Chemotherapy of Bone and Soft Tissue Sarcomas
Rashmi Chugh
Scott M. Schuetze
Sarcomas are an uncommon group of malignancies that arise from connective tissue and can be located in any part of the body. Most develop in mesodermal tissues, with the exception of malignant peripheral nerve sheath tumors, and primitive neuroectodermal tumors that are of ectodermal origin.1 Sarcomas have a wide age distribution, occurring in infants to elderly, and various histologic patterns. They, likewise, exhibit a spectrum of clinical behavior, ranging from nonaggressive tumors of well-differentiated cells to pleomorphic tumors that rapidly metastasize to distant organs. Sarcomas comprise approximately 0.86% of all new cancers in the United States each year.2 There are an estimated 10,500 new cases of soft tissue sarcomas (STSs) diagnosed annually and 2,600 new cases of bone sarcoma.2 Of these, 40% to 60% will go on to develop metastatic disease, for which chemotherapy is often the best option for treatment.
BONE SARCOMAS
Primary bone malignancies are uncommon, whereas metastatic spread of carcinoma to bone is quite common. Among the most common primary sites for bone metastasis are cancers of the breast, prostate, kidney, and thyroid. A primary bone cancer is any neoplasm that arises from the tissues or cells within bone and has the capacity to metastasize to distant organs. Many tumor types are possible, including cancers derived from osteoblasts, cartilage cells, fibrous tissue, fat, vascular elements, and hematopoietic and neural tissues. Multiple myeloma is a malignant plasma cell disorder that primarily arises within bone and has an incidence over six times of that seen from bone sarcomas. The most common primary bone sarcoma is osteosarcoma followed by chondrosarcoma. Ewing family of tumors comprises a group of malignant neoplasms usually containing characteristic chromosomal translocation involving the EWS locus that may arise in bone, soft tissues (previously called primitive neuroectodermal tumor), or chest wall (Askin tumor). Strikingly, in the last 30 years, the cure rate for patients with osteosarcoma or Ewing sarcoma has dramatically improved from 5% to 20% to 50% to 60%. This improvement principally resulted from the integration of orthopaedic oncologists with pediatric and/or medical oncologists.
Epidemiology
In the United States, malignant tumors of bone are diagnosed in approximately 2,600 patients yearly and 1,500 die of disease.2 The peak age incidence for osteosarcoma and Ewing sarcoma is in the second decade of life (typically around the age of 15 coincident with adolescent growth spurt),1 and chondrosarcoma has a peak incidence in the seventh decade. Osteosarcoma is the most common bone tumor in persons under the age of 20, and chondrosarcoma the most common in persons over the age of 50. Both osteosarcoma and Ewing sarcoma incident rate is the same for both sexes until age 13 after which point men are more commonly affected.3 Osteosarcoma occurs with the same prevalence in every country.3 Ewing sarcoma is 10 times more common in whites than in blacks (American or African) or Asians.3
Etiology
The increased incidence of bone sarcomas in teenage years supports the assumption that skeletal neoplasms arise in areas of rapid growth, which may be more susceptible to transformation. The most common location of primary bone sarcomas is the metaphyseal region adjacent to the growth plate and is the area of long bones with the most proliferation and remodeling. Approximately two-thirds of all bone sarcomas occur near the knee (distal femur or proximal tibia).4 Prolonged growth or overstimulation of bone metabolism from chronic conditions such as Paget disease, osteomyelitis, or bone infections can also lead to a bone sarcoma.5 In addition, external radiation treatment and bone-seeking radioisotopes from occupational or medicinal therapies have been linked to osteogenic sarcoma and chondrosarcoma.6
Chromosomal translocations that create fusion proteins can promote tumorigenesis. Almost all Ewing sarcoma cases are associated with a translocation between chromosomes 22 and 11 or 21, which result in a fusion between the EWS and FLI1 or ERG genes, respectively, and a translated chimeric protein that can function as a transcription activator.7,8 Loss of tumor suppressor function is also an important mechanism of cancer development. The two most studied tumor suppressor genes are RB and TP53; both are involved in osteosarcoma. Patients with hereditary retinoblastoma are at great risk for developing a second cancer, which is osteosarcoma in half of the cases. Almost one-third of patients with osteosarcoma, but without retinoblastoma, have mutation in or loss of expression of the RB gene.9,10 Similarly, a significant percent of patients with osteosarcoma have mutations of TP53.11,12 The MDM2 binds to TP53 and inhibits normal DNA binding.13 A minority of patients with osteosarcoma have overexpression of MDM2.14
OSTEOSARCOMA
Pathology
Primary bone sarcomas are classified according to their cytologic features and cellular products (i.e., osteoid or chondroid matrix). Osteosarcoma is the most common primary malignant bone sarcoma.15 It is typically composed of spindle cells and osteoblasts that produce immature bone or osteoid. It may arise on the surface of bone (parosteal and periosteal) or within bone (intramedullary) and may be low or high grade. Although osteosarcomas can occur as low- or high-tumor grade lesions (based on mitotic count; pleomorphism, and cellular atypia), approximately 90% are high grade. Osteoblastic, chondroblastic, and fibroblastic variants are recognized common subtypes of high-grade, intramedullary osteosarcoma. Parosteal and periosteal osteosarcomas are lower-grade neoplasms. Tumor grade is the most important factor in considering chemotherapy because high-grade osteosarcomas frequently metastasize and result in markedly poorer survival rates than low-grade osteosarcomas when treated with surgery alone.
Presentation and Initial Evaluation
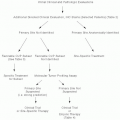
Patients with osteosarcoma typically present with localized pain that is progressive in intensity and may have a hard mass on examination. Serum alkaline phosphatase and/or lactate dehydrogenase may be elevated. Radiography is often helpful to differentiate between a benign and malignant process involving bone and should be performed in all cases of suspected malignancy.16 CT provides detailed information about the extent of tumor involvement in the cortex of bone and adjacent soft tissues. MRI can delineate tumor relationship with adjacent neurovascular structures, extent of marrow involvement, and skip metastasis, which usually involve intramedullary spaces, within bone. CT and MRI are considered complementary studies in the evaluation of suspected malignant bone tumors. CT of the lungs and bone scintigraphy are essential to accurately stage osteosarcoma. Arteriography is used at times to determine vascular anatomy and displacement in the evaluation for limbsparing surgery.
Prognosis
A number of prognostic factors for disease outcomes in patients treated for high-grade osteosarcoma with surgery and chemotherapy have been identified. Elevated levels of serum alkaline phosphatase and lactate dehydrogenase at diagnosis have been associated with reduced disease-free and/or overall survival.17,18,19,20 An analysis of the influence of patient age, sex, anatomic tumor location, tumor size, and extent of tumor necrosis following chemotherapy in multiple studies reporting on moderate sample sizes suggested that patient age, sex, and anatomic location do not affect survival.21 After multivariate analysis, the extent of tumor necrosis following chemotherapy most consistently correlated with disease-free survival. Patients with <10% residual viable tumor after neoadjuvant chemotherapy fared better than patients with a poorer tumor response. Meyers et al.19 reported a multivariate analysis of 279 osteosarcoma patients without metastasis at diagnosis, which identified axial location of the primary tumor as an independent risk for reduced disease-free survival. A more recent multivariate analysis that included 1,700 patients with high-grade osteosarcoma identified the presence of metastasis at diagnosis, macroscopic residual disease after surgery, tumor site (axial vs. appendicular skeleton), and poor histologic response to preoperative chemotherapy as predicting reduced survival rates at 10 years after diagnosis.22 Less than 10% of patients with macroscopic residual (unresected) disease survived longer than 5 years versus >60% of patients rendered free of disease by surgery; therefore, complete resection of osteosarcoma, when feasible, is vital to best management. At present, prognostic factors in osteosarcoma are important in clinical trial design and analysis but do not directly impact clinical practice recommendations. Patients with >10% residual microscopic tumor (poor response) after preoperative chemotherapy in the retrospective multivariate analysis were more than twice as likely to die of osteosarcoma as patients with a better histologic response. An ongoing, multinational, randomized trial (EURAMOS-1) European and American Osteosarcoma Study Group is attempting to answer whether integration of additional chemotherapy agents into the postoperative treatment plan for patients with a poor tumor response to preoperative chemotherapy improves patient outcome.
Adjuvant Chemotherapy for Osteosarcoma
Adjuvant chemotherapy is generally reserved for the treatment of bone sarcomas that are high grade. Early studies of adjuvant chemotherapy after amputation suggested that there was an improvement in disease-free survival or delay in time to distant recurrence of disease in the series of patients treated with single-agent doxorubicin or methotrexate compared with historical groups of patients treated with amputation alone.23,24 Later studies of adjuvant chemotherapy that combined drugs demonstrated reduced rates of osteosarcoma recurrence.25,26 Randomized trials confirmed that multiagent chemotherapy including doxorubicin, methotrexate, cyclophosphamide, bleomycin, and dactinomycin compared with no chemotherapy significantly reduced risk of disease recurrence and improved survival in patients with localized osteosarcoma.27,28
Rosen et al at Memorial Hospital in New York developed a series of neoadjuvant chemotherapy protocols that provided time for surgical planning and construction of osseous and joint prostheses, and early initiation of systemic therapy to treat micrometastatic disease and to evaluate the outcomes related to histologic response of tumor to chemotherapy. Meyers et al.19 from Memorial Hospital reported a 76% probability of disease-free survival at 5 years from diagnosis in patients <22 years treated at a single institution with the T10 protocol using methotrexate, doxorubicin, cyclophosphamide, dactinomycin, and bleomycin. This compared favorably to the historical data suggesting less than a 20% survival rate for patients treated with surgery alone.
A randomized study performed in multiple centers by the European Osteosarcoma Intergroup suggested that the more complicated and protracted chemotherapeutic regimen, T10, did not result in significantly better disease-free or overall survival than treatment with 75 mg/m2/cycle of doxorubicin and
100 mg/m2/cycle of cisplatin given for six cycles.29 Progression-free survival (PFS) and overall survival at 5 years were 44% and 55%, respectively, and similar in patients treated with the T10 regimen or doxorubicin/cisplatin. The results from the multicenter study were inferior to the single institution results reported by Meyers et al., but compliance with and delivered chemotherapy dose intensity in patients treated with the T10 regimen in the randomized study was poor. In addition, for practical reasons, the multicenter study did not alter adjuvant therapy based on tumor histologic response that may have biased the results. A randomized study of doxorubicin, methotrexate, and cisplatin versus doxorubicin, methotrexate, cyclophosphamide, dactinomycin, and bleomycin reported equivalent disease-free survival rates of 75% at 30 months supporting a place for cisplatin in osteosarcoma therapy.30
100 mg/m2/cycle of cisplatin given for six cycles.29 Progression-free survival (PFS) and overall survival at 5 years were 44% and 55%, respectively, and similar in patients treated with the T10 regimen or doxorubicin/cisplatin. The results from the multicenter study were inferior to the single institution results reported by Meyers et al., but compliance with and delivered chemotherapy dose intensity in patients treated with the T10 regimen in the randomized study was poor. In addition, for practical reasons, the multicenter study did not alter adjuvant therapy based on tumor histologic response that may have biased the results. A randomized study of doxorubicin, methotrexate, and cisplatin versus doxorubicin, methotrexate, cyclophosphamide, dactinomycin, and bleomycin reported equivalent disease-free survival rates of 75% at 30 months supporting a place for cisplatin in osteosarcoma therapy.30
A Southwest Oncology Group (SWOG) trial of doxorubicin, ifosfamide (with 2-mercapto-ethane sulphonate [MESNA]), and dacarbazine (MAID regimen) in advanced metastatic sarcomas showed a poor 26% response rate and median survival of 10 months in adults with osteosarcoma.31 The authors concluded that cisplatin should be included in therapy for osteosarcoma. Other studies have demonstrated that doxorubicin is an important component of chemotherapy for osteosarcoma; favorable histologic response and metastasis-free survival rates were worse when doxorubicin was omitted. Loss of hearing in the high-frequency range is a long-term toxicity concern from treatment with cisplatin.32 Hearing loss detected by audiometry has been reported in 50% of patients with osteosarcoma treated with cisplatin; the magnitude and incidence of high-frequency hearing loss increased with increasing cumulative doses of cisplatin, especially with doses33 above 240 mg per m2. A significantly higher incidence of hearing loss was found in pediatric patients receiving cisplatin administered in 1 day (incidence 78%) versus 2 days (incidence 30%) per cycle.34 Hearing loss severe enough to interfere with normal activities is uncommon occurring in <10% of patients. Many of the current osteosarcoma chemotherapy protocols recommend administering cisplatin over 4 hours daily for 2 days or over a 48- to 72-hour infusion to minimize adverse impact on hearing.
The value of methotrexate in the treatment of osteosarcoma in adults is controversial. The European Osteosarcoma Intergroup conducted a randomized comparison of doxorubicin 75 mg/m2/cycle with cisplatin 100 mg/m2/cycle for six cycles to doxorubicin 75 mg/m2/cycle, cisplatin 100 mg/m2/cycle and methotrexate 8 g/m2/cycle for four cycles to ask whether methotrexate improves outcome.35 Overall survival was not different between the groups, and disease-free survival was better in the group that did not receive methotrexate. The group treated with methotrexate received a lower total dose of doxorubicin and cisplatin, which may have biased the results. A European Osteosarcoma Intergroup randomized trial of doxorubicin and cisplatin versus a modified T10 regimen did not detect a significant improvement in survival with the use of methotrexate, but the delivered dose intensity in the group receiving the T10 regimen was compromised by noncompliance.29
Favorable outcomes associated with the use of high doses of methotrexate by the Memorial Hospital group and others prompted additional investigation into the potential dose-response relationship of methotrexate combined with other agents. Two randomized studies did not detect a difference in disease-free survival between moderate doses (200 mg per m2) versus high doses (2 to 8 g per m2) of methotrexate combined with doxorubicin.36,37 A Cooperative Osteosarcoma Study Group trial suggested that peak serum methotrexate levels above 1,000 µm after infusion of 12 g per m2 over 4 hours was associated with better disease-free survival in osteosarcoma than lower concentrations, but this finding has not been confirmed by others.37,38,39 Methotrexate, especially at high doses, is generally more difficult to administer in adults than children because of the potential for reduced methotrexate clearance rates and reduced renal function from comorbid diseases and prior exposure to nephrotoxic agents.40 The total dose of methotrexate administered per course is usually capped at 20 g and close attention must be paid to renal clearance of the drug via methotrexate serum levels and leucovorin rescue to minimize the risk of life-threatening toxicity. The toxicity rate from high-dose methotrexate in adults is substantial.41 Despite the mixed results, high-dose methotrexate with leucovorin rescue given by experienced physicians especially to young patients seems warranted.
Ifosfamide and etoposide have significant antitumor activity in relapsed osteosarcoma and as first-line therapy for metastatic disease in children.42,43 However, the addition of ifosfamide to a regimen of cisplatin, doxorubicin, and methotrexate for treatment of localized disease did not improve the rate of favorable histologic response (<10% residual viable tumor) or 5-year event-free survival in a randomized comparison with cisplatin, doxorubicin, and methotrexate.13 Patients received 4 courses of cisplatin 120 mg per m2, 6 courses of doxorubicin 75 mg per m2, and up to 12 courses of methotrexate 12 g per m2 (dose capped at 20 g per course) with or without 5 courses of ifosfamide 9 g per m2. Analysis of outcome results from 677 children with localized osteosarcoma showed a 64% and 53% probability of event-free survival at 5 years without or with inclusion of ifosfamide, respectively. An SWOG phase II single-arm study in 63 adults with localized osteosarcoma treated with doxorubicin, cisplatin, and ifosfamide did not demonstrate improvement in survival over prior studies using doxorubicin and cisplatin alone.44 One half of the patients experienced disease relapse, median time to progression was 18 months, and probability of survival at 5 years was 58%. The European Osteosarcoma Intergroup also failed to demonstrate an advantage to the addition of ifosfamide to doxorubicin and cisplatin in a phase II study.45 The probability of survival at 5 years was 62%.
Altering Dose Intensity and Frequency
Intensification of chemotherapy using standard agents does not seem to be a promising means of reducing disease recurrence rates. A single institution nonrandomized study using a dosedense schedule of doxorubicin, cisplatin, and ifosfamide in 19 adults with localized osteosarcoma showed a 70% probability of event-free and overall survival at 5 years from study registration, which did not appear to be significantly different from outcomes of studies delivering treatment every 3 to 4 weeks.46
Likewise, intensification of chemotherapy doses has not produced superior results over the use of more standard doses and has been more toxic. Dose intensification of ifosfamide to 15 g/m2/course in patients up to age 40 (median age of treated patients was 14 years) with localized osteosarcoma resulted in a 64% and 77% probability of 5-year event-free and overall survival, respectively, and resulted in severe thrombocytopenia and nephrotoxicity in 31% and 10% of patients, respectively.47 Dose intensification of ifosfamide to 10 g/m2/course in adults (median age 38 years) with poor prognosis osteosarcoma resulted in an approximate 45% probability of survival at 5 years. As discussed in the preceding text, intensification of the methotrexate dose has not improved disease-free or overall survival in randomized comparisons.36,48
Likewise, intensification of chemotherapy doses has not produced superior results over the use of more standard doses and has been more toxic. Dose intensification of ifosfamide to 15 g/m2/course in patients up to age 40 (median age of treated patients was 14 years) with localized osteosarcoma resulted in a 64% and 77% probability of 5-year event-free and overall survival, respectively, and resulted in severe thrombocytopenia and nephrotoxicity in 31% and 10% of patients, respectively.47 Dose intensification of ifosfamide to 10 g/m2/course in adults (median age 38 years) with poor prognosis osteosarcoma resulted in an approximate 45% probability of survival at 5 years. As discussed in the preceding text, intensification of the methotrexate dose has not improved disease-free or overall survival in randomized comparisons.36,48
Biologic Therapy
Investigators at the Karolinska Hospital explored the activity of interferon before widespread adoption of multiagent adjuvant chemotherapy for the management of localized high-grade osteosarcoma. Eighty-nine patients received interferon-α as adjuvant treatment for 18 months to 5 years. With follow-up of >10 years from diagnosis, overall survival was 43% at 10 years and is provocative for antitumor activity of interferon-α in osteosarcoma. However, a randomized trial of adjuvant chemotherapy with or without interferon-β failed to show a survival difference between arms.30 The ongoing EURAMOS study is attempting to answer in a randomized design whether the administration of interferon alfa-2B for 18 months after the completion of standard chemotherapy for patients with a good histologic tumor response will improve the relapse-free survival rate.
Mifamurtide (liposomal muramyl tripeptide phosphatidyl ethanolamine) is a synthetic analog of a component of the cell wall of Bacille Calmette-Guerin that activates macrophages and monocytes. Mifamurtide has activity in canine osteosarcoma.49 A nonrandomized study suggested that the administration of mifamurtide for 24 weeks after resection of lung metastasis prolonged time to subsequent relapse of osteosarcoma compared with an historical control group.50 A randomized study of mifamurtide administered with chemotherapy after resection of localized osteosarcoma in patients <30 years of age detected an 8% improvement in the overall survival rate at 6 years from study entry compared to standard chemotherapy alone.51 There was a trend in improvement in event-free survival in the group receiving mifamurtide that did not reach the level of statistical significance. The potential causes of a significant impact on overall survival without an effect on event-free survival in patients treated with mifamurtide have raised controversy. Mifamurtide has been approved for use in children and young adults with nonmetastatic osteosarcoma by regulatory agencies in Europe but remains investigational in the United States.
Tailoring Therapy to Tumor Response
Increasing the number of agents used in neoadjuvant chemotherapy of osteosarcoma is associated with higher proportions of patients obtaining a good histologic response of tumor to treatment (often defined as <10% residual viable tumor) but has not similarly improved survival.52,53,54 A different approach to improve outcomes is tailoring adjuvant therapy to the tumor response to preoperatively administered drugs. Rosen et al.55 described favorable outcomes for patients with poor histologic response to methotrexate-containing induction therapy after substitution of cisplatin for methotrexate. But, longer follow-up failed to demonstrate an advantage to altering adjuvant treatment for poor responses.19 Bacci et al. at the Rizzoli Institute for Orthopaedic Surgery in Bologna administered two cycles of neoadjuvant methotrexate, doxorubicin, and cisplatin to 164 patients with osteosarcoma localized to an extremity. Patients with a good histologic response received adjuvant methotrexate, doxorubicin, and cisplatin, whereas patients with a poor histologic response received ifosfamide and etoposide in addition to the aforementioned drugs.56 With a reported average follow-up of 54 months, 57% of the patients with poor histologic response were free of disease, which was significantly better than the 32% event-free survival in the group’s preceding study of methotrexate and cisplatin. The event-free survival in patients with a good histologic response was similar between studies at approximately 70%. To our knowledge, it has not been established that altering chemotherapy in the adjuvant setting for patients with residual viable tumor after neoadjuvant chemotherapy reduces the risk of metastasis. Residual osteosarcoma after induction therapy probably represents innate resistance to drugs, which may not be altered by the administration of other conventional cytotoxic chemotherapy. The ongoing EURAMOS randomized trial of the same versus alternate chemotherapy as given preoperatively in poorly responding tumors may help to address this hypothesis. However, patients with poor tumor response to chemotherapy fare better than historical groups treated with surgery alone; therefore, postoperative chemotherapy should not be withheld for a perceived lack of treatment effect on the primary tumor.
Therapy for Advanced/Recurrent Osteosarcoma
The combination of ifosfamide and etoposide is active in relapsed or metastatic osteosarcoma, resulting in objective responses in approximately 60% of patients.42,43 The projected 2-year progression-free and overall survivals were 43% and 55%, respectively. The combination can be very myelosuppressive, and prophylactic growth factor support is usually administered. Treatment of relapsed osteosarcoma with ifosfamide, etoposide, and methotrexate after primary doxorubicin and cisplatin therapy resulted in a similar response rate of 62%.57 With a relatively short duration of follow-up, the 2-year survival was estimated to be approximately 50%; therefore, the addition of high-dose methotrexate to ifosfamide and etoposide in the relapsed setting does not appear to improve responses or prolong survival. Patient participation in clinical studies for treatment of advanced or recurrent osteosarcoma should be encouraged to identify additional active agents.
Retrospective reviews of patient outcomes following relapse of osteosarcoma have demonstrated a clear benefit from complete surgical resection of pulmonary metastasis.58,59,60 Long-term disease-free survival can be achieved in 7% to 30% of patients with the lung as the first site of recurrence. In multivariate analysis, >2
years from diagnosis to first relapse and the presence of one or two metastatic nodules were significantly associated with higher post-relapse event-free survival.58,60 Achieving remission with surgery is necessary for long-term survival and patients treated with chemotherapy alone will ultimately succumb to disease. Thoracotomy with wedge metastasectomy has been the preferred surgical approach. To our knowledge, a randomized comparison of open thoracotomy to thoracoscopic metastasectomy in osteosarcoma has not been reported. The role of chemotherapy in the control of disease after metastasectomy has not been defined, but the use of high-dose ifosfamide (>10 g/m2/cycle) has been reported and treatment with chemotherapy has been associated with improved event-free survival.60 Second pulmonary recurrences should be resected if surgical remission can be accomplished. Long-term survivorship has been reported after four or more episodes of recurrence in the lungs managed by metastasectomy.61 Patients who experience isolated local recurrence or solitary metastasis in the bone should be treated with surgery if a second completed remission can be achieved.59,62 In advanced cases of osteosarcoma in which complete resection is not possible, chemotherapy can extend survival but is not curative.
years from diagnosis to first relapse and the presence of one or two metastatic nodules were significantly associated with higher post-relapse event-free survival.58,60 Achieving remission with surgery is necessary for long-term survival and patients treated with chemotherapy alone will ultimately succumb to disease. Thoracotomy with wedge metastasectomy has been the preferred surgical approach. To our knowledge, a randomized comparison of open thoracotomy to thoracoscopic metastasectomy in osteosarcoma has not been reported. The role of chemotherapy in the control of disease after metastasectomy has not been defined, but the use of high-dose ifosfamide (>10 g/m2/cycle) has been reported and treatment with chemotherapy has been associated with improved event-free survival.60 Second pulmonary recurrences should be resected if surgical remission can be accomplished. Long-term survivorship has been reported after four or more episodes of recurrence in the lungs managed by metastasectomy.61 Patients who experience isolated local recurrence or solitary metastasis in the bone should be treated with surgery if a second completed remission can be achieved.59,62 In advanced cases of osteosarcoma in which complete resection is not possible, chemotherapy can extend survival but is not curative.
Radiotherapy for Osteosarcoma
High-grade osteosarcoma is generally believed to be only moderately sensitive to radiation and is not a routine part of multidisciplinary care. However, in selected cases, radiation may contribute to long-term control of disease and be worth considering when complete resection is not feasible, for example, osteosarcoma occurring in the axial skeleton, or if surgery is refused.63,64 Primary radiation is likely to substantially benefit only patients with low-tumor burden or a good response to chemotherapy. The use of protons, carbon ions, or infusion of bone-seeking radioisotopes can increase the tumor-to-normal tissue radiation dose. Palliation of symptoms, but only limited effect on tumor progression, was reported from the use of samarium, with or without gemcitabine as a tumor radiosensitizer, in metastatic osteosarcoma.65,66
OSTEOSARCOMA VARIANTS
Osteosarcoma of the Jaw
Osteogenic sarcoma arising in the maxilla or mandible accounts for approximately 5% of osteosarcomas. Osteosarcoma of the jaw occurs in older patients than osteosarcoma involving long bones, with a mean age of 35.67,68 Craniofacial radiation is a predisposing factor and radiation-induced osteosarcoma of the jaw generally follows a more aggressive clinical course. More of the osteosarcomas of the jaw are high grade rather than low grade, but the frequency of metastasis is lower at approximately 20% than in osteogenic sarcomas of long bones.67 Radical resection of tumor to achieve clear surgical margins offers the best chance for cure; uncontrolled local disease is the main cause of death. Patients with completely resected low-grade osteosarcomas of the jaw do well without adjuvant chemotherapy. Adjuvant chemotherapy seems to improve disease-free and overall survival in patients with high-grade lesions.68
Surface Osteosarcoma
Parosteal osteosarcomas are low-grade tumors arising from the surface of bone. Complete resection without chemotherapy is usually curative. Dedifferentiation of tumor occurs in a minority of cases and is associated with a high risk of tumor-related mortality.69 Periosteal osteosarcomas are bone surface tumors of intermediate malignant potential. The most common site for tumor involvement is the lower extremity (femur followed by tibia). The probability of long-term overall survival after complete resection is >80%, and improvement in outcome from adjuvant chemotherapy has not been demonstrated.70 However, data from randomized clinical trials is lacking and metastases occur in up to 10% of cases. High-grade surface osteosarcoma is a rare subtype that is distinct from parosteal and periosteal osteosarcoma and accounts for <10% of malignant surface bone tumors. Similar to periosteal osteosarcoma, high-grade surface osteosarcoma most commonly occurs in the lower extremity during the second decade of life.71 Pulmonary metastases frequently arise, and localized disease should be treated with wide resection and doxorubicin-based chemotherapy.71 Histologic response to neoadjuvant chemotherapy predicts improved survival.71
Extraskeletal Osteosarcoma
Extraskeletal osteosarcoma is a rare, highly malignant tumor producing an osteoid or chondroid matrix and without direct attachment to bone or periosteum. Most cases arise deep to muscle fascia and in the lower extremity.72,73 The median age at presentation is older than with conventional osteosarcoma. Long-term event-free and overall survival after treatment with complete resection and doxorubicin-based chemotherapy approaches 50%. Investigators at MD Anderson have recommended doxorubicin and ifosfamide as the preferred regimen.73 With a relatively short median follow-up of 3 years, the Cooperative Osteosarcoma Study Group has reported an estimated 5-year event-free survival of 69% for patients achieving a completed surgical resection and treated with doxorubicin, ifosfamide, and cisplatin.72 Conclusions regarding the role of radiation therapy in the management of extraskeletal osteosarcoma cannot be made because of limited available data.
Malignant Fibrous Histiocytoma of Bone
Malignant fibrous histiocytomas of bone (MFH-B) are highgrade spindle cell lesions without osteoid or chondroid formation arising in bone. They account for approximately 5% of bone tumors.74 Treatment of MFH-B with surgery and chemotherapy in a similar manner as high-grade osteosarcoma results in a high rate of tumor response assessed histologically and patient survival. A European Osteosarcoma Intergroup Study of surgery and six cycles of doxorubicin and cisplatin chemotherapy for treatment of localized, extremity MFH-B resulted in a 56% probability of disease-free survival at 5 years from diagnosis.74 Similar results have been obtained using regimens incorporating ifosfamide. MFH-B should be treated aggressively using surgery and chemotherapy.
EWING FAMILY OF TUMORS
Ewing sarcoma is an undifferentiated bone tumor that can also arise in the soft tissues (primitive neuroectodermal tumor) and the chest wall (Askin tumor), a spectrum of neoplastic diseases known as the Ewing sarcoma family of tumors. Ewing sarcoma accounts for 10% to 15% of malignant bone sarcomas.15 The cause is unknown, and it is not associated with exposure to radiation or familial cancer syndromes. The morphologic appearance of Ewing sarcoma is that of a primitive undifferentiated neoplasm with monotonous sheets of small round blue cells. There is usually extensive necrosis with viable tumor around blood vessels. Ewing sarcoma is a high-grade neoplasm. Although no routinely used histochemical stains can uniformly distinguish Ewing sarcoma from other small round blue cell neoplasms, the vast majority express high levels of a cell surface glycoprotein (CD99), which is encoded by the MIC2 gene, and FLI-1.75 CD99 expression in the cytoplasmic membrane and expression of FLI-1 are sensitive but not specific markers for Ewing. Fluorescence in situ hybridization and PCR assays to detect the EWS gene rearrangement, which is present in most of Ewing cases, are available to aid in the differential diagnosis of challenging cases.
Patients often present with constitutional symptoms such as fever, fatigue and weight loss, pain, and a firm mass. Ewing usually occurs in the second or third decades of life, but older individuals can be affected. Outcomes in individuals >40 years of age treated aggressively appear to be as good as in younger patients.76 The outcome in adults treated with abbreviated course (6 to 10 cycles, median 10 cycles) of chemotherapy compared with pediatric patients receiving a standard course (10 to 17 cycles, median 16 cycles) appeared to be significantly worse in a retrospective analysis performed in Canada.77 Common sites of involvement are femur (22%), pelvis (19%), tibia/fibula (15%), and humerus (10%).4 Early hematogenous dissemination gives rise to metastasis in the lungs, and less frequently bone. Rarely are lymph nodes, abdominal organs, brain, and marrow involved. Approximately one-third of patients initially present with detectable metastatic disease; however, most, if not all, Ewing patients have micrometastatic disease at the time of diagnosis. Relapse rates after local therapy alone are in excess of 90%. The presence of fever, anemia, or an elevated serum lactate dehydrogenase at diagnosis, a pelvic primary tumor, and large tumor volume are poor prognostic factors in patients with localized disease.78,79,80 The worse prognosis associated with pelvic Ewing may be related to larger size of tumor compared with extremity lesions at diagnosis.81,82 Favorable histologic response of Ewing to neoadjuvant chemotherapy correlates with survival.78,79,81,82,83 Mutation inp53 and/or deletion of p16/p14ARF correlate with poor histologic response to chemotherapy and a lower survival rate.84
Primary Therapy for Ewing
The first Intergroup Ewing’s Sarcoma Study (IESS) demonstrated a clear advantage for the addition of doxorubicin to cyclophosphamide, dactinomycin, and vincristine in patients with Ewing sarcoma localized in an extremity in a randomized trial.85 The second IESS demonstrated a survival advantage for 1,400 mg per m2 of cyclophosphamide administered every 3 weeks over 500 mg per m2 of cyclophosphamide given weekly.86 In a nonrandomized study, substitution of cyclophosphamide by ifosfamide at a biologically higher dose and an increase in the dose of doxorubicin from 50 to 60 mg per m2 resulted in improved survival for patients with localized or metastatic disease.87 Incorporation of ifosfamide and etoposide with cyclophosphamide, doxorubicin, and vincristine has improved significantly 5-year event-free and overall survival rates for patients with localized, but not for patients with metastatic disease and can be considered a standard therapy.80 Cyclophosphamide 1,200 mg/m2, doxorubicin 75 mg/m2, and vincristine 2 mg/course administered in alternating cycles every 3 weeks with ifosfamide 1.8 g/m2/day and etoposide 100 mg/m2/day for 5 days per course for up to 17 cycles or approximately 1 year resulted in a 72% and 34% probability of survival at 5 years for patients with localized and metastatic disease, respectively. Dactinomycin was substituted for doxorubicin when a total doxorubicin dose of 375 mg per m2 was reached. Compression of the frequency of chemotherapy cycles from every 3 weeks to every 2 weeks using granulocyte-colony stimulating factor support may improve the relapse-free survival rate by 5% to 10% in patients with localized Ewing at diagnosis, but the final report from the study evaluating this approach is pending.88 Symptomatic cardiomyopathy occurring years after chemotherapy for Ewing has been reported but is rare.83 Azoospermia is more likely to develop than permanent amenorrhea.83 Late recurrence of disease >5 years after diagnosis is not a rare event, and patients should be followed long-term for recurrence and secondary malignancies.
Local control of disease is achieved using surgery or radiation usually after the fourth cycle of chemotherapy. Complete resection of tumor is preferred. Radiation is administered adjuvantly for treatment of microscopic residual disease or if surgery cannot be performed. Radiation doses above 40 Gy are associated with lower rates of local failure when applied as primary local therapy for tumors <8 cm in size. Chemotherapy, with the exception of doxorubicin and dactinomycin, is generally administered during radiation but attenuated doses of cyclophosphamide are given if a significant portion of the marrow is within the radiation field. Radiation-induced sarcomas were reported to have occurred in 3% of the patients surviving >5 years from diagnosis of Ewing.83
Despite advances in chemotherapy treatment of localized Ewing, the outcome remains poor for patients with metastatic disease at diagnosis. Patients with extrapulmonary metastasis have the lowest rate of survival.89 In a multivariate analysis of 114 patients with metastatic Ewing not involving extrapulmonary sites at diagnosis, complete histologic response of the primary tumor to chemotherapy, unilateral lung involvement, and whole lung radiation were independently associated with improved event-free survival.89 The probability of 5-year event-free survival in patients who received whole lung radiation was approximately 35%. Pulmonary metastasectomy for Ewing does not seem to impact survival. Intensification of cyclophosphamide and ifosfamide did not improve outcome compared
with previous studies.90 High-dose chemotherapy and/or total body irradiation supported by transplanted autologous stem cells failed to improve outcomes in patients with metastatic Ewing.91,92 Euro-Ewing Intergroup EE99 is a currently accruing randomized study of protracted chemotherapy versus consolidation using high-dose busulfan and melphalan with an accrual goal of 1,200 patients that may help define the role of high-dose therapy in Ewing.
with previous studies.90 High-dose chemotherapy and/or total body irradiation supported by transplanted autologous stem cells failed to improve outcomes in patients with metastatic Ewing.91,92 Euro-Ewing Intergroup EE99 is a currently accruing randomized study of protracted chemotherapy versus consolidation using high-dose busulfan and melphalan with an accrual goal of 1,200 patients that may help define the role of high-dose therapy in Ewing.
Chemotherapy for Relapsed/Refractory Ewing
Ifosfamide combined with etoposide is active in relapsed Ewing not previously treated with the agents.93 Cyclophosphamide followed by topotecan daily for 5 days is more active than topotecan alone and is a reasonable option.90,94 Irinotecan combined with temozolomide may have schedule-dependent synergy and has been evaluated in pediatric patients. Analyses of patients with relapsed Ewing treated with irinotecan 10 to 20 mg per m2 daily days 1 to 5 and 8 to 12 along with temozolomide 100 mg per m2 daily on days 1 to 5 of each cycle suggests clinically meaningful transient control of disease in about 50% of patients.95,96 Irinotecan combined with vincristine is under study in early phase trials. Inhibitory monoclonal antibody to the insulin-like growth factor-1 receptor has demonstrated single-agent activity and very good tolerability in patients with relapsed Ewing and likely will be further developed in combination with chemotherapy.97 High-dose therapy may improve survival in specific subsets, for example, patients with chemotherapy-sensitive relapsed disease.98 Survival rate at 5 years from relapse may approach 20% and appears to be better in patients with >2 years from initial diagnosis to relapse. Prognosis is very poor for patients with primary resistant disease, and participation in phase II studies is encouraged to help identify additional active agents.
CHONDROSARCOMA
Malignant tumors that produce pure hyaline cartilage are chondrosarcomas and the second most common bone sarcoma in adults.99 Chondrosarcomas, unlike most other primary bone sarcomas, have increased frequency with increased age, and the tumors frequently involve the axial skeleton. Although chondrosarcomas may be primary, they are also frequently secondary, developing in preexisting benign lesions such as enchondromas and osteochondromas.100,101,102 In syndromes with multiple occurrences of these lesions such as enchondromatosis (Ollier disease) or hereditary multiple exostosis, the incidence of malignant transformation of one or more of these lesions can be as high as 25%.103,104 Although secondary chondrosarcomas are rare in people <50, they frequently occur in young adults with Ollier or multiple hereditary osteochondromatosis. Biologic behavior is based on tumor grade (e.g., low, intermediate, or high) as well as location. More centrally located chondrosarcomas have a greater propensity to metastasize as do high-grade lesions. Recognized major subtypes are conventional chondrosarcoma, dedifferentiated chondrosarcoma, mesenchymal chondrosarcoma, and clear cell chondrosarcoma. Clear cell chondrosarcoma is a rare, low-grade variant managed by complete excision. Incompletely excised tumor can recur and occasionally metastasize.
Conventional chondrosarcomas arise in previously normal bone and account for approximately 90% of malignant cartilage tumors. The pelvis is most frequently involved followed by the proximal femur and proximal humerus. Local pain is the most common presenting symptom. En bloc excision is the recommended treatment. Local recurrence develops in approximately 20% of cases and is associated with intralesional or marginal surgical margins, tumor in the shoulder or pelvis, and highergrade lesions.105 Multiple recurrent disease often presages metastases. Metastasis develops in approximately 15% of cases and is associated with grades 2 and 3 tumors.105 Grade 1 (low grade) chondrosarcoma has an excellent prognosis. Conventional chondrosarcoma is relatively resistant to chemotherapy and radiation therapy and is therefore not indicated outside of a clinical trial.
Dedifferentiated chondrosarcoma is a highly aggressive malignancy containing clearly defined regions of a low-grade cartilage tumor adjacent to a high-grade spindle cell neoplasm and accounts for approximately 10% of chondrosarcoma cases. Dedifferentiated chondrosarcomas usually arise during the sixth or seventh decade of life.106 Metastases, usually involving lung, develop in >90% and chemotherapy does not appear to affect survival.106 Median survival is <1 year, and few patients survive 5 years from diagnosis. Chondroblastic osteosarcoma should not be mistaken for dedifferentiated chondrosarcoma because patients with the former benefit from chemotherapy.
Mesenchymal chondrosarcoma is an uncommon variant composed of small round undifferentiated cells interspersed with islands of well-differentiated hyaline cartilage. A hemangiopericytomatoid variant has also been described.107 The peak incidence is in the second and third decades of life, and tumor may arise in bone, extraskeletal sites, or the CNS.108 The bones most commonly affected are mandible, spine, pelvis, and ribs. The course of disease is unpredictable and may be very protracted. Survival appears to correlate inversely with the mitotic rate of tumor assessed by detection of Ki-67 antigen.109 Metastases involving lung, nodes, or bone ultimately develop in more than half of cases, and the 10-year survival rate is generally <30%.108 Patients may benefit from treatment with radiation and Ewing or osteosarcoma chemotherapy, but methotrexate does not appear to be active.107,110
GIANT CELL TUMOR OF BONE
Giant cell tumor (GCT) of bone is a benign neoplasm that results in a predominantly lytic process in the ends of long bones and represents approximately 5% of bone tumors. It is comprised of a mixture of mononuclear stromal cells and osteoclastic, multinucleated giant cells. The stromal cells are thought to be the malignant element, but the morbidity of the tumor arises from lysis of bone by the activated osteoclast-like cells. GCTs arising in soft tissues have been described.111 Bone lesions are usually treated using curettage and application of a high-speed burr to the cavity wall with or without an adjuvant such as cryotherapy, instillation of phenol or alcohol, or
cementation using polymethymethacrylate. Occasionally en bloc excision is required for complete removal. Local recurrence occurs in approximately 15% to 25% of cases after curettage. Malignant transformation is an exceedingly rare event but is predisposed by radiation. Lung metastasis can occur in 2% to 5% of cases of benign GCT of bone.112 In most of the cases, metastasis follows local recurrence. Patients often live for years after appearance of metastasis with indolent growth of pulmonary nodules. Spontaneous regression of metastatic GCT involving the lung has been reported.112 Chemotherapy and radiation do not seem to impact the clinical course of metastatic GCT and have been associated with significant morbidity.112,113 Thoracotomy with wedge resection may render patients clinically free of metastasis but relapse in lung occurs in up to 50% of cases.112,113
cementation using polymethymethacrylate. Occasionally en bloc excision is required for complete removal. Local recurrence occurs in approximately 15% to 25% of cases after curettage. Malignant transformation is an exceedingly rare event but is predisposed by radiation. Lung metastasis can occur in 2% to 5% of cases of benign GCT of bone.112 In most of the cases, metastasis follows local recurrence. Patients often live for years after appearance of metastasis with indolent growth of pulmonary nodules. Spontaneous regression of metastatic GCT involving the lung has been reported.112 Chemotherapy and radiation do not seem to impact the clinical course of metastatic GCT and have been associated with significant morbidity.112,113 Thoracotomy with wedge resection may render patients clinically free of metastasis but relapse in lung occurs in up to 50% of cases.112,113
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree