Figure 1 In human body, the arcuate nucleus (ARC) is the major target for short-term signals that regulate human appetite. ARC contains two distinct neuronal subtypes expressing the anorexigenic (appetite decreasing) neuropeptides, POMC, and CART or expressing orexigenic (appetite stimulating) neuropeptides, NPY and AgRP (Hagan and Niswender, 2012; Parker and Bloom, 2012). Long-term signals are generated for metabolic energy needs of body, regulated by hormonal systems as leptin and insulin (Harrold, 2004). Leptin is predominantly synthesised by WAT, reduces food intake and increases energy expenditure, resulting in body weight loss. Insulin is secreted by pancreatic β-cells in response to elevated blood glucose. Thus, glucose is metabolised and excess energy stored as glycogen (Pliquett et al., 2006). Leptin and insulin inhibit anabolic neural circuits and stimulate catabolic circuits to increase energy expenditure.
Endocrine alterations in obesity
Recently, the endocrine alterations in adrenal and pituitary glands, gastrointestinal system (stomach and pancreas), gonads, thyroid and AT are attracting interest to understand the pathological mechanisms of obesity. An overview of the endocrine alterations in many physiological systems is given in Table 1. These dysfunctions in the obesity influences energy homoeostasis and neuroendocrine activities, provided a link between obesity and obesity-related diseases.
Table 1 Endocrine alterations in many physiological systems in obesity (Burman et al., 2009; Alvarez-Castro et al., 2011)
| Endocrine gland | Correlation | Considerations |
| Adrenal gland | ↓ Cortisol-binding globulin (CGB) levels as Cushing’s syndrome | Patients with Cushing’s syndrome display a number of clinical features include abdominal obesity, hypercortisolism, insulin resistance, impaired glucose homoeostasis, hypertension and lipid abnormalities |
| ↑ Urinary free cortisol excretion and total cortisol production reported in women with abdominal obesity | It has been suggested that a dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis could potentially be a cause for abdominal obesity. Furthermore, corticotropin releasing hormone (CRH) administration or meal ingestion increases basal cortisol and ACTH levels in obese women with abdominal fat distribution | |
| ↑ Cortisol response to ACTH in obese men and obese premenopausal women, but not in obese post-menopausal women | It indicates a possible role for oestrogens in cortisol response to ACTH | |
| ↑ Cortisol production both in obese women with PCOS and without | ||
| Pituitary gland | ↓ GH secretion in obesity ↓ GH secretory response to higher fat mass ↓ GH secretion by up to 6% per each unit increase in BMI | The reduction in GH levels in obesity is believed to result from abnormal pituitary function due to decrease in the number of somatotroph cells in the pituitary gland |
| ↓ IGF-1 levels in subjects with abdominal obesity compared to total obese individuals or healthy controls | Hyperinsulinemia commonly found in obesity and it has been suggested that high insulin levels may be decrease GH release | |
| ↑ Secretion of free fatty acids | Free fatty acids have a decreasing effect on GH release in obesity | |
| ↑ Leptin levels in obesity | There is no evidence that the high leptin levels are related to low GH secretion in obese subjects | |
| Stomach | ↓ Ghrelin levels in obesity | It has determined decreased ghrelin levels in obese individuals and circulating ghrelin levels are negatively correlated with the degree of adiposity in humans |
| Pancreas | ↑ Hepatic glucose production and ↓ peripheral glucose uptake in visceral abdominal obesity | Due to elevated free fatty acids (FFA) levels released by abdominal fat |
| ↑ Insulin secretion compared to leans (three to four-folds) and hyperinsulinemia in obese subjects | Obesity, in particular, abdominal or visceral obesity, is strongly associated with insulin resistance, and increases the risk for type 2 diabetes in obese patients. In experimental animal studies have shown either insulin resistance or hyperinsulinemia can precede the development of obesity. Moreover, there is a significant correlation between waist to hip ratio and insulin sensitivity, which is inversely correlated with visceral abdominal | |
| Ovary | ↓ Oestrogen levels in obesity ↑ Androgen levels in obesity | In women, menopause is associated with the development of obesity because of its suppression effect upon ovarian function and precipitation effect upon abdominal weight gain |
| ↑ Androgen production rate and free testosterone and estradiol levels in visceral adiposity ↓ Sex hormone binding globulin (SHBG) levels and the 17-beta estradiol/free testosterone ratio | An excess accumulation of visceral fat contributed to the metabolic syndrome marked by insulin resistance, hyperinsulinaemia, dyslipidaemia, hypertension, pro-thrombosis and a pro-inflammatory state. Furthermore, visceral adipose tissueproduces more of adipokines enhanced weight gain and obesity | |
| ↑ Accumulation of intraabdominal fat and hyperinsulinemia in 20–69% of women with polycystic ovarian syndrome (PCOS) ↑ Oestrogen production in obese women with PCOS | PCOS is the most common ovarian pathology in premenopausal women, which is defined by the presence of obesity, anovulation and hyperandrogenism. Hyperinsulinemia is believed to be the main aetiological factor behind the development of PCOS. By the way, hyperinsulinemia decreases the levels of SHBG and increases the ovarian production of androgens. In obese women with PCOS is also accompanied with enhanced oestrogen production because of increased aromatisation of adrenal androgens into oestrogens in the adipose tissue | |
| ↑ The ratio of serum LH to FSH in women with PCOS | Leptin levels are higher in obese women with PCOS. Higher leptin levels might have a modulatory effect on GnRH by mechanisms probably involving nitric oxide activation in the pituitary | |
| Testis | ↓ Total or free testosterone and SHBG levels in obese men ↑ Serum levels of estradiol and estrone in obese men | The conversion of androgens to oestrogens through the aromatase action of the adipose tissue, which partially suppress the pituitary and cause mild hypogonadism (i.e. impairment of fertility or sperm count) in very obese men. Circulating leptin levels are found correlated to total and free testosterone, suggesting that elevated levels of leptin may contribute to the development of reduced androgens in male obesity |
| Thyroid | ↑ TSH, free and total T3, as well as thyroid volume in obesity | It might be related to enhanced carbohydrate intake (positive energy balance state). In particular, leptin has been shown to alter the HPA axis and abnormal TSH secretion has been suggested as the reason for elevated leptin levels in obesity. Furthermore, increases in TSH and peripheral thyroid hormones may be an adaptation process to increased basal energy metabolism, and thus to energy expenditure |
| ↑ Modest weight gain and ↓ thermogenesis and metabolism in hypothyroidism | There has been shown to be a linear association between hyperlipidemia and hypothyroidism |
Adipose tissue
There are two adipose tissue types in humans, as WATBAT. White WAT is main site of energy storage, whereas BAT is mainly found to regulate body temperature in new-borns. Although AT can be considered as inert organ, which only stores excess energy in the form of fat, adipocytes synthesised and released substances or hormones called as adipocytokines or adipokines to mediate many important functions. In addition to adipocytes, the WAT also contains endothelial cells, fibroblasts, leucocytes and most importantly macrophages. AT mainly produces adipokines as leptin, apelin and adiponectin or cytokines as TNF-α, IL-6 and MCP-1. Some adipokines act through autocrine and paracrine mechanisms to control the growth of AT. Obesity is associated with profound changes in the structure and function of AT (i.e. hyperplasia and hypertrophy in adipocytes, expanding of the extracellular matrix, angiogenesis and macrophage infiltration). Adipokines also act in endocrine manner to control energy homoeostasis and body weight regulation, and has important effects in many physiological systems. Obesity can be considered as a result of alterations in the physiological mechanisms that control body weight or disruptions in adipokine gene synthesis. Furthermore, it was shown until recently that an excess accumulation of visceral fat contributing to the obesity and metabolic disorders (i.e. insulin resistance, hyperinsulinaemia and hyperglycaemia), cardiovascular disease (i.e. dyslipidaemia and hypertension) and inflammation.
Main adipokines will review in this issue, some of adipokines (apelin, angiotensin II, acylation stimulating protein (ASP), leptin, plasminogen activator inhibitor-1 (PAI-1), monocyte chemoattractant protein-1 (MCP-1), resistin, visfatin, proinflammmatory cytokines and glucocorticoids) are reported to increase in human obesity, whereas the others (adiponectin and omentin) decrease with increasing fat mass. Molecular properties, expressions, correlations, administrative effects and main functions of obesity hormones in regulation of energy homoeostasis and body weight are summarised in Table 2.
Table 2 Molecular properties, expressions, correlations, administrative effects and main functions of obesity hormones in energy homoeostasis and body weight
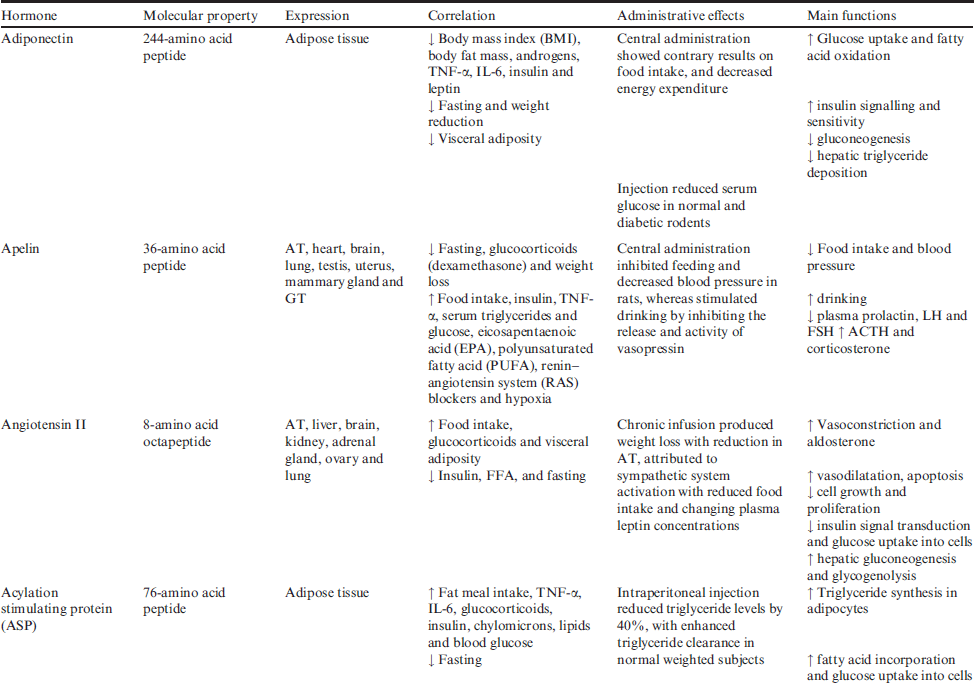
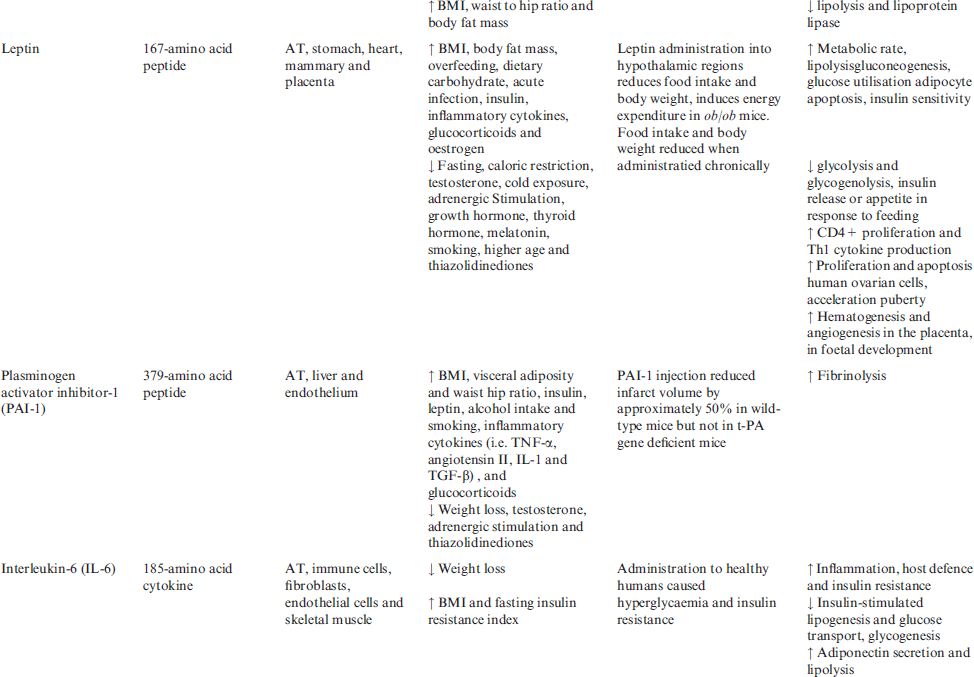
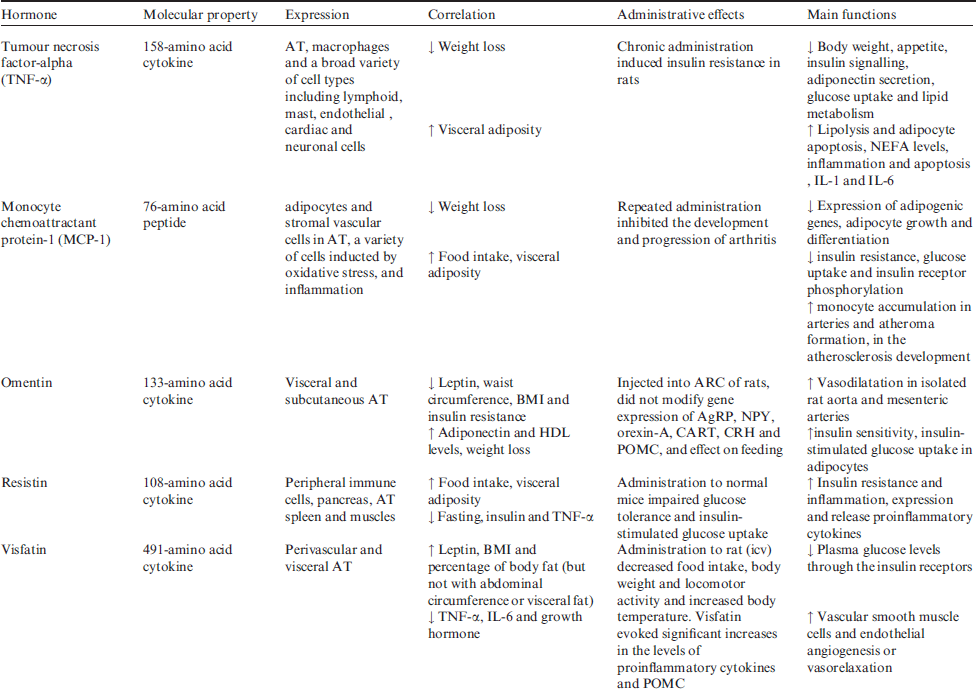

Adiponectin
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree