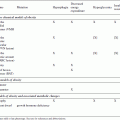
In a typical experiment, animals of different genotypes or in different experimental conditions are challenged with one or more experimental diets, and their biological responses under these diets are compared to those of animals on a control chow. While it is not possible to outline a single protocol to cover all such experiments, key parameters to consider include using appropriate numbers of age- and gender-matched mice (see Critical Parameters for more details) and maintaining the diets for sufficient times to induce the desired responses. Typical times required for the induction of various pathologies are indicated in Table 29B.5.1.
Most of the diets described in Table 29B.5.1 are generally formulated by commercial animal food suppliers. Alternatively, experimenters with specific requirements may want to have custom diets formulated either by commercial food suppliers that offer this service or by academic facilities that specialize in animal nutrition. In any case, it should be kept in mind that most of these diets are only partially defined diets, and variations in the composition of certain constituents can modify the biological response in vivo. For example, a high-fat diet is most commonly made from animal fat using lard, but the nature of lard triglycerides can vary according to the specific origin and can thereby affect the biological outcome.
BASIC PROTOCOL 2
ADMINISTRATION OF A TEST COMPOUND THROUGH THE DIET
Incorporating a compound in the diet to test for its physiological actions is an easy and efficient way to achieve reproducible daily exposure with minimal stress to the animals. The type of diet in which the compound is mixed should be chosen, on the one hand, according the appropriate diet for the purpose of the study and, on the other hand, according to the chemical properties and bioavailability of the compound itself. Indeed, the polarity of the compound can influence its bioavailability. For example, in the case of a lipophilic molecule, the fat content of the diet can modify its intestinal absorption by increasing its incorporation into lipid micelles. In addition, technical considerations relating to the amount of compound to incorporate, its polarity, and the type of diet chosen should be evaluated in order to achieve a homogeneous incorporation, which will ensure constant and homogeneous dosing of the animals (see Critical Parameters and Fig. 29B.5.3).
Materials
Determine daily food intake
The accuracy of the doses administered through food relies on the correct measurement of food intake (FI), which directly controls the amount of compound ingested. Therefore, FI should be evaluated, always housing the same number of animals per group.

Equation 29B.5.1
Calculate test compound dose
A test compound is generally administered at a constant dose normalized to body weight (mg compound/kg body weight). Since accomplishing this for each individual animal would require individual housing and preparation of a diet with a concentration of compound adapted to each mouse, a widely accepted simpler approach consists of using the average body weight (BW) and food intake (FI) as an estimate for each animal. Alternatively, in the experimental case where both the diet and the treatment are expected to minimally affect body weight, the compound can be administered at a constant concentration in the diet. The concentration should be calculated the same as for a constant dose, but only once, using the BW and FI calculated before the beginning of the treatment or from estimates of these parameters derived from pilot studies performed in the exact same experimental setting.
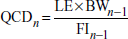
Prepare pellets containing test compound
Administer test compound in diet
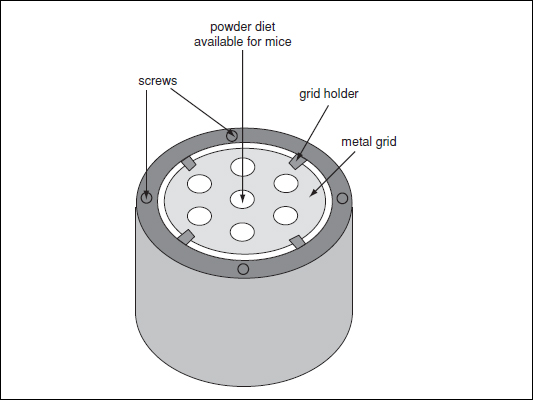
Figure 29B.5.1 Example of a food trough for powdered diet. Powdered diet is placed in the barrel-shaped container and the metal grid (which limits food spoiling and contamination) is positioned on top of the diet and secured with screws and a grid holder. Mice access food via the small holes of the grid.
BASIC PROTOCOL 3
CALORIC RESTRICTION
Caloric restriction (CR) is the main nongenetic manipulation known to extend lifespan in a wide variety of animal models. Although various CR strategies have been experimentally tested, with different levels of feeding restriction, CR is typically defined as a 33% to 50% decrease in total caloric intake. In order to maintain a balanced nutritional supply, diet composition is unchanged to ensure that all sources of nutrients, vitamins, and minerals are provided, and only the quantity of food the animals can access daily is held at levels lower than ad libitum.
The altered metabolic rate induced by this dietary manipulation has been demonstrated to produce a metabolic profile desirable for treating diseases of aging in both animal models and humans (e.g., metabolic disorders or neurodegeneration; Koubova and Guarente, 2003; Masoro, 2005). CR has also been shown to reduce the incidence and progression of spontaneous and induced tumors (Weindruch and Walford, 1988). It is thus not surprising that caloric-restricted mice are a model used extensively to understand the fundamental mechanisms underlying these prevalent human pathologies, and that developing pharmacological caloric restriction mimetics is a current research strategy for antineurodegenerative and anticancer interventions (Roth et al., 2005; Elliott and Jirousek, 2008).
An easily applicable method for restricting caloric intake is to feed the CR group with the same diet as a control group fed ad libitum, but to fast them for 24 hr on alternate days. This method leads to a 30% to 40% decrease of the global caloric intake (Weindruch et al., 1986). However, it generates cycles of lipolysis and fat storage that are not representative of a constant reduction in caloric intake.
The most reliable method for restricting caloric intake consists of pair-feeding CR mice at 50% to 67% of a control group fed ad libitum by restraining their daily food intake. A caloric restriction of 40% is recommended (i.e., pair-feeding at 60% of the ad libitum control). Ideally, mice should be housed in individual cages. If this is not possible, they should at least be housed at the same number of animals per cage. The quantity of food to be administered to the CR group should be evaluated daily as described in the steps below.
Materials
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree