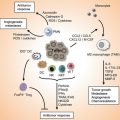
Fig. 20.1
Schematic overview of complex network of diet-immunity-cancer
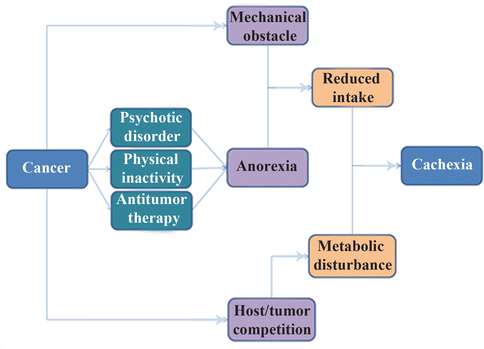
Fig. 20.2
The casual pathways of cachexia occurrence after malignancy
It should be noted that antitumor agents with their side effect on cells with high turnover may exacerbate malnutrition. This could be explained by the competition between cancerous regions and normal cells of the gastrointestinal system to use nutrients to repair the adverse effects of antitumor drugs (hypermetabolic state). Briefly, impaired caloric intake, side effects of therapy, changes in taste and mood, pain and other adverse consequences of eating, obstruction, fistula, and malabsorption all promote malnutrition in cancer patients [73–77]; therefore, well-nourished patients with intact gastrointestinal integrity have lower morbidity and mortality than others [78].
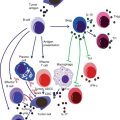
It should be noted that cachexia after cancer differs from cachexia following starvation. Increased protein and glucose turnover, high whole body synthesis and catabolism, accelerated hepatic protein production (especially acute phase agents), increased serum free fatty acid levels, and depletion of fat stores were reported only in cancer patients. However, metabolic abnormalities and, paradoxically, impaired immune response are probable consequences of cancer cachexia, as explained in the previous section [79, 80]. Increased levels of immunosuppressive mediators (e.g., TGF-β), decreased C3 and delayed hypersensitivity response, and diminished numbers and activity of (NK) cells are the most common changes in the defense system of patients with cancer cachexia, leading to more infectious complications and poor prognosis [81]. Neutrophil chemotaxis, monocyte phagocytosis and killing, number of T cells, and proliferation of lymphocytes are also defective in patients with lung cancer [82]. Phagocytic and bactericidal activities of neutrophils were low in hepatocellular carcinoma patients [83]. In addition, surgical stress in cancer patients enhances Th2 and compromises the Th1/Th2 balance and expression of HLA-DR on monocytes, which is considered to be a central marker of immune paralysis after surgical trauma [84]. Most of these immune parameters are also reduced during radiotherapy and chemotherapy because of their side effects on bone marrow. However, these factors are reversible after nutrition improvement [85].
20.5 Role of Nutritional Support in Immune Restoration of Cancer Patients
Adjuvant therapy of cancer patients by different nutritional support strategies (dietary counseling, oral nutritional supplements, enteral tube feeding, and parenteral tube feeding) is the mainstream recommendation to increase their quality of life and to obviate the risks associated with gastrointestinal complications and reverse malnutrition [86]. However, there is no comprehensive approach based on the needs of cancer patients with cachexia or those with increased nutrient requirements [87]. Several studies have shown the effectiveness of nutritional supply in groups of patients with malignancy that resulted in weight gain, increased appetite, increased energy and protein intake, reduced gastrointestinal toxicity, and enhanced immune function [88–90]. In the clinical setting with standard treatment protocols, it turns out that the implementation of nutrition support in patients with cancer is most effective when it is limited to special, well-described circumstances. Nonetheless, the potential advantages of some specific nutrients have been described and are outlined below.
20.5.1 Arginine
Arginine is a semi-essential amino acid with immunomodulatory potential such as stimulated thymic growth and mononuclear cell response to mitogens, which enhances lymphokine-activated killer cell generation via a nitric oxide-mediated mechanism and stimulates the release of polyamines by the small intestine. In one randomized trial of malnourished patients with head and neck cancer, follow-up at 10 years indicated better survival in those who received supplemental arginine preoperatively [91, 92].
20.5.2 Glutamine
Glutamine is the most abundant amino acid in the human body and the preferential fuel of rapidly dividing cells such as lymphocytes and macrophages [93]. However, supplementing glutamine in the diets of patients with cancer may be counterproductive because glutamine (which is essential for fast growing cells in culture) may promote accelerated tumor growth [94]. A meta-analysis of studies that used parenteral glutamine postoperatively showed it was associated with a shorter hospital stay and a lower incidence of infectious complications [95].
20.5.3 Branched Chain Amino Acids
L-valine, L-leucine, and L-isoleucine can improve the immune response and maintain serum albumin level in the course of hepatocellular carcinoma recurrence [96].
20.5.4 Nucleotides, Long-Chain Omega-3 Polyunsaturated Fatty Acids, and Eicosapentaenoic Acid
20.5.5 Fructooligosaccharides
This group of functional fibers associated with increased lactic acid bacteria acts as an immunomodulator by stimulating IgA synthesis, promoting mucin production, modulating inflammatory cytokines, and decreasing Ag absorption [101].
20.5.6 Bioactive Compounds
Agaricaceae fungus consisting of ergosterol, oleic acid, and triterpenes may inhibit neovascularization induced by tumors and therefore attenuate cancer progression [102].
20.5.7 Vitamins C and E
20.5.8 Vitamin A
20.6 Concluding Remarks
In summary, due to the safety and cost-effectiveness of oral dietary therapies, nutrition counseling and the implementation of nutritional supplements should be the initial approaches to nutritional support [107]. Even though parenteral nutrition may also lead to weight gain and improvement in nitrogen balance in patients with cancer, it does not clearly improve serum albumin levels or alter whole body protein turnover even with prolonged administration. Therefore, when nutrition support is chosen as a therapy, the use of enteral nutrition is preferred if the gastrointestinal tract is functional [108, 109]. The use of parenteral nutrition should be limited to malnourished cancer patients who are receiving active anticancer treatment, whose gastrointestinal tract is not functional or who cannot tolerate enteral nutrition, and who are anticipated to be unable to meet their nutrient requirements for 14 days or more [108].
Moreover, it is proposed that preoperative and postoperative immune-nutrition intervention by total parenteral nutrition using a lipid-based regimen is the method of choice in cancer patients who have undergone major surgery to reduce immune dysfunction without enhancing tumor growth (increased augmentation of lymphocyte blastogenesis and production of helper T-lymphocyte lymphokine IL-2, increased ICAM-1 level, and decreased IL-4 and IL-10 values) [111–113]. This observed preference of parenteral nutrition is marginal, and enteral methods are always the preferable route for cancer patients with an intact digestive system. It is also reported that complement components and lymphocyte response may be better with enteral rather than parenteral nutrition [110, 113].
References
1.
Dossus L, Kaaks R. Nutrition, metabolic factors and cancer risk. Best Pract Res Clin Endocrinol Metab. 2008;22(4):551–71.PubMed
2.
Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112(12):1785–8.PubMedCentralPubMed
3.
Schaffler A, Muller-Ladner U, Scholmerich J, Buchler C. Role of adipose tissue as an inflammatory organ in human diseases. Endocr Rev. 2006;27(5):449–67.PubMed
4.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808.PubMedCentralPubMed
5.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–83.PubMed
6.
Matsubara M, Namioka K, Katayose S. Decreased plasma adiponectin concentrations in women with low-grade C-reactive protein elevation. Eur J Endocrinol. 2003;148(6):657–62.PubMed
7.
Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2413–8.PubMed
8.
Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38(10):1647–53.PubMed
9.
Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33(4):335–51.PubMedCentralPubMed
10.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7.PubMed
11.
Campos FG, Logullo Waitzberg AG, Kiss DR, Waitzberg DL, Habr-Gama A, Gama-Rodrigues J. Diet and colorectal cancer: current evidence for etiology and prevention. Nutr Hosp. 2005;20(1):18–25.PubMed
12.
Brockman DA, Chen X, Gallaher DD. Consumption of a high β-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur J Nutr. 2013;52(7):1743–53.PubMed
14.
Bourdon I, Yokoyama W, Davis P, Hudson C, Backus R, Richter D, et al. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. Am J Clin Nutr. 1999;69(1):55–63.PubMed
15.
Hekkens WT, Haex AJ, Seeder WA. Clinical and biochemical analysis of glutentoxicity. V. The origin of fecal fatty acids on a fat-free, gluten-free diet in patients with idiopathic steatorrhea. Gastroenterologia. 1964;101:13–9.PubMed
16.
Sandhu MS, White IR, McPherson K. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol Biomarkers Prev. 2001;10:439–46.PubMed
17.
Norat T, Lukanova A, Ferrari P, et al. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002;98:241–56.PubMed
18.
Langer CJ, Hoffman JP, Ottery FD. Clinical significance of weight loss in cancer patients: rationale for the use of anabolic agents in the treatment of cancer-related cachexia. Nutrition. 2001;17(1 Suppl):S1–20.PubMed
19.
Proceedings of a conference on nutrition and immunity. Atlanta, Georgia, May 5–7, 1997. Nutr Rev. 1998;56(1 Pt 2):S1–186.
20.
Meydani SN, Erickson KL. Nutrients as regulators of immune function: introduction. Fed Am Soc Exp Biol. 2001;15(14):2555.PubMed
21.
Beisel WR. History of nutritional immunology: introduction and overview. J Nutr. 1992;122(3 Suppl):591–6.PubMed
22.
Gogos CA, Kalfarentzos F. Total parenteral nutrition and immune system activity: a review. Nutrition. 1995;11(4):339–44.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree