced, escalation of use, addiction, and withdrawal if drug us is discontinued. In context of food intake, overeating leads to down-regulation DA-mediated reward circuitry (Johnson and Kenny, 2010). Further, acute food deprivation and a chronic restricted diet resulted in increased DA release at feeding and increased D2 receptor binding in rats respectively (Thanos et al., 2008). Therefore, it is reasonable to hypothesise that the down-regulation of DA-based reward circuitry associated with obesity results from repeatedly overeating highly rewarding foods. Data suggest that this process is more pronounced for energy dense foods that are highly palatable (Alsio et al., 2010). If true, consuming high energy density foods would contribute to overeating and the development of obesity in two ways: by altering food reward neurobiology in a manner that increases intake of those foods, and also by contributing excess calories during intake. This is particularly relevant to the obesity epidemic, given that innate and conditioned food preferences are positively related to the energy density of foods (Johnson et al., 1991) and omnipresence of energy dense, highly palatable foods in the surrounding environment.
Development of hyper-reward and incentive responsivity
Animal experiments also reveal that after repeated intake of palatable food, DA-signalling in reward regions shifts from occurring in response to receipt of the food to occurring in response to conditioned cues that predict food receipt (Schultz et al., 1993). For example, naïve monkeys showed activation of mesotelencephalic DA neurons in response to a rewarding food taste, yet, after conditioning, maximal DA signaling was elicited by the conditioned cue that predicted food receipt rather than by actual food receipt (Schultz et al., 1993). Similarly, the greatest DA activation occurred in an anticipatory fashion as trained rats approached and pressed the bar that produced food reward, whereas reward-related activation decreased during actual food intake (Kiyatkin and Gratton, 1994). There is also evidence that a history of elevated sugar intake may contribute to excess elevations in anticipatory reward from food in rats (Avena et al., 2005). Collectively, these data indicate that repeated intake of palatable food results in a ‘shift’ of the DA-signalling from intake of the palatable food to cues that predict future receipt of the food, consistent with the incentive salience theory of addiction (Robinson and Berridge, 2000). The incentive salience theory posits that reward from anticipated receipt and receipt operate in tandem with the development of the reinforcing value of food, but after repeated presentations of food, hedonics decrease, while anticipatory reward increases. In support of this theory, one prospective study found that individuals who showed greater activation in the OFC in response to a cue signalling impending delivery of chocolate milkshake showed elevated future weight gain over one year (Figure 3; Yokum et al., 2011). Additionally striatal and insular responsivity to images of energy dense foods predicted lack of success in a weight loss programme over a nine-month follow-up (Murdaugh et al., 2012). Interestingly, exercise-induced fat loss over a six-month period was associated with a reduction in insula and attention region responsivity to appetising food images relative to baseline (Cornier et al., 2012). Together these data suggest that hyper-responsivity to food cues increases risk for future weight gain and resistance to weight loss efforts, and further, that a negative energy balance may also affect responsivity of reward, gustatory, and attention regions to food stimuli.
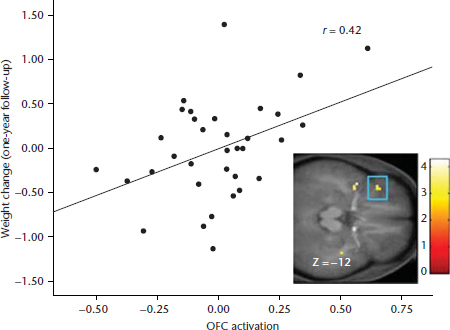
Figure 3 Activation in the orbitofrontal cortex in response to initial orientation to appetising food images (contrasted with pictures of glasses of water) related to weight change over a one-year period (Yokum et al., 2011).
In sum, these animal and human data point toward reward neuroplasticity as a function of food intake. Specifically, overeating (possibly of energy dense foods) may lead to: (1) reductions in reward-related responsivity in line with the opponent-process theory and/or (2) the hyper-responsivity of regions that encode the incentive salience of food cues akin to the incentive salience theory. Despite this evidence, most human neuroimaging studies are cross-sectional and investigate individuals that are already overweight or obese, making it challenging to identify the initial vulnerability factors of overeating and obesity from the consequences of being overweight or obese.
Dynamic Vulnerability Model of Overeating
As a whole, findings seem patently incompatible with both the reward deficit and reward surfeit models of obesity. This state of affairs has prompted us to propose a new working Dynamic Vulnerability Model of obesity (Stice et al., 2011). This model (Figure 4) postulates that individuals at risk for obesity experience initial hyper-reward responsivity from food intake, thereby driving individuals to overeat, which reduces striatal DA circuitry and signalling during subsequent food intake. Concurrently, there is an emergence of hyper-responsivity of regions that encode the incentive salience of food cues. These two processes taken together may drive further overeating in a feed-forward fashion. This model accords with the thesis that hyper-responsivity of reward circuitry increases risk for overeating (Dawe and Loxton, 2004), as well as the hypotheses that repeated consumption leads to: (1) reduction of reward responsivity to food intake analogous to the opponent-process theory (Solomon and Corbit, 1974) and (2) increased incentive valuation when anticipating reward parallelling the incentive salience theory (Robinson and Berridge, 2000).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree