2 VU University Medical Centre, Amsterdam, The Netherlands
3 Academic Medical Centre, Amsterdam, The Netherlands
- Disease-related malnutrition is a frequent clinical finding in hospital populations.
- Improvement in nutritional intake can be achieved with food fortification and dietary counselling, oral nutritional supplements, enteral feeding, parenteral nutrition, or a combination of these approaches.
- Nutritional support can effectively contribute to improved functional and clinical outcomes, not only in hospitals, but also in the community setting.
9.1 Introduction
‘Nutritional support’ refers to the provision of adequate nutrients to meet the requirements of patients at risk of developing malnutrition. This can be in the form of oral diet, diet and nutritional supplements, or artificial nutritional support, such as enteral or parenteral feeding. Identifying people at risk of undernutrition is the first step in management. Many studies have shown that a substantial proportion of hospitalised patients are at risk of developing malnutrition. This proportion may increase in certain population groups. Assessing nutritional status and assessing nutritional requirements is necessary before optimal nutritional support can be instigated.
Causes and consequences of malnutrition and the process of assessing nutritional status are discussed in Chapter 2. Specific guidelines for disease groups are discussed in the chapters specific to those conditions. Ethical issues should always be considered before considering nutritional support, and these are covered very comprehensively in Chapter 10. This chapter aims to outline general considerations in providing nutritional support and the methods and products available to do so.
9.2 Meeting nutritional needs
Energy, macronutrients, minerals, vitamins, trace elements, fluid, and electrolytes are all necessary for optimal body function. Without sufficient energy, fat and protein stores will be mobilised and these fuels will be oxidised in order to meet energy needs. Since the loss of protein stores directly affects body function, it is important to administer sufficient amounts of energy and protein. Protein synthesis and protein degradation occur simultaneously in all body tissues; the difference between these two processes determines whether the body is anabolic or catabolic. Whereas in the diseased patient protein synthesis can be stimulated by feeding, protein intake cannot influence whole-body protein breakdown that occurs during inflammation. In severely ill patients, an increased protein intake of 1.5 g/kg body weight per day (normally 0.8 g/kg body weight/day) optimally stimulates protein synthesis, resulting in the least-negative nitrogen balance.
Carbohydrates are not essential nutrients as they can be produced from amino acids and glycerol. However, carbohydrates yield energy, and delivering carbohydrates in food prevents unnecessary stimulation of gluconeogenesis and thus slows down the rate of protein breakdown.
Fat is an excellent energy source that yields, on a weight basis, more than twice the energy of carbohydrates (9 vs. 4 kcal/gram). Dietary fat is also a source of essential fatty acids that cannot be synthesised by the body. Essential fatty acids maintain biomembrane structure, influence coagulation characteristics, and are precursors for leukotrienes and prostaglandins. The minimal fat allowance to ensure an adequate intake of fat-soluble vitamins and essential fatty acids is 10–15% of energy intake.
During disease, fluid and electrolyte balances can become disturbed. Fluid retention will affect body weight, and if not accounted for, can lead to inaccuracies in assessing nutritional requirements. Therefore, fluid/electrolyte balance should be carefully monitored and intake should be adapted accordingly (see Chapter 3).
In the past, hyperalimentation (the delivery of energy in excess of requirements) was thought to be efficient in improving nutritional status. However, hyperalimentation has been shown to induce severe metabolic abnormalities such as hyperglycaemia, hyperlipidaemia, and increased carbon dioxide production. Patients receiving nutritional support should be fed to meet their requirements. In clinical practice, basal energy expenditure is often calculated using empirical formulas, which are often regression equations, computed on the basis of energy expenditure and some anthropometric variables. Selected methods for estimating energy requirements are shown in Box 9.1. The Schofield equation is another frequently used formula; it is used in Chapter 25 (Table 25.1). An often-used simple guideline for estimating the daily energy needs of a patient is 25–35 kcal/kg body weight. One should realise that all of the equations, including the fixed factor of 25–35 kcal/kg, provide only rough estimates of the resting energy requirements, providing accurate predictions in only ~50% of patients and prediction errors as large as 20–30% (200 kcal/day or more). In addition, the extra factor for activity and disease needed to obtain an idea of total energy expenditure also carries large uncertainties. Therefore, the equations can be used as a starting point for nutritional therapy, but monitoring the effects of the therapy is of greater importance.
Men: 66.4730 + (13.7516 × W) + (5.0033 × H) − (6.7550 × A)
Women: 655.0955 + (9.5634 × W) + (1.8496 × H) − (4.6756 × A)
W, weight in kg; H, height in cm; A, age in years.
To predict total energy expenditure (TEE), add an illness/activity factor of 1.2–1.8, depending on the severity and nature of illness.
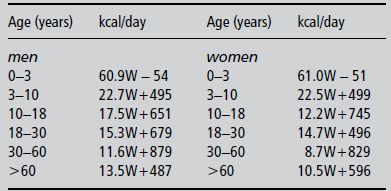
Men: 879 + (10.2 × W)
Women: 795 + (7.18 × W)
For adults aged from 19 to 77 years.
Men: 10W + 6.25H − 5A + 5
Women: 10W + 6.25H − 5A − 161
W, weight in kg; H, height in cm; A, age in years.
Energy requirements are strongly dependent on body composition. The body can be divided into fat mass and fat-free mass. Of the fat-free mass, the body cell mass is metabolically the most active; it consumes, on a weight basis, more energy than the fat mass. Patients with a higher body cell mass have higher energy needs than patients with a lower body cell mass. For example, young adult men who are tall and heavy need more energy compared with women or elderly, small, or slim people. This can be explained by the fact that men have a higher body cell mass than women and need more energy even if they are of the same age, height, and weight. Women have 10% more fat mass than men. During ageing, both men and women lose body cell mass, which is replaced by fat mass, leading to a decrease in overall energy expenditure. Obese people have considerable amounts of fat mass but their body cell mass is also increased, resulting in a higher energy requirement than they would have if they had a normal body weight. When applying equations in obese persons, a correction for optimal body weight does not seem to be necessary; the current body weight is the best predictor of energy expenditure.
Basal energy expenditure, together with additional factors such as physical activity levels, has to be assessed to calculate total energy expenditure. In the clinical situation, additional disease-associated factors should be taken into account during the calculation of the required energy needs. These include disease stress factor, activity factor, and temperature factor. During fever, basal energy expenditure is raised approximately 10% per degree of body temperature. Energy and nutrient losses from malabsorption should be taken into account when present.
In view of differences in nutritional needs between patients, it is always important to assess needs on an individual basis.
9.3 Oral feeding and oral nutritional supplements
Nutritional screening is an effective way of identifying patients at nutritional risk. Once these patients are identified, a decision can be made with regard to oral feeding and nutritional supplements.
Regular hospital food should contain sufficient energy and nutrients for the vast majority of people, and this should be the first option for feeding wherever possible. The macronutrient ratio of standard hospital food (the general menu), for hospital patients not at risk of malnutrition, corresponds to the requirements of optimal nutrition and should consist of 45–55% carbohydrate (of which 20–30 g is fibre), 30–35% fat, and 15–20% protein. Energy should be distributed as follows: 20% at breakfast, 30% at lunch, and 25% in the evening. The remaining 25% should be distributed over the course of the day in the form of snacks.
If a patient can be fed orally and is malnourished or at risk of developing malnutrition, oral intake must be optimised. The purpose of this diet is to increase energy and protein intake in order to meet the recommended nutritional needs. Consumption of food rich in protein and fat should be encouraged due to the high energy density of fat, and the fact that illness is often accompanied by an increased energy and protein need. The energy and protein intake can be improved by the supply of additional appetising and nourishing normal foods and snacks, supply of modular products, and alteration of food consistency or sip feeding. Other solutions to improve intake include tailoring food provision to meet the demands of patients (i.e. guaranteeing that the hospital meals meet the requirements of patients) and having patients make their food choice at the point of consumption, for example by changing food catering from a plated system (ordering choices and portion size in advance) to a bulk system (ordering at the moment of consumption).
One novel way of ensuring at least 30 minutes of undisturbed meal time is the ‘protected meal times’ policy, which has been promoted by the UK Hospital Caterers Association (HCA). Protected meal times require a minimisation of interruptions, such as medical or drug rounds and cleaning, and the rescheduling of procedures to avoid the three appointed times. Nursing staff are thus able to provide assistance and encouragement with eating where necessary, and will have immediate knowledge of patients’ eating habits or difficulties.
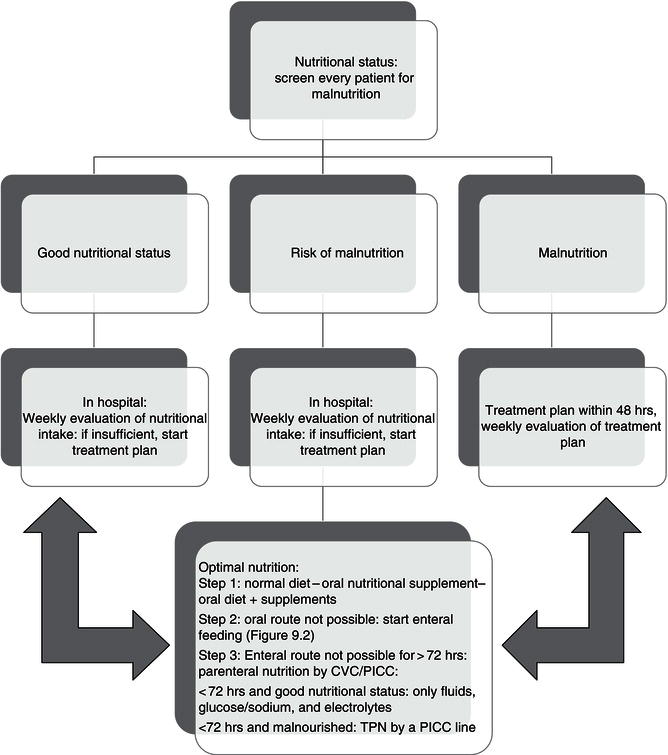
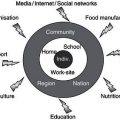
In Figure 9.1, the different options for feeding are shown. All patients should be screened at admittance, and nutritional intake should be assessed by a dietitian/clinical nutritionist at regular intervals so that nutritional support can be adjusted if necessary.
If the addition of normal foods does not improve nutritional intake, commercially available modular products can be used. Modular products are single macronutrients and are used to augment oral feeding. They exist as glucose polymers, protein powders, and fat emulsions. Typically modular products do not provide a source of micronutrients.
If sufficient energy intake cannot be achieved with fortified foods, many nutritional supplements are available which can be taken in addition to the diet to provide extra macro- and micronutrients. Most of these oral nutritional supplements (ONS) are supplied in the form of a drink and are nutritionally complete, providing protein, carbohydrate, fat, vitamins, and minerals. These drinks can be used during the day in between meals and have been shown to have beneficial effects on nutritional status and outcome.
The majority of ONS formulas are commercially available either over the counter in supermarkets and pharmacies or on prescription. Homemade liquidised supplements have the advantage of being more palatable but the disadvantage of being of unknown nutritional composition.
ONS drinks have been demonstrated to increase energy intake in a variety of patient groups compared to a normal oral diet. Most patients use two or three portions (around 500 kcal/20–40 g protein) a day if they use normal meals as well. In most studies they have had little suppressive effect on food intake, and in some patients groups they have been shown to actually stimulate appetite and food intake. Although ONS drinks can promote weight gain, changes in body composition have been infrequently assessed. Functional changes, for example in muscle strength, activity levels, and physical and mental well-being, have been shown to improve with supplementation. Although the impact of supplements may be limited in patients with severe end-stage disease, mortality rates are significantly lower in other malnourished patients receiving ONS compared with those not receiving supplements. Complication rates are also lower and length of hospital stay is reduced, resulting in considerable cost savings with the appropriate use of ONS. They have also been shown to have a beneficial role in the community setting, with improved intake and weight as well as improved functional and clinical outcomes.
9.4 Enteral tube feeding
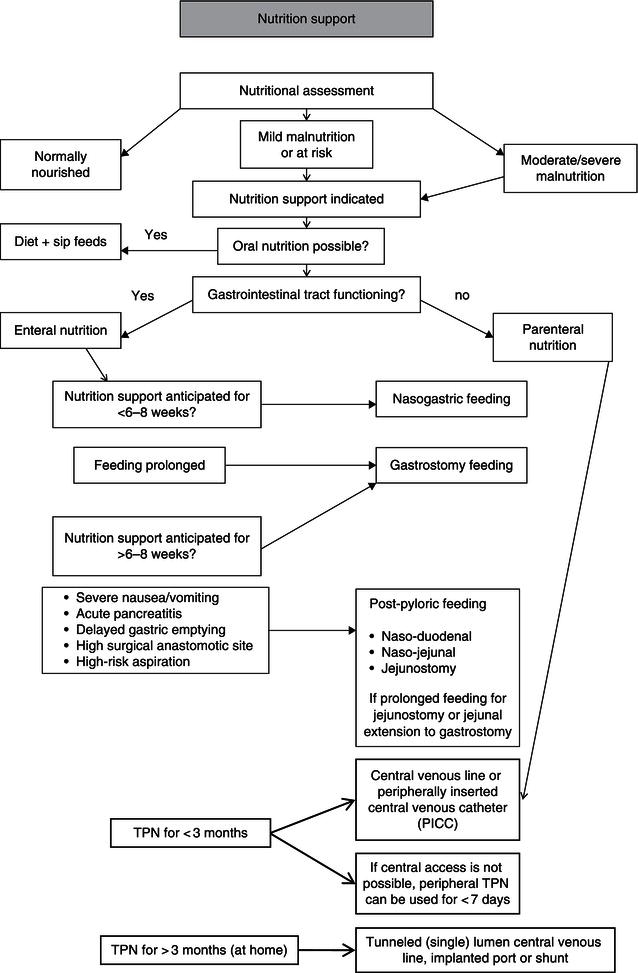
If oral intake is insufficient to meet nutritional requirements, or if it is contraindicated due to dysphagia, obstruction, facial injuries, artificial respiration, GI operation, malabsorption, or lack of consciousness, enteral tube feeding (ETF) should be considered (Figure 9.2). In both hospitals and the community, tube feeding is used in a variety of clinical conditions and ages and over varying periods of time, from weeks to years. It can be used as the sole source or as a supplementary source of nutrition. ETF has been shown to increase nutritional intake compared to a normal oral diet, attenuate loss of body weight and lean tissue, and improve functional and clinical outcomes. Patients with a functional gastrointestinal (GI) tract who will not, cannot, or should not eat, and are candidates for nutritional support, should be fed enterally. If possible, enteral feeding is always preferred over parenteral nutrition because of the benefits of feeding the gut, such as stimulation of the immune function and prevention of bacterial overgrowth and bacterial translocation. If full enteral nutritional support is not feasible, it is advisable to use minimal enteral feeding next to parenteral nutrition.
ETF is contraindicated in patients with intestinal obstruction distal to the tube, high-output fistulas (>1000 ml/day), GI bleeding, or bowel ischaemia. A mechanical obstruction distal to the tube results in accumulation of enteral feeding proximal to the obstruction. This can lead to severe bowel extension, abdominal pain and even bowel rupture. Enteral feeding is also contraindicated in patients with paralytic ileus due to the lack of peristalsis. Enteral feeding in patients with a high-output fistula or enterostomy stimulates the production of GI juices. Losses of water and electrolytes through the fistula can be replaced, but stimulation of the gut by enteral feeding can lead to a high fistula output, resulting in considerable losses of fluids and electrolytes through the fistula or enterostomy. These patients should be limited in their enteral intake in order to reduce electrolyte and fluid losses, in spite of feelings of thirst, to a maximum of 10–15 ml/kg. Fluids can be given by infusion. During bowel ischaemia there should be no enteral provocation, as the feed cannot be absorbed and may increase ischaemia. Enteral nutrition should be avoided in patients with active GI bleeding and nutritional support should only commence once a patient is haemodynamically stable.
Enteral nutrition should be given as early as possible. In critically ill patients, starting enteral nutrition within 24 hours after admission to the intensive care unit (ICU) seems to have positive effect on several outcome parameters. Although there is currently insufficient evidence, early enteral nutrition in an appropriate amount and with the aim of avoiding gut failure is recommended.
Feeding routes
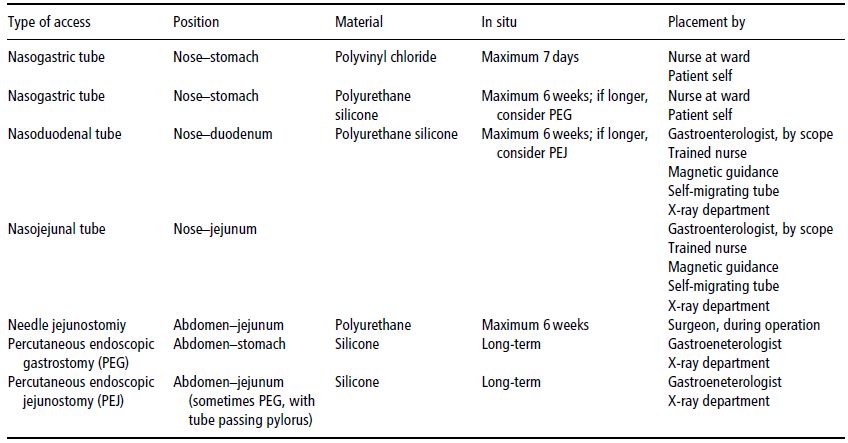
Access routes for enteral feeding vary according to the individual patient. In deciding which route to use, the anticipated length of feeding and the presence of delayed gastric emptying are two major considerations. Access to the GI tract via the nasal route, by for example nasogastric (into the stomach), nasoduodenal (into the duodenum), or nasojejunal (into the jejunum) tubes, is usually short-term (less than 8 weeks). During tube placement, patients are asked to swallow the tube after advancing it through the nose. Nasogastric tubes are usually placed at the bedside. Naso small-bowel tubes can be placed in the endoscopy unit or under fluoroscopic guidance in the X-ray department, but some units have success in placing small-bowel feeding tubes at the bedside with the aid of a motility agent such as erythromycin or metoclopramide hydrochloride. The latest technique is the use of an electromagnetic guidance system to guide the tube through the pylorus.
Feeding tubes can increase the incidence of gastroesophageal aspiration, as the cardiac sphincter at the top of the stomach cannot fully close. Placing the patient’s bed at an angle of 30° can reduce the incidence of aspiration. With ongoing problems, a motility agent can be given or the tube can be placed post-pylorically.
Table 9.1 Choice of artificial enteral access.
- disturbed coagulation;
- neoplasms in the stomach;
- morbid obesity;
- gastric varices;
- progressive ascitis or peritonitis.
When enteral feeding is anticipated for a longer period of time, a percutaneous endoscopic gastrostomy or jejunostomy (PEG/PEJ – see Table 9.1) should be considered. This is a more-invasive category of enteral feeding, in which the tube accesses the GI tract through the abdominal wall. This procedure can be carried out in the endoscopy unit or the radiology department. Access can be to the stomach or small bowel. Complications associated with these procedures are infrequent, but include reaction to anaesthesia, perforation of adjacent organs, bleeding, and infection. Special precautions should be taken when feeding into the small bowel, due to the lack of gastric volume or acidity. Contraindications for enterostomy access are summarised in Box 9.2.
Post-pyloric feeding
A specific problem with post-pyloric feeding is the so-called dumping syndrome, which results from the administration of a high-osmolar solution directly into the small bowel. This leads to an increased fluid secretion from the bowel in order to dilute the solution. Dumping syndrome may cause diarrhoea, dizziness, and an exaggerated insulin response and ensuing hypoglycaemia. This situation is worsened when the administration rate is increased too quickly. It is generally recommended that the infusion rate of small-bowel feeding in adults does not exceed 125 ml/hour.
Complications of enteral feeding
Aspiration pneumonia
Many patients experience problems with swallowing, which can cause aspiration pneumonia. It is very important to identify these as soon as possible to minimise the risk. Patient groups at risk include those with head and neck cancer and those with a tracheostomy, stroke, or a neurological disease such as motor neurone disease or multiple sclerosis. A swallowing assessment should be carried out by qualified personnel (ideally a speech and language therapist) and the consistency of the diet should be altered as necessary. It was former practice to check for possible aspiration by adding blue food dye to feeds. This has been shown to be ineffective as a clinical tool and potentially life threatening, and should not be used. Although postpyloric feeding reduces the chance of aspiration, it can not fully exclude aspiration, so all patients with artificial enteral feeding should be monitored in order to prevent this complication.
Diarrhoea and constipation
Following the introduction of ready-to-use feeding systems, bacterial contamination of feeds is rarely seen. Some feeds may need to be decanted or reconstituted prior to feeding, which increases the incidence of bacterial contamination. The manufacturer’s guidelines on feed hanging times should be referred to in all cases. Usually, giving sets should be changed every 24 hours, but they may need to be changed more frequently in critically ill or immunocompromised patients.
Diarrhoea in the enterally fed patient can be drug-related, infection-related (due to Clostridium difficile), disease-related, or caused by hyperosmolar feeds being administered too fast. The offending cause should be removed, if possible. The key medications which cause diarrhoea include antibiotics, sorbitol-containing medications often present in syrups, and magnesium-containing medications. First-line treatment of diarrhoea should be fluid and electrolyte replacement. If C. difficile infection is suspected, a stool sample should be sent for analysis and the patient should be started on treatment as required.
Constipation may be caused by many drugs. A typical and common example is opioids. If possible, the offending agent should be discontinued. Factors that can be used to reduce constipation include:
- increased water intake;
- the use of a high-fibre feed to increase faecal bulk;
- laxatives and stool softeners.
Nausea and vomiting
This should be assessed medically. Patients who have an impaired gastric function are at risk of aspiration if they vomit. It is important to confirm placement of the feeding tube. The patient may just need a couple of hours with the feed disconnected, but long periods of discontinuation of the feed should be avoided to prevent malnutrition. Antiemetic drug can be used, and in extreme cases post-pyloric feeding or parenteral nutrition may be required. Long-term diabetes mellitus patients and neurological patients are at risk for gastric-emptying disturbances.
Physical characteristics of enteral feeding tubes
Clinical nutritionists and dietitians will need a working knowledge of the physical characteristics of enteral access devices, including tubes, connectors, giving sets, and feeding pumps, in order to be able to identify the devices best suited to the needs of a patient. Nasoenteric tubes for adults are available in lengths from 94 cm (36 in) (enteral) to 156 cm (60 in) (the longest for feeding postpylorically). Gastrostomy tubes are shorter and are available in different options, including very-short gastrostomy skin-level devices called ‘buttons’, which are very useful for GI access in patients who are young or aware of the cosmetic appearance of the tube, or in agitated, confused patients who are at risk of pulling out the tube.
Table 9.2 Advantages and disadvantages of different enteral feeding routes.
| Feeding route | Advantages | Disadvantages |
| Nasogastric tube or nasoenteral tube | Less invasive Quick Cheap Feeding can be initiated immediately after tube placement and confirmtion of tube location | Oropharyngeal and esophageal irritation Increased risk of sinusitis, esophagitis, nasopharyngitis Swallowing may be painful and difficult Increased risk of reflux Coughing, vomiting, or sneezing may result in migration of the tube into the oesophagus or pharynx, with an increased risk of aspiration Abnormalities in the nose, neck, or oesophagus area may prevent tube placement Tube can be placed in the trachea The tube can easily be removed by disoriented patients Stigmatising Tubes should be replaced on a regular basis (PVC 7 days, PUR 6 weeks) Location of the nasoenteral tubes often requires an endoscopic approach and/or X-ray confirmation |
| Through abdominal wall: Percutaneous endoscopic gastrostomy Gastrostomies Enterostomies Jejunostomy catheters | Less stigmatising Better psychosocial acceptance Less migration of tube Less tube removal Less reflux or aspiration No oropharyngeal and oesophageal irritation Surgical options can be performed when disorders in the nose, neck, or oesophagus are present No difficulties with swallowing Rare replacement of tubes | Invasive access method with increased risks of postoperative complications Sedation and antibiotics may be necessary Placement may be time-consuming Skin around the tube can be irritated Leakage of nutrients or intestinal juices into the abdomen Translocation of the bowel around the jejunostomy catheter Occlusion of the bowel, caused by haematomas Jejunostomy catheter can dislocate and clog Jejunostomy catheter requires X-ray confirmation Abnormalities in the oropharyngeal-oesophageal area may prevent percutaneous endoscopic gastrostomy placement |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree