Ocular Side Effects of Cancer Therapeutics
Ravi Bobba
Mark Klein
The increased use of chemotherapeutic agents combined with longer patient survival has led to a higher incidence and recognition of ophthalmologic side effects. Although such side effects are relatively uncommon, they may be quite severe, disabling, and irreversible.1 The clinician must be aware of potential ocular complications of chemotherapeutic agents, and where appropriate inform the patient of possible side effects and perform a baseline ophthalmologic examination prior to the initiation of therapy. This chapter reviews the incidence, clinical manifestations, and pathophysiology associated with ocular toxicity of the common chemotherapeutic agents.
ALKYLATING AGENTS
Busulfan
Carboplatin
Carboplatin may cause maculopathy and optic neuropathy weeks after intravenous administration.10,11 Intracarotid therapy can cause severe ocular and orbital toxicity.12 Left intracarotid administration of etoposide phosphate and carboplatin inferior to the ophthalmic artery in a patient with glioblastoma multiforme resulted in a glaucoma in the ipsilateral eye within 7 hours. This was treated with cycloplegics. Subsequently, there was severe orbital inflammation that resulted in decreased visual acuity, proptosis, optic neuropathy, and total external ophthalmoplegia in the ipsilateral eye requiring a lateral cantholysis and high-dose intravenous corticosteroids. Anterior uveitis developed 2 weeks later and was treated with topical corticosteroids.12 Subtenon injection has been associated with limitation of ocular motility.13 Higher doses of carboplatin than what is recommended was associated with vision loss with the recovery being either complete or incomplete after the drug was stopped.14 Papilledema was reported with high doses of carboplatin area under the curve (AUC) 12,11 but was also noted at the standard dose of AUC 5,15 which resolved after stopping the carboplatin chemotherapy and the initiation of steroid treatment.15
Chlorambucil
Ocular toxicity is rare; keratitis, diplopia, bilateral edema, and retinal hemorrhages have been reported.16
Cisplatin
Toxicities associated with intravenous administration of cisplatin have included papilledema,17 unilateral retrobulbar neuritis,17 bilateral retrobulbar neuritis,18 bilateral optic nerve swelling,19 optic neuritis,20 transient cortical blindness,21,22 sudden blindness,23 transient left homonymous hemianopsia,24 and altered color perception.25 Intracarotid artery bolus infusion may result in mild blurring of vision or permanent ipsilateral blindness secondary to retinal damage, periorbital pain, thrombosis of the central retinal artery, and retrobulbar neuritis.26,27,28,29 Infusion distal to the ophthalmic artery does not guarantee that the eye will be protected and can be associated with increased risk of cerebral toxicity.30,31,32,33,34
It is not known if cisplatin has a direct toxic effect on nerve fibers or if a vascular disturbance leads to optic nerve inflammation. Interestingly, a patient with cisplatin-induced hypomagnesemia had reversible cortical blindness,35 suggesting another potential mechanism.
Cyclophosphamide
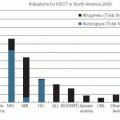
Intravenous cyclophosphamide (CY) may cause blepharoconjunctivitis or blurred vision.36 The 7-year long-term outcomes analysis of 316 patients with chronic myeloid leukemia (CML) and 172 with patients with acute myeloid leukemia (AML) in the randomized studies comparing the conditioning regimens before transplantation with either CY and total body irradiation (TBI) or Bu and CY found increased incidence of cataracts in the CML CY-TBI.37 Cataract incidence in the AML groups was (Bu-CY vs. CY-TBI) 12.3% and 12.4%, and 16% and 47% (P = .0003) among CML patients (Bu-CY vs. CY-TBI).37
Ifosfamide
Blurred vision and florid conjunctivitis were reported in a patient on the third day of treatment with combination therapy consisting of ifosfamide, mesna, vincristine, and doxorubicin.38 The ocular side effects lasted for several hours and resolved with the end of ifosfamide infusion.
Nitrogen Mustard
Nitrogen mustard has never been reported to have ocular side effects when given intravenously. Following intracarotid injection, however, patients have developed severe necrotizing uveitis in the ipsilateral eye.39
Nitrosoureas
The ocular side effects of nitrosoureas (bis-chloronitrosourea, BCNU; lomustine, CCNU; methyl-CCNU) in humans are not
well established, although transient blurring, loss of depth perception, and retinopathy have all been reported.40,41,42 Intracarotid infusion of BCNU has resulted in both transient and permanent ophthalmic complications felt to reflect both immediate vascular injury within the distribution of the ophthalmic artery and chronic ocular ischemia.43,44 In conjunction with autologous bone marrow transplantation, high-dose BCNU has been associated with nerve fiber-layer infarcts45 and ischemic microvascular lesions of the retina and/or optic disc.46 BCNU in combination with procarbazine was associated with optic neuroretinitis.41 Carmustine wafer implantation can be associated with visual field defects, diplopia, and eye pain can be seen in patients.47
well established, although transient blurring, loss of depth perception, and retinopathy have all been reported.40,41,42 Intracarotid infusion of BCNU has resulted in both transient and permanent ophthalmic complications felt to reflect both immediate vascular injury within the distribution of the ophthalmic artery and chronic ocular ischemia.43,44 In conjunction with autologous bone marrow transplantation, high-dose BCNU has been associated with nerve fiber-layer infarcts45 and ischemic microvascular lesions of the retina and/or optic disc.46 BCNU in combination with procarbazine was associated with optic neuroretinitis.41 Carmustine wafer implantation can be associated with visual field defects, diplopia, and eye pain can be seen in patients.47
ANTIBIOTICS
Doxorubicin (Adriamycin)
ANTIMETABOLITES
Cytosine Arabinoside
High doses of cytosine arabinoside (≥2 g per m2) can result in keratoconjunctivitis,53,54 ocular pain, tearing, foreign body sensation, photophobia, and blurred vision.55,56,57 On examination, patients have bilateral conjunctival hyperemia and fine corneal epithelial opacities. Symptoms typically begin 4 to 8 days after initiation of therapy and last 2 to 9 days. The incidence of side effects increases with increasing duration of therapy. Glucocorticoid eye drops given before the first dose of cytosine arabinoside may ameliorate or prevent side effects.58 Higa et al.54 has reported that artificial tears are as effective as glucocorticoid drops, suggesting that eye drops may decrease toxicity by diluting intraocular concentrations of the drug. The mechanism of the ocular toxicity of cytosine arabinoside is unclear, but may be due to inhibition of corneal epithelial DNA synthesis.59 Visual loss60 and optic neuropathy61 have been reported.
5-Fluorouracil
Topical 5-fluorouracil (5-FU 1%) is used as an adjuvant treatment after surgical excision in the treatment of localized noninvasive ocular surface squamous neoplasia.62,63 When topical preparations of 5-FU are applied near the eye, circumorbital edema, eyelid inflammation, conjunctival irritation, and corneal erosions have resulted.62,64,65 Systemic use of 5-FU alone or in combination chemotherapy regimens may cause canalicular mucosal inflammation, acute and chronic conjunctivitis that may lead to lacrimal duct stenosis and ectropion of the lower lids.33,66,67,68,69,70,71,72,73 5-FU may also potentiate radiation effects on the eye.74 It should be noted that the uncommon dihydropyrimidine dehydrogenase deficiency increases the toxicity of the 5-FU.75 In patients with 5-FU associated ocular toxicity, the 5-FU levels in the tears are the same as in plasma, and the same is not true in asymptomatic patients.76 The 5-FU tear concentration levels did not differ between asymptomatic or symptomatic ocular toxicity patients receiving CY, methotrexate and 5-FU.77 A complete eye and lacrimal system examination should be performed on all patients receiving 5-FU who develop symptoms. Cromolyn eye drops78 and cryotherapy79 have been reported as beneficial for 5-FU-associated conjunctivitis. For lacrimal gland inflammation, a trial of topical antibiotics/steroids to decrease inflammation may be warranted if therapy is nearly complete. If further 5-FU therapy is necessary, patients may be considered for silastic tubing intubation of the lacrimal system.66 Crystalline keratopathy from corneal deposits are noted to develop after adjunctive subconjunctival 5-FU application.80,81 There is a risk of conjunctival filtering bleb leak with local 5-FU use during trabeculectomy in patients with glaucoma filtering surgery.82,83
Methotrexate
Conjunctivitis, increased lacrimation, blurred vision, photophobia, and ocular pain may occur in patients who receive systemic methotrexate despite leucovorin rescue.84,85 Symptoms resolve with cessation of therapy.
There are two reports of optic nerve atrophy in patients with acute lymphoblastic leukemia who received systemic methotrexate along with intrathecal cytosine arabinoside and 2,400 rads of total brain irradiation as part of induction chemotherapy.86,87 A single case report describes an association of intrathecal administration of methotrexate and cytarabine with a diagnosis of posterior leukoencephalopathy.88
HORMONAL AGENTS
Corticosteroids
Since 1960, the relationship between systemic corticosteroids and the formation of posterior subcapsular cataracts has been generally accepted.93,94,95,96 Once cataracts form, they are usually stable in development with cessation of therapy. However, small numbers of patients have demonstrated cataract reversal, whereas others have shown progressive changes following reduction of dosage or discontinuation of steroids.
The pathology of steroid-related cataracts is similar to that of Bu- or age-related cataracts.97 A number of mechanisms may be involved, including inhibition of the sodium-potassium ATPase pump mechanism,95 binding of corticosteroids to lens proteins with alteration in the native configuration of the lens crystallins,98 and oxidation of protein groups with aggregation of lens crystallins.95
Tamoxifen
Tamoxifen-associated eye disease has been reviewed by Nayfield and Gorin.99 Prior to 1992, ocular toxicity, including retinopathy and bilateral optic neuritis, had been reported in only eight patients.100,101,102,103,104,105 Since then, multiple investigators have reported ocular side effects, including cataract formation, as additional patients received tamoxifen as therapy for breast cancer.106,107,108,109
In 1993 the National Surgical Adjuvant Breast and Bowel Project began Protocol P-1E to determine the prevalence of adverse ocular changes associated with the long-term use of tamoxifen.108 Although there was no vision-threatening ocular toxicity, there was a trend toward an increased frequency of intraretinal crystals with tamoxifen use; reports were more frequent in those women who had taken tamoxifen for the longest period. The incidence of posterior subcapsular opacities was also increased in patients who received tamoxifen.
A second study included women enrolled on the Breast Cancer Prevention Trial.109 Women at increased risk for breast cancer were randomized to tamoxifen or placebo. Information collected from 12,232 women demonstrated that the rate of cataract development among women who were cataract-free at the time of randomization was 21.72 per 1,000 women in the placebo group and 24.82 per 1,000 in the tamoxifen group (RR = 1.14; 95% CI = 1.01 to 1.29). In the placebo and tamoxifen groups, the rates of developing cataracts and undergoing cataract surgery were 3.00 and 4.72 per 1,000 women, respectively (RR = 1.57; 95% CI = 1.16 to 2.14).
Little is known about the pharmacodynamics of tamoxifen and its metabolites in ocular tissues. Case reports in breast cancer patients of stabilization of optic nerve head metastases and reduction in the size of retinal metastases with tamoxifen therapy suggest that the drug does penetrate the choriocapillaris and is exposed at least to the basal surface of the pigment epithelium.110 Pathologically, lesions have been confined to the nerve fibers and inner plexiform layers of the retina; electron microscopy demonstrates features suggestive of axonal degeneration.111 Tamoxifen and its derivatives are high-affinity blockers of specific chloride channels, which are essential for maintaining normal lens hydration and transmittance, suggesting a molecular mechanism by which tamoxifen can cause cataract formation.112
Anastrozole
The ATAC (Arimidex, Tamoxifen, Alone, or in Combination) trial randomized patients with hormone-receptor positive localized breast cancer to 5 years of anastrozole or tamoxifen.113 Cataracts were described in 6% of patients receiving anastrozole versus 7% receiving tamoxifen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree