2 University Hospital Southampton, UK
3 Hôpital Antoine-Béclère (Assistance Publique-Hôpitaux de Paris), France
13A DIABETES MELLITUS
- The recent increase in the prevalence of diabetes, which currently affects about 5% or more of the populations of many countries and which is expected to continue to increase in the future, is largely attributable to the rapid growth of obesity. Prevention and treatment of obesity can prevent and treat diabetes or make its management easier.
- Poor metabolic control in those with diabetes increases the risk of short-tem complications (e.g. hypoglycaemia and hyperglycaemia, both of which can produce coma) and long-term complications, such as retinopathy, nephropathy and neuropathy. Good metabolic control, which intimately involves diet, can prevent or delay the appearance of these complications.
- Establishing good metabolic control involves balancing dietary intake, which elevates the blood glucose concentration, with physical activity and insulin/oral hypoglycaemic agents, both of which lower the blood glucose concentration. The diet recommended for diabetes is generally similar to the healthy diet recommended for the general population, but the temporal pattern of food ingestion is more important in those with diabetes than those without.
- Special dietary regimens are required when metabolic instability has developed, for example in diabetic ketoacidosis, or when major changes in dietary intake, physical activity, and insulin sensitivity are expected to occur (e.g. after elective or accidental injury or severe infection).
- In women of child-bearing age with diabetes, poor glycaemic control at the time of conception and early pregnancy are associated with a several-fold increased risk of developing malformations in the offspring. Good glycaemic control during this period can prevent the malformations. The infant of the diabetic mother is prone to neonatal hypoglycaemia, which can be prevented by precautionary regimens.
13A.1 Introduction
Prevalence
Diabetes mellitus is a chronic condition caused by inherited and/or acquired deficiency in the production of insulin by the pancreas or by the ineffectiveness of the insulin produced. The consequent hyperglycaemia and associated metabolic disturbance cause damage to the body systems, in particular the blood vessels and nerves.
Diabetes is a major clinical and public health problem, with important nutritional implications with respect to both its causes and its consequences. The estimated prevalence of diabetes worldwide in 2011 was 366 million, and by 2030 it is expected to rise to 552 million. This projected increase is largely due to the obesity ‘epidemic’, which is affecting both low- and high-income countries, but especially low-income countries, where the propensity to obesity-induced diabetes is greater. Indeed, obesity can increase the risk of type 2 diabetes by as much as 50–100 times, which helps explain the distribution of diabetes both within countries and between countries. The International Diabetes Federation estimates that in 2011, the five countries with the largest number of people with diabetes were China, India, the United States of America, Russia, and Brazil. In the UK, it is estimated that more than 1 in 20 people have diabetes (diagnosed or undiagnosed). In 2011, those with diagnosed diabetes in the UK amounted to 2.9 million people, and by 2025 it is estimated that this will increase to 5 million people. Low-birth-weight babies are more likely to develop type 2 diabetes in adult life (in both developed and developing countries), implying that early nutritional influences in utero programme individuals to develop maturity-onset diabetes (and cardiovascular problems) several decades later.
Pathogenesis and types of diabetes
Two distinct pathways for the pathogenesis of the condition have been identified. Diabetes mellitus caused by the absolute or near-absolute deficiency of insulin is known as type 1 diabetes, which usually presents in childhood. Diabetes mellitus associated with insulin resistance is known as type 2 diabetes, which usually presents in adults. However, the distinction between the two types is not absolute, since patients with type 2 diabetes may require insulin, and those with type 1 diabetes may present in adulthood, although not usually beyond 30 years of age. While the nomenclature of diabetes continues to change, it is reasonable to regard types 1 and 2 diabetes as being at the extreme ends of a spectrum, in which there is an overlap in the middle. Monogenetic causes of diabetes account for a small proportion of the total. One of the genetic causes is maturity-onset diabetes of the young (MODY), which arises from mutations of an autosomal dominant gene, and is associated with presentation of diabetes before the age of 25 years.
Type 1 diabetes occurs because the beta cells of the pancreas, which produce insulin, are destroyed by the body’s own immune system. Type 1 sufferers are therefore dependent on exogenous administration of insulin for survival. It is less common than type 2 diabetes and accounts for around 10% of all people with the condition.
Type 2 diabetes, formerly known as non-insulin-dependent diabetes, develops when the beta cells are able to produce insulin but there is resistance to the action of insulin, so that hyperglycaemia occurs, often in association with an elevated circulating insulin concentration. Unlike in type 1diabetes, in which the pancreas cannot be stimulated to produce insulin, in type 2 diabetes the pancreas can be stimulated to produce more insulin.
Other forms of diabetes also exist, including gestational diabetes mellitus (GDM; also called type 3 diabetes). GDM is largely attributed to insulin resistance, which typically presents in the second trimester of pregnancy and progresses into the third, when insulin sensitivity can be reduced by as much as 80%. The incidence of GDM may range from 1 to 14%, and it accounts for 90% of all diabetes in pregnancy, the remainder being due almost exclusively to type 1 and type 2 diabetes.
Glycaemic control, nutrition, and insulin
In healthy people, the circulating glucose concentration is tightly controlled within the range of 3.4–7.8 mmol/l, despite intermittent ingestion of food containing varying amounts of carbohydrate, episodes of fasting – which may be prolonged for 14 hours or more – and episodes of exercise of varying intensity. Between meals, most of the circulating glucose is largely derived from glycogen that has previously been deposited in the liver and muscle following meal ingestion. After an overnight fast, glucose is released into the circulation at a rate of about 200 g per day, the majority of which arises from glycogen, and the remainder from gluconeogenesis, mainly in the liver and to a lesser extent the kidney. Under normal circumstances, the brain uses glucose (about 120 g per day) as its major energy source, although it can also use ketone bodies during starvation. Hypoglycaemia in the absence of hyperkeonaemia implies that the brain is deprived of nutritional fuel, which can rapidly cause confusion and unconsciousness. Prolonged hypoglycaemia can cause permanent brain damage. Acute hyperglycaemia also has adverse effects, including fluid shifts, dehydration, and even coma and death.
Insulin has a key role in glucose homeostasis in both health and disease. The so-called ‘diabetes of injury’ arises from systemic stress or inflammation and is generally associated with insulin resistance. As a major regulatory hormone, insulin is normally released from the pancreas in response to dietary nutrients (mainly glucose), so that its concentration increases many times above fasting values, facilitating, along with other regulatory hormones or signals, glucose homeostasis. In diabetes, this regulation is disturbed. Key aspects of glucose transport into cells, insulin-signalling pathways, and insulin resistance are briefly summarised in this section.
Glucose transporters
Due to the presence of the lipid bilayer, water-soluble molecules such as glucose do not directly cross cell membranes. Glucose transport is facilitated by a group of proteins (GLUT transporters), of which more than a dozen have been identified. The first four have been well characterised. GLUT-1 and GLUT-3 are found in most cells. They have a high affinity for glucose, which means that glucose uptake can be maintained even at low circulating concentrations, and so account for basal non-insulin-mediated glucose uptake (GLUT-3 is the main glucose transporter for the brain). GLUT-2 transporters are found on the beta cells of the pancreas (and hypothalamus and other cells) and are involved in glucose sensing. Unlike GLUT-1 and GLUT-3, which show little or no increase in transport at higher ambient glucose concentrations, GLUT-2 transporters have a low affinity for glucose (high Michaelis constant (Km) – that concentration of a substrate that gives half-maximal activity), which means that transport increases as glucose concentration rises to high levels, so that the concentration in the beta cell reflects that in the circulating, especially since the transport is bidirectional. Although GLUT-3-mediated transport is not stimulated by insulin, it triggers release of insulin from beta cells, which has multiple effects on insulin-sensitive tissues. GLUT-4, which is found in skeletal muscle, cardiac muscle, and adipose tissue, is insulin-sensitive and is involved in insulin-regulated glucose storage. Insulin stimulates the movement of cytoplasmic vesicles containing GLUT-4 (which is synthesised by ribosomes and sequestered into vesicles by the Golgi apparatus) so that they fuse with the cell membrane to increase the density of GLUT-4 transporters on the cell membrane and facilitate increased glucose uptake into cells.
Insulin-signalling pathways
To exert metabolic effects, insulin has first to bind to the insulin receptor. This receptor, which consists of four subunits, has a molecular weight of ~320 kDa and is encoded by a single gene, located on chromosome 19. The activation of the insulin transmembrane receptor leads to phosphorylation of the amino acid tyrosine in specific proteins within the cell, which in turn initiate a cascade of three major intracellular signalling pathways. One of these ultimately leads to the translocation of GLUT-4 from within the cell to the outer part of the membrane of insulin-sensitive tissues (see above), such as muscle and adipose, with resulting facilitation of glucose uptake into cells. Another signalling pathway involves phophatidylinositol 3-kinase (PI3K), which mediates many of the metabolic functions of insulin. The third major pathway, the MAP kinase (mitogen-activated protein kinase) pathway, is linked to enhanced cell growth. Control of these insulin-signalling pathways is complex and not entirely understood, although they can be viewed as being partly autoregulatory, with a negative feedback from downstream changes produced by the cascade(s), and partly non-autoregulatory, involving signals from unrelated pathways (e.g. those associated with inflammation). A better understanding of these pathways may provide deeper insights into insulin resistance and new drug therapies for treating diabetes.
Insulin resistance
Despite extensive research to elucidate the mechanism of glucose intolerance and insulin resistance in type 2 diabetes, the processes involved are still not fully understood. It seems that altered insulin-receptor density on cell membranes is not the major cause of the common variety of insulin resistance in type 2 diabetes or obesity. At least part of it is related to disturbances in signals generated in the PI3K pathway. Signals involved in inflammatory pathways are also involved. Triglycerides and fatty acids, both of which are elevated in obesity and type 2 diabetes, are also thought to play a key role, especially since fatty acids compete with glucose for oxidation. Although there are still aspects of these competitive interactions that are not fully elucidated, part of the fatty acid effect appears to involve GLUT-4 receptors, which can be rate-limiting to glucose uptake in at least some circumstances. The exact nature of the lipid moiety responsible for insulin resistance caused by fatty acids is unclear. Exercise can reduce insulin resistance, and part of this process involves insulin-induced GLUT-4 translocation (see earlier in this section). However, muscle activity can stimulate glucose uptake in the absence of increasing insulin levels. Insulin sensitivity to glucose does not necessarily mirror insulin sensitivity to fatty acid and amino acid metabolism, which emphasises further the complexity of the processes involved.
Consequences of poor metabolic control
In the short term, poor metabolic control of diabetes may lead to confusion and collapse, and in the long term it may lead to the development of comorbidities, such as cardiovascular disease and debilitating complications that affect the nerves, eyes, kidneys, and other organs. Some of the long-term complications of diabetes are considered to be due to damage caused to large vessels (macrovascular damage, producing atherosclerosis, which predisposes to common conditions such as myocardial infarction, stroke, and hypertension) and small vessels (microvascular complications, which are much more specific to diabetes, and which can affect the retina, leading to blindness, renal glomeruli, leading to renal failure, and nerves, leading to neuropathy). At least some of this damage is mediated by the presence of an abnormal lipid profile: elevated cholesterol, low-density lipoprotein (LDL), and triacylglycerol concentrations, and reduced HDL concentrations.
Good metabolic control can help prevent both short-term problems and long-term complications, which may appear decades later.
13A.2 Presentation and diagnosis
Presentation
Type 1 diabetes usually presents with a 2–8 week history of polyuria (induced by glycosuria, which develops as a consequence of hyperglycaemia), thirst (due to dehydration), and weight loss. Ketone bodies, formed from the breakdown of fat as a result of insulin deficiency, can cause a ‘pear drop’-like smell from the breath. Ketone bodies can be readily detected in urine.
Type 2 diabetes can present with a diverse range of complications, such as repeated skin and urine infections (associated with hyperglycaemia), retinopathy, tingling and numbness of the feet (neuropathy), cardiovascular disease, and erectile dysfunction. Subacute presentations over months or longer can also occur, with a mixture of polyuria and thirst on the one hand and other complications, such as infections, on the other.
Diagnosis
The diagnosis of diabetes is based on history and examination, and on biochemical tests involving measurement of glucose (see Table 13A.1).
Alternative laboratory criteria for diagnosing diabetes mellitus are also available. One of these concerns haemoglobin, which is glycated with glucose. If the circulating glucose concentration has been high in recent weeks, the extent of glycation (typically reported as % HbA1c or mmol/mol Hb) is increased. Since red cells survive for about 120 days, the % HbA1c is influenced by the circulating glucose concentration over this period. In normal subjects, the values range from 4 to 6% (20 to 42 mmol/mol), and in diabetics it is desirable for HbA1c levels to be less than 6.5% (48 mmol/mol), although they may be considerably higher.
According to a World Health Organization (WHO) report published in 2011, HbA1c can be used as a diagnostic test for diabetes, providing that:
- stringent quality assurance tests are in place;
- assays are standardised to criteria aligned to international reference values;
- there are no conditions present that preclude its accurate measurement.
Table 13A.1 Diagnostic criteria of diabetes mellitus based on glucose concentration (WHO, 2006).
1.Diabetes symptoms (i.e. polyuria, polydipsia and unexplained weight loss) plus:
2.With no symptoms, diagnosis should not be based on a single glucose determination but requires confirmatory plasma venous determination. At least one additional glucose test result on another day with a value in the diabetic range is essential: either fasting, from a random sample, or from the 2-hour post-glucose load. If the fasting or random values are not diagnostic, the 2-hour value should be used. |
An HbA1c cut-off point of 6.5% is recommended for the diagnosis of diabetes. A value of less than 6.5% does not necessarily exclude diabetes diagnosed using a glucose tolerance test.
The diagnostic use of HbA1c needs to be interpreted with caution in some situations, such as recent onset of type 1 diabetes or recent onset of diabetes due to acute illness or drugs such as steroids or antipsychotic agents (included in the mixed category of type 4 diabetes). In these situations there has been insufficient time for HbA1c to increase. Vitamin supplements, such as vitamin C and E, and high cholesterol concentration may also influence the results.
HbA1c is often used to monitor long-term control of glucose in those with established diabetes
13A.3 Principles of diabetic management and the role of diet
The management of diabetes is underpinned by observations that good metabolic control can not only prevent short-term problems, such as those associated with hypo- and hyperglycaemia, but also long-term complications. However, it is recognised that beyond a certain stage of structural damage, for example beyond a certain stage of proliferative retinopathy, it may be difficult or impossible to attenuate the progression of diabetes through good metabolic control. This means that early preventive measures need to be implemented, and nutrition has a key role to play in this respect.
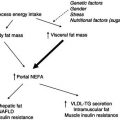
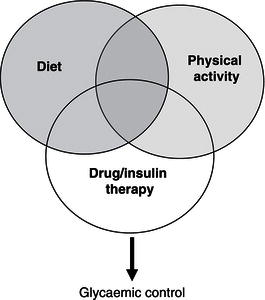
The sophisticated mechanisms associated with metabolic regulation of glucose and other nutrients in healthy subjects cannot be easily replicated in those with diabetes. These regulatory mechanisms are complex and are adapted to take into account variations in dietary intake and physical activity (and interactions between them), as well as variations in physical and psychological stresses associated with activities of daily living. Nevertheless, in diabetes attempts are made to balance an appropriate dietary intake (amount and composition of food) with an appropriate type and amount of insulin and physical activity. An increase in dietary intake, particularly of carbohydrate, while physical activity and drug therapy remain unaltered can cause hyperglycaemia, while a decrease in dietary intake can cause hypoglycaemia. An increase in physical activity can produce hypoglycaemia, while a decrease can produce hyperglycaemia. The individual components of a triad comprising drug therapy, physical activity, and dietary intake play a key role in glycaemic control (Figure 13A.1). Although they are individually considered later, it should be remembered that they operate simultaneously in an interactive manner.
In addition to these three factors, other variables can influence glycaemic control, including psychological stress and acute disease. For example, not only can gastroenteritis with vomiting reduce dietary intake, which tends to decrease the circulating glucose concentration, but it may also be associated with insulin resistance, which increases the glucose concentration, making glucose control difficult. Major elective surgery and accidental injury are also typically associated with a decrease in dietary intake and insulin resistance, and careful management is necessary in some circumstances. Following surgery (see Chapter 20 of Surgery and Trauma in this series), there is insulin resistance, and several of the postoperative complications are similar to those that occur in diabetes. Insulin resistance is also associated with low-grade inflammatory disease, for example in renal failure. Special nutritional problems, including those involving nasogastric and intravenous feeding, are discussed later. Guidelines for the effective management of both type 1 and type 2 diabetes have been issued by various national and international organisations, including the National Institute for Health and Clinical Excellence (NICE) in the UK.
Figure 13A.1 Diet, physical activity, and drug/insulin therapy interact in regulating the circulating glucose concentration.
13A.4 Insulin/drugs
Insulin regimens
There are several different types of insulin, which vary in their speed of action and duration of activity.
- Rapidly-acting analogues (e.g. NovoRapid, Humalog) can be injected just before, during, or after food ingestion, and have a peak action at between 0 and 3 hours. They tend to last between 2 and 5 hours – only long enough to cover the effects of individual meals.
- Long-acting analogues (e.g. Lantus, Levemir) tend to be injected once daily in order to provide background insulin lasting approximately 24 hours. They can be administered independently of food as they do not have an obvious peak action.
- Short-acting insulins (e.g. Actrapid, Humulin S) should be injected 15–30 minutes before a meal to cover the rise in blood glucose levels that occurs after eating. Their peak action is between 2 and 6 hours and can last for up to 8 hours.
- Medium- and longer-acting insulins (e.g. Insulatard, Humulin I) are injected once or twice daily to provide background insulin or in combination with short-acting insulin/rapid-acting analogue. Their peak activity is between 4 and 12 hours and they can last up to 30 hours.
- Mixed insulin (e.g Humulin M3, Insuman Comb 50) is a combination of medium- and short-acting insulin.
- Mixed analogue (e.g. NovoMix 30, Humalog Mix 25) is a combination of medium-acting insulin and a rapid-acting analogue.
These insulin types may be given alone or in combination according to lifestyle, including mealtime routine. The ‘basal bolus’ regimen consists of combining a long-acting insulin, to provide lasting background cover, with a rapid-acting insulin, to cover the effects of carbohydrate meals/snacks, which are usually eaten three times daily (breakfast, lunch, and evening meal). This popular regimen can be modified to suit individuals according to lifestyle. For example, the dose of a rapidly-acting insulin can be increased before the large meal is taken. Another regimen involves the continuous subcutaneous administration of insulin, often rapid-acting insulin, using an insulin pump; ‘boluses’ are given by the patient at meal times. The pump attempts to imitate the healthy pancreas, although it delivers insulin into the peripheral circulation, whereas the pancreas delivers it into the portal circulation (where the insulin concentration is much higher than in peripheral blood), which produces important ‘first-pass’ metabolic effects in the liver. The main advantage of using insulin-pump therapy is that it allows flexibility in coping with different patterns of dietary intake and physical activity, both of which can vary from day to day. However, the pump is expensive, its use requires training, and local skin infections can occur. An alternative but less flexible option is the ‘twice-daily fixed-dose insulin regimen’, which involves a combination of a short- and an intermediate/longer-acting insulin injected twice daily, typically 20–30 minutes before breakfast and before the evening meal. This regimen is suitable for individuals who have a fairly fixed routine, in which physical activity and meals occur at similar times and in similar amounts each day.
Oral drugs
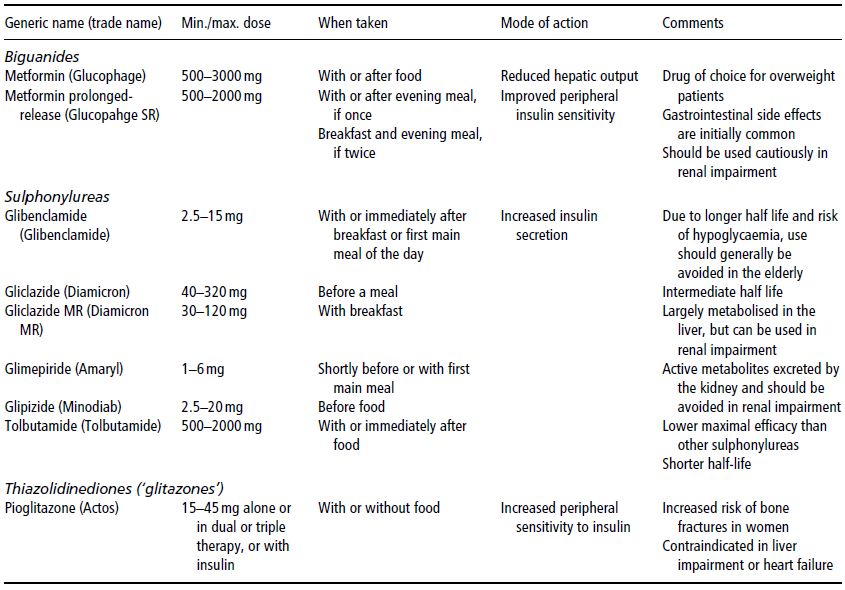
There are three main groups of oral drugs that are commonly used in the treatment of type 2 diabetes: biguanides, sulphonylureas, and thiazolidinediones (often referred to as ‘glitazones’). The properties of some of these drugs are shown in Table 13A.2. The biguanide metformin, which increases insulin sensitivity, at least partly because it suppresses hepatic gluconeogenesis, is often used as the initial oral hypoglycaemic agent (in conjunction with an appropriate lifestyle change). This is partly because weight loss in overweight or obese individuals is more likely to be facilitated by metformin, which does not stimulate insulin secretion, than sulphonylureas, which do stimulate insulin secretion. Metformin has also been shown to have clear cardioprotective properties, to be as effective as sulphonylurea or insulin in reducing microvascular risk, and to have some beneficial effects independent of its hypoglycaemic actions. If metformin is inadequate in controlling the blood glucose concentration, another class of drug, such as a sulphonylurea or a glitazone, can be added; if these are insufficient, insulin may be used either alone or in combination with oral hypoglycaemic agents, which allows a lower dose of insulin to be administered.
Other drugs
New drugs (intravenous and oral) for the treatment of diabetes continue to be developed. Among these are the postprandial regulators, such as nateglinide and repaglinide, which are oral hypoglycaemic agents with predominant effects in the postprandial period. Another group of drugs of particular nutritional interest was developed from physiological observations that orally administered glucose produces greater insulin response than intravenous glucose. This is believed to be largely due to the release of two gastrointestinal peptides (glucose-dependent insulinotropic peptide (GIP) and glucagon-like-peptide-1 (GLP1)) which are clearly stimulated by oral and not intravenous administration of glucose. This so-called incretin effect appears to be attenuated in type 2 diabetes, and therefore injectable incretin analogues have been developed, with the aim of achieving better glycaemic control in type 2 diabetes (GIP and GLP1 are also putative physiological signals that suppress appetite). Inhibitors of the enzyme dipeptidyl peptide 4 (DPP4), which rapidly destroys GLP-1 (e.g. linagliptin and saxagliptin), are also available. The thiazolinediones are another group of agents that reduce insulin resistance. They are commonly used in some centres, but they have a tendency to cause weight gain, and one of them (rosiglitazone) has been withdrawn from formularies because it was suspected of increasing risk of cardiovascular disease.
Table 13A.2 Examples and properties of some oral drugs commonly used for the treatment of type 2 diabetes.
13A.5 Physical activity
Physical activity has clear beneficial effects on cardiovascular risk and can improve glycaemic control in diabetes, especially in the postprandial period. Physical activity has been shown to reduce mortality in diabetes and can be safely used in patients with type 2 diabetes treated by diet alone or in conjunction with oral hypoglycaemic agents. In those treated with insulin or drugs that stimulate insulin secretion, precautionary measures must be taken to prevent exercise-induced hypoglycaemia. Such precautionary measures may involve consuming additional carbohydrate in amounts that depend on the duration and intensity of the activity and on the blood glucose reading before the activity begins, or reducing the amount of insulin administered before activity. The blood glucose concentration should optimally be in the normal range before the activity is undertaken.
13A.6 Diet
Dietary intake has a special role to play in the management of diabetes, not only because it is one of the three main components influencing glycaemic control and metabolic function, but because it is the only one of the three components illustrated in Figure 13A.1 that can be used to treat hypoglycaemia (by taking a snack or drink containing a rapidly absorbed sugar). The other two components, physical activity and drug/insulin therapy, tend to reduce the blood glucose concentration and may predispose to hypoglycaemia.
Dietary advice for diabetes should be broadly based on the healthy eating principles that apply to the general population. However, there are specific considerations for diabetes, which relate to the timing of food intake. In a patient with a good 24-hour profile of glucose concentration, changes in the timing and distribution of the same diet during the day can cause hypoglycaemia and/or hyperglycaemia, which are not seen in healthy people. Personal preferences and willingness to change lifestyle, including dietary habits, need to be considered carefully when devising a care plan for patients with diabetes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree