Nonepithelial Malignancies of the Breast
Francisco J. Esteva
Carolina Gutiérrez
Nonepithelial malignancies of the breast account for less than 1% of breast tumors. The most common primary nonepithelial breast cancers are sarcomas and lymphomas. Young et al. (1) evaluated the demographic and tumor characteristics of all malignant non-carcinomas of the breast using 26 population-based registries in the United States and found that of 363,801 women diagnosed with malignant breast tumors diagnosed between 1994 and 1998, only 1,401 women (0.4%) had tumors that were nonepithelial in origin. All but nine of the nonepithelial breast cancers in that study were some form of soft-tissue sarcoma. The most common nonepithelial cancer was malignant phyllodes tumor, which accounted for 61% of these diagnoses. In addition to the 363,801 malignant cancers classified as breast tumors, another 613 tumors in Young’s study arose in the breast but were classified as myelomas or lymphomas; two as solitary myelomas, two as Hodgkin lymphoma, and the remaining 609 as non-Hodgkin lymphoma (1). Cutaneous melanomas arising in the breast or the skin over the breast have been reported. Despite the infrequent presentation of these nonepithelial breast malignancies, knowledge of their unique features, clinical characteristics, pathology, molecular biology, appropriate diagnostic evaluation, proper staging, and treatment is important to provide optimal patient care. Metastasis to the breast from other organs is another presentation of a mass that can be confused with primary breast cancer. When the primary site is unknown, establishing this diagnosis requires extensive pathologic examination using conventional histology, special immunohistochemistry, flow cytometry, cytogenetics, and electron microscopy. Limited data are available regarding the molecular biology of most nonepithelial malignancies of the breast.
PRIMARY SARCOMAS OF THE BREAST
Primary sarcomas of the breast are malignant tumors arising from the connective tissue within the breast and account for less than 1% of all breast malignancies. According to the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, the annual incidence of breast sarcomas is 4.5 cases per million women (2). Sarcomas can arise de novo (primary) or as a consequence of treatment of an epithelial breast cancer (secondary) (3, 4). Radiation therapy for breast carcinoma can lead to the development of secondary sarcomas with a latency of up to 20 years (4, 5).
Malignant mesenchymal tumors of the breast are broadly composed of malignant phyllodes tumor and soft-tissue sarcoma. The stroma of phyllodes tumor develops from the hormonally sensitive periductal and intralobular stroma of the mammary gland that undergoes malignant change. Primary soft-tissue sarcomas of the breast arise from interlobular mesenchymal elements that comprise the supporting mammary stroma and exhibit histologic subtypes that do not differ from sarcomas seen in other sites in the body. In general, fibrosarcoma, angiosarcoma, malignant fibrous histiocytoma (MFH), liposarcoma, osteosarcoma, and stromal sarcoma comprise the major histological subtypes (6). The histologic distinction is still important as new molecular classification and targeted therapies are developed. Although the etiology of most soft-tissue sarcomas remains unknown, angiosarcoma of the breast has increasingly been associated with prior external beam radiation therapy of the breast and with lymphedema that occurs after radical surgery, with or without radiation, for primary breast cancer. Because of the rarity of breast angiosarcoma, only a small series of patients have been reported (7, 8 and 9).
Primary breast sarcomas typically present clinically with a unilateral mass with a growth rate that often is rapid when compared to epithelial breast cancer. The size of these tumors is variable, ranging from 1 to 40 cm in most studies, with an average median size of 5 to 6 cm (10, 11). The gross appearance of these tumors is influenced in part by the specific histological features, but, in general, they consist of firm, fleshy, tan to gray tissue with variable soft, cystic, and hemorrhagic areas.
Pathological grading plays a critical role in the prognosis of mammary sarcomas. The tumors vary from hypercellular,
fairly uniform spindle cell fibroblastic proliferations to atypical, highly anaplastic cells, and most tumors are intermediate to high grade. Increased mitotic activity (more than 10/10 HPF, range 0-43) and necrosis are additional findings. A diagnosis of primary breast sarcoma must be established only after a range of benign and malignant spindle cell lesions, such as fibromatosis, nodular fasciitis, fibrous histiocytoma, sarcomatoid carcinoma, and metaplastic carcinoma, have been excluded. The distinction is important for treatment and for prognosis. The pathologic evaluation of primary breast sarcomas must include extensive sampling and, in some instances, markers of differentiation, cell surface markers, immunohistochemical studies, cytogenetics, and, in some cases, electron microscopy (12, 13).
fairly uniform spindle cell fibroblastic proliferations to atypical, highly anaplastic cells, and most tumors are intermediate to high grade. Increased mitotic activity (more than 10/10 HPF, range 0-43) and necrosis are additional findings. A diagnosis of primary breast sarcoma must be established only after a range of benign and malignant spindle cell lesions, such as fibromatosis, nodular fasciitis, fibrous histiocytoma, sarcomatoid carcinoma, and metaplastic carcinoma, have been excluded. The distinction is important for treatment and for prognosis. The pathologic evaluation of primary breast sarcomas must include extensive sampling and, in some instances, markers of differentiation, cell surface markers, immunohistochemical studies, cytogenetics, and, in some cases, electron microscopy (12, 13).
Breast sarcomas differ clinically from primary breast epithelial tumors. The most common mode of spread is hematogenous, and axillary lymph node involvement is not as frequent as it is with epithelial breast tumors. The most frequent sites of initial metastases are the lungs, bone marrow, and liver (11). Breast imaging studies are nonspecific except that microcalcifications are rare, the mass is often well circumscribed, and tumors tend to be heterogeneous because of the presence of necrosis within the tumor (14, 15). Diagnosis of a primary breast sarcoma requires a core biopsy, incisional or excisional. A fine needle aspiration is not adequate. Excisional biopsy is preferred, with attention to negative surgical margins. Tumor size, the presence of regional and/or distant metastases, and the tumor grade are important factors to determine the stage and prognosis (13, 16).
The treatment for primary breast sarcomas is wide excision that allows adequate margins free of cancer cells (17). Axillary lymph node dissection is not recommended unless there are enlarged lymph nodes or lymph nodes that appear suspicious under ultrasound or magnetic resonance imaging (MRI). Radiation therapy and chemotherapy may be considered in patients with angiosarcomas and high-grade sarcomas because these lesions have a tendency to recur locally and can also metastasize. However, the role of adjuvant therapy in this setting is controversial. A retrospective review of 55 patients with primary breast sarcoma treated at the Mayo Clinic between 1975 and 2001 reported that adjuvant chemotherapy and radiation therapy did not improve survival although an advantage could easily be missed in such a small study (6). Responses have been reported in patients with metastatic sarcoma of the breast using anthracycline-and ifosfamide-based chemotherapy (18). The treatment regimen should be individualized and a multidisciplinary approach involving a close collaboration among the surgeon, radiation oncologist, and medical oncologist is mandatory.
Angiosarcoma of the Breast
Angiosarcoma arises in the breast more often than in any other organ, and it is also the most common soft-tissue sarcoma involving the breast (8). Angiosarcoma of the breast tends to occur in younger women at a median of 38 years. Because the disease affects younger women, an association with pregnancy has been observed; however, there is no evidence for a hormonal basis for breast angiosarcoma. A correlation has also been suggested between prior radiation therapy in the setting of breast conserving surgery and the development of angiosarcomas (11). The SEER program data compiled by the National Cancer Institute included more than 194,000 women who were treated for breast carcinoma. Among patients in the radiotherapy cohort, the relative risk of developing angiosarcoma was 15.9 (5). The median latency period between radiation for breast cancer and the diagnosis of angiosarcoma has been estimated to be about 6 years. One study failed to confirm the observation that prior radiation increased the risk of developing angiosarcoma (19). In that series, only nine cases of angiosarcoma were documented, which represents a prevalence of 5 per 10,000. Cutaneous angiosarcoma of the chest wall after mastectomy and radiation therapy may also occur (7).
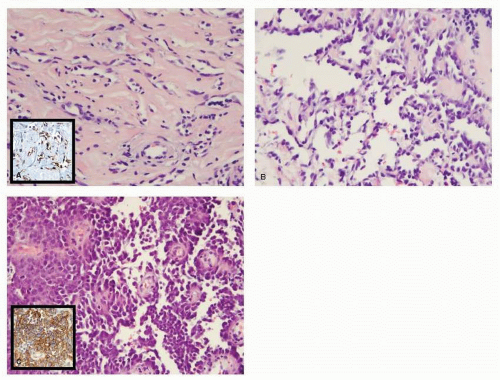
Typically, patients present with a rapidly growing painless breast mass. The overlying skin may have blue or purple discoloration (20). In the largest series, containing 69 patients with breast angiosarcoma, tumor size varied between 1 and 14 cm with a median of 5.5 cm (21). In the majority of cases, the angiosarcoma forms a friable, firm, or spongy hemorrhagic tumor. In high-grade lesions, cystic hemorrhagic necrosis is usually present. Hemorrhagic discoloration in the surrounding breast tissue may indicate that the tumor extends beyond the evident mass. In some cases, the tumors have been described as poorly defined areas of thickening or induration. Microscopically, three distinct patterns of growth have been described; they reflect the degree of differentiation and were thought to correlate with prognosis although one report did not find a relationship between grade and patient outcome (9). Low-grade or type I tumors (Fig. 64-1A) are composed of open, anastomosing vascular channels that invade mammary glandular tissue and fat, producing atrophy of the terminal duct lobular units. Some prominent hyperchromatic endothelial nuclei may be found, but most often they have inconspicuous nuclei. The endothelial cells are distributed in a flat monolayer around the vascular spaces without papillary endothelial proliferation, and rare mitoses are seen. Intermediate-grade or type II angiosarcoma (Fig. 64-1B) shows scattered focal areas of more cellular proliferation consisting of well-developed papillary endothelial proliferation that may combine polygonal or spindle cells. Infrequent mitoses may be found in cellular or spindle areas. Type III or high-grade angiosarcoma (Fig. 64-1C) exhibits highly malignant features that comprise more than 50% of the tumor (22). These tumors consist of prominent epithelial tufting and solid papillary formations with cytologically malignant endothelial cells. Mitoses are readily found. Areas of necrosis and hemorrhage, so called “blood lakes,” are only found in high-grade angiosarcomas. The high-grade tumors have infiltrative borders that feature low-grade vascular channels, which may lead to the erroneous diagnosis of a low-grade lesion on a core biopsy. Immunohistochemically, angiosarcomas are positive for Factor VIII related antigen, thrombomodulin, B72.3, CD31, and CD34. These reagents are useful for distinguishing angiosarcomas from carcinoma and other neoplasms. It has been reported that the level of the cell cycle protein SKp2 and the Ki-67 index as a measure of proliferation can be used to distinguish benign vascular lesions such as hemangiomas from malignant low-grade angiosarcomas (23).
Postirradiation Angiosarcomas of the Skin
The histological features of post-radiation angiosarcomas differ from primary breast angiosarcomas and mainly involve the skin with or without occasional invasion of the subjacent breast parenchyma (24, 25). High-grade areas are solid, epithelioid, or spindle cell foci with slit-like spaces with intraluminal or extravasated red blood cells. In addition, regardless of the microscopic pattern, malignant cells in post-radiation angiosarcoma have poorly differentiated nuclei with prominent nucleoli and mitotic activity. Angiosarcoma in the skin and breast after radiotherapy must be distinguished from benign vascular lesions that arise in the same clinical setting and that are called atypical vascular lesions (AVL) (24). These lesions appear 1 to 17 years after radiotherapy as single or multiple skin nodules measuring 5 mm or less in
diameter. Histologically, a focal proliferation of anastomosing vascular channels lined by a single layer of endothelial cells with occasional hyperchromatic nuclei is seen. The vascular spaces are usually empty and are limited to the superficial and mid-dermal areas. There is insufficient information to determine definitively whether AVLs can progress to sarcomas. One report suggests that AVLs may be precursors of angiosarcomas (25). Molecular analysis in a series showed that post-radiation cutaneous angiosarcoma is characterized by increased expression and amplification of MYC, whereas atypical vascular lesions do not show this alteration, questioning their role as precursors; however, cases of AVLs progressing to angiosarcoma were not studied in this report (26).
diameter. Histologically, a focal proliferation of anastomosing vascular channels lined by a single layer of endothelial cells with occasional hyperchromatic nuclei is seen. The vascular spaces are usually empty and are limited to the superficial and mid-dermal areas. There is insufficient information to determine definitively whether AVLs can progress to sarcomas. One report suggests that AVLs may be precursors of angiosarcomas (25). Molecular analysis in a series showed that post-radiation cutaneous angiosarcoma is characterized by increased expression and amplification of MYC, whereas atypical vascular lesions do not show this alteration, questioning their role as precursors; however, cases of AVLs progressing to angiosarcoma were not studied in this report (26).
Treatment of Angiosarcomas of the Breast
The optimal surgical treatment of breast angiosarcoma is segmental mastectomy if negative margins can be achieved or total mastectomy if the former is not possible (27, 28). Axillary dissection is not recommended. Patients with angiosarcomas have a worse prognosis than patients with other types of sarcoma (6). The most important prognostic markers are histologic grade (subtype) and tumor size although histologic grade was not prognostic in one study (9). However, the roles of adjuvant chemotherapy and radiation therapy are unclear. Several series suggest that adjuvant therapies do not improve disease-free or overall survival. A review of 69 patients with angiosarcoma of the breast treated at the MD Anderson Cancer Center found no improvement in survival of patients with angiosarcoma of the breast treated with neoadjuvant chemotherapy or radiation therapy. However, in this study, the response rate to anthracycline- and gemcitabine-based chemotherapy in the metastatic setting was 48%, suggesting that breast angiosarcoma is potentially a chemosensitive disease (21).
Osteogenic Sarcoma of the Breast
Extraskeletal osteosarcoma of the breast is an extremely rare tumor, accounting for 12.5% of mammary sarcomas (29). Primary breast osteosarcomas are considered highly aggressive tumors with early local recurrence and hematogenous spread (most commonly to the lungs). The most common presentation is a circumscribed and movable mass that on mammography may show osseous trabeculae or coarse calcifications. Silver et al. (30) reported a series of 50 patients with osteogenic sarcoma of the breast diagnosed at the Armed Forces Institute of Pathology (AFIP) between 1957 and 1995. The median patient age at presentation in that study was 63.2 years, and the tumor size varied from 1.4 to 13 cm at the time of diagnosis. The histological features are similar to other skeletal osteosarcomas. The most common variants observed are fibroblastic, osteoblastic, and osteoclastic. In the osteoblastic osteosarcoma, the osteoid is deposited in a fine, ramifying, lacelike, or coarsely trabecular pattern
and sometimes in sheaths of osteoid or bone. Atypical cartilage has been reported in 36% of primary breast osteosarcomas. Necrotic foci can be identified in 30% of the cases. Multinucleated osteoclastic giant cells are usually present in areas of bone formation. Immunohistochemistry is helpful in ruling out a metaplastic carcinoma with heterologous elements, which expresses CAM 5.2, pancytokeratin, and high molecular weight keratin (Fig. 64-2).
and sometimes in sheaths of osteoid or bone. Atypical cartilage has been reported in 36% of primary breast osteosarcomas. Necrotic foci can be identified in 30% of the cases. Multinucleated osteoclastic giant cells are usually present in areas of bone formation. Immunohistochemistry is helpful in ruling out a metaplastic carcinoma with heterologous elements, which expresses CAM 5.2, pancytokeratin, and high molecular weight keratin (Fig. 64-2).
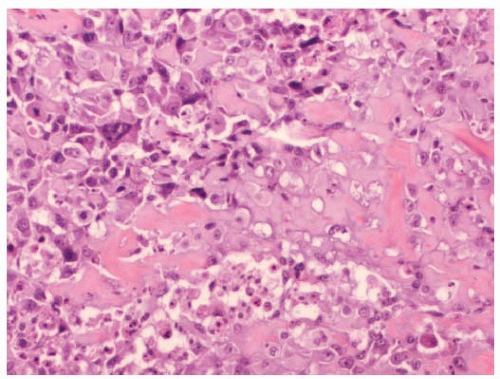 FIGURE 64-2 Primary osteosarcoma of the breast. Malignant cells with round nuclei and prominent nucleoli with lacelike osteoid are seen. |
The relationship between prior breast or chest wall irradiation and breast osteosarcoma is not clear. One of the patients in the AFIP series had received radiotherapy for ipsilateral breast carcinoma 9 years before presentation, but none of the other patients had been exposed to radiation therapy (30). Like other sarcomas, spread to regional lymph nodes is uncommon with breast osteosarcoma. No axillary lymph node involvement was noted in 20 patients who underwent axillary lymph node dissection in the AFIP series. Of 39 patients with follow-up, locally recurrent (n = 11) or metastatic disease (n = 15) was documented at a mean of 10.5 and 14.5 months from diagnosis, respectively, in the same study. Adjuvant radiation therapy and chemotherapy are not recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree