Many effective cancer chemotherapeutic agents are destructive to the normal bone marrow cells. Therefore, bone marrow toxicity is often a limiting factor in the administration of adequate chemotherapy doses for curative intent. Hematopoietic stem cell transplantation (HSCT) allows the administration of supralethal chemo/radiotherapy to increase the percentage of malignant cells destroyed while rescuing the patient from hematopoietic toxicity with the transplant of the hematopoietic stem cells. Additionally, the healthy new cells transplanted may allow the replacement of an intact immune system to provide an antitumor effect or, in the case of bone marrow transplants for congenital diseases, to provide cells that are no longer deficient in certain vital components.
The first report of a procedure that was similar to a transplant was in 1939, when a patient received 18 ml intravenous marrow from his brother as a treatment for aplastic anemia.
1 The beginning of modern bone marrow transplantation can be traced to work showing that rodents could be protected against lethal hematopoietic injury by the intravenous infusion of bone marrow.
2 The subsequent identification of the human leukocyte antigen (HLA) system and the development of adequate storage techniques for hematopoietic cells laid the groundwork for clinical trials.
High-dose chemotherapy with bone marrow or peripheral blood progenitor (stem cells) transplant is now increasingly used for the treatment of many hematologic, immunologic, and neoplastic diseases.
3,4,5,6 Hematopoietic stem cells can be obtained directly from the bone marrow by multiple aspirations from the pelvic bones while the patient is under general anesthesia (bone marrow transplantation) or from the peripheral blood after stimulation by hematopoietic growth factors by the process called
leukapheresis (peripheral blood stem cell transplantation). Several sources of hematopoietic stem cells can be used for the transplant. These include allogeneic bone marrow cells from an HLA-identical sibling donor, a partially matched family donor, or an unrelated donor obtained through the National Marrow Donor Program; related or unrelated placental/umbilical cord blood; syngeneic bone marrow from an identical twin donor; or autologous cells derived from either the bone marrow or the peripheral blood progenitor compartment. The chemo/radiotherapeutic regimen and the source of the hematopoietic stem cells that are used for reconstitution are chosen on the basis of the disease type and other patient characteristics (
Table 11-1).
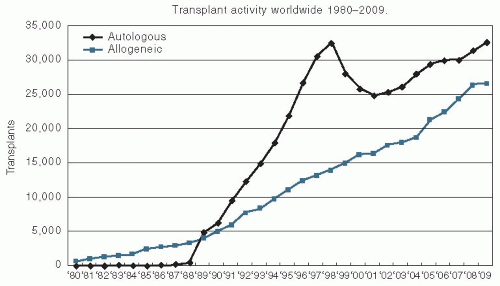
The use of this treatment has been growing since the 1990s. The most recent figures from the Center for International Blood and Marrow Transplant Research demonstrate that >30,000 autologous transplants were performed and reported to the registry in 2009, and approximately 25,000 allogeneic transplants were performed and reported to the registry in that same year (
Fig. 11.1).
The expansion in the use of HSCT is the consequence of steady improvements in safety and of our ability to offer this technology to older patients and to patients with new indications such as chronic lymphocytic leukemia (CLL) or autoimmune diseases. This chapter outlines the diseases treated with HSCT, the therapy and supportive care used during the transplant, and the complications that can occur during the various types of transplantation.
ALLOGENEIC AND SYNGENEIC TRANSPLANTATION
Allogeneic bone marrow or peripheral blood transplantation involves the transfer of stem cells from a donor to another person. A syngeneic transplant is the special case of a donor and a recipient who are genetically identical twins. Allogeneic transplants are considered for patients up to age 70, and occasionally even older patients are treated. The results tend to be poorer in older patients because of the increasing incidence of graft-versus-host disease (GVHD) and other comorbidities with age. However, the decision about the transplant in any individual patient must take into account all factors, including the patient’s comorbidities, not just the chronologic age. The chances of having an HLA match from a sibling are one in four for each sibling. However, because of the relatively small size of families in the United States, only approximately 30% of patients have an HLA-matched sibling.
For patients who lack an HLA-identical sibling donor, there are two possible solutions: to identify an unrelated but closely HLA-matched person through the National Marrow Donor Program or to use a partially matched related donor. Many HLA phenotypes are possible, which makes the identification of a matched unrelated donor sometimes difficult and time consuming. The National Marrow Donor Program (http://www. marrow.org/) has been developed to facilitate the search for unrelated donors in the United States. Depending on the ethnic descent, in the current national registry, which today includes approximately 9 million potential donors, the chances of finding an HLA-matched unrelated donor are between 50% and 80%.
7,8 Owing to advances in HLA-typing and improved supportive care over the last decade, current results of matched unrelated donor transplants are no different in success rate when compared with similar patients who received matched sibling donor transplant.
9An alternative approach is to identify a related person who shares some, but not all, of the patient’s HLA antigens.
10 Successful allogeneic transplantation can be performed with marrow from such donors, although the risks of graft rejection and GVHD may be increased.
Since 1988, an allogeneic sibling or unrelated partially matched placenta/umbilical cord blood has been increasingly explored as an alternative source of hematopoietic stem cells. The safe conduct of this procedure is still limited to a few specialized centers. Advantages of such stem cells are faster availability, no risk for the donor, and less GVHD as a result of the immature system of such donors.
11Once a suitable donor has been identified, the patient is prepared for the allogeneic transplant with chemotherapy alone or combined radio/chemotherapy. The purpose of this treatment is to destroy any remaining malignant cells, to provide sufficient immunosuppression to allow engraftment of the new cells, and to clear the marrow space for engraftment of the new cells. Only certain chemotherapeutic agents can be dose escalated in this manner. The agents chosen must have toxicities, other than hematologic ones, that are dose limiting at levels well above the hematologic effects that allow adequate escalation. For example, high doses of some anthracyclines are difficult to use in transplantation because of the cardiac toxicity that is apparent at relatively low doses. Most regimens consist of total body irradiation (TBI) combined with alkylating agents, etoposide, and/or cytarabine. Some non-TBI-containing regimens have also been developed using multiple alkylating agents, most commonly cyclophosphamide and busulfan.
In the 1990s, it was discovered that relapse of leukemia after allogeneic stem cell transplantation could, in some cases, be controlled by further infusions of lymphocytes from the same marrow donor.
12 The donor T lymphocytes initiate the destruction of leukemia cells by an immune mechanism called the
graft-versus-leukemia effect. A similar mechanism against solid tumors has been subsequently demonstrated in animal experiments and in some humans and is called the
graft-versustumor effect. These observations led, during the late 1990s, to the development of a new approach to the allogeneic stem cell transplantation: the nonmyeloablative or reduced intensity transplantation regimens. The whole concept of nonmyeloablative regimens is based on the philosophy that allogeneic stem cell transplantation is, in fact, a successful form of immunotherapy in which the donor lymphocytes play a main role in eradicating the malignant disease and very high doses of chemotherapy and irradiation are not necessary for success. Subsequently, preparative regimens could be significantly decreased in intensity and were just enough to allow sufficient immunosuppression for the allogeneic stem cells to engraft (
Table 11-2). The decreased cytoreductive component is a
possible limitation of nonmyeloablative transplants, especially in patients with diseases that are rapidly proliferating or less susceptible to the allogeneic graft-versus-malignancy effect. The nonmyeloablative approach in allogeneic transplantation is associated with markedly reduced transplant-related toxicity and allows transplanting patients who would not be able to tolerate conventional high-dose regimens, such as the elderly, those who are receiving second transplants, or those with poor performance status.
13
AUTOLOGOUS BONE MARROW TRANSPLANTATION
Autologous bone marrow transplantation involves the use of the patient’s own hematopoietic cells to reestablish bone marrow function after the administration of high-dose chemo/radiotherapy. These reinfused hematopoietic cells can come from the patient’s bone marrow, peripheral blood, or a combination of the two. This approach has several advantages and several disadvantages compared with allogeneic transplantation. Because a major limitation to the use of allogeneic bone marrow transplantation is the fact that not all patients will have an HLA-matched sibling donor, the use of autologous hematopoietic cells increases the number of patients who are eligible for transplantation. Autologous transplantation can also safely be used in an older patient population because of the lack of risk of GVHD, which is always a concern as the age of the patient rises. The relative trends in the use of autologous and allogeneic transplantation are shown in
Figure 11.1. The transient drop in the use of autologous transplantation is mainly because of the sharp drop of number of transplants performed for a breast cancer indication. Currently, the main diseases that are most common indications for autologous transplantation are multiple myeloma and lymphoma.
A concern in using autologous hematopoietic cells is the risk of contamination of the graft with viable tumor cells. Most studies demonstrate that populations who are undergoing autologous transplant have higher relapse rates than those with allogeneic transplants. However, with the increased risk and complications of allogeneic transplantation, the outcome is often similar in long-term follow-up.
14 Numerous methods, including in vitro treatment with chemotherapeutic agents, monoclonal antibodies and complement, or positive selection of CD34 antigen-positive progenitors, have been attempted to decrease the tumor contamination and therefore decrease the possibility of relapse.
15,16,17,18,19 Retrospective analyses have suggested that patients who received autologous grafts that were negative by molecular testing for residual disease may have better outcomes than individuals with grafts that were positive by these techniques.
16 However, most relapses occur at sites of previous disease, raising the question of whether resistance to treatment and overall increased tumor burden resulted in the relapse or the reinfusion of tumor cells caused the relapse. Initial trials with gene-marking experiments have demonstrated that in at least some of the patients, who underwent autologous transplantation for leukemia and neuroblastoma, reinfused cells did contribute to the relapse in some of these individuals.
20 Allogeneic and autologous transplantations are compared in
Table 11-3.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access