Smoking
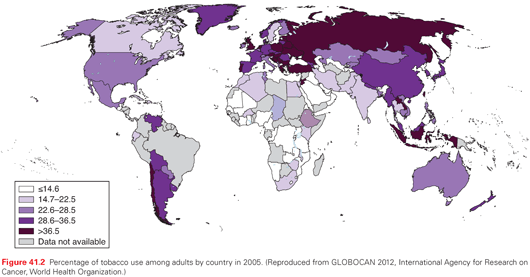
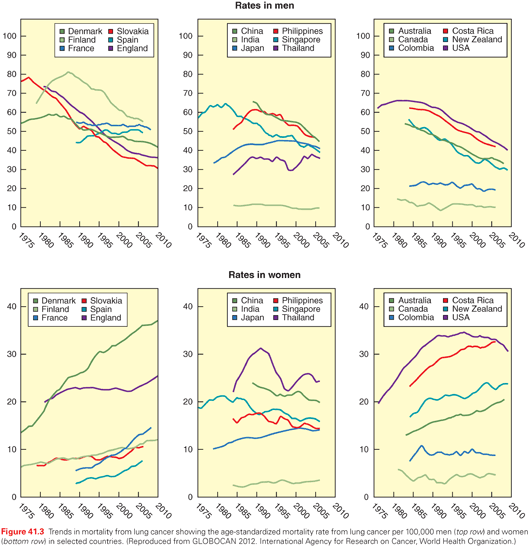
At the turn of the 20th century, lung cancer was a rare malignancy. In an extensive autopsy review in the United States and Western Europe in 1916, Adler found that lung cancers represented <0.5% of all cancer cases.6 Over the next several decades, a substantive increase in the incidence of lung cancer was noted; at the same time, observations of the adverse health effects of smoking were made. In 1941, Ochsner and DeBakey stated, “it is our definite conviction that the increase in the incidence of pulmonary carcinoma is due largely to the increase in smoking, particularly cigaret (sic) smoking.”7 Two landmark case-control studies published in 1950 by Doll and Hill8 in the United Kingdom and Wynder and Graham9 in the United States established a causal link between cigarette smoking and bronchogenic carcinoma. The Royal College of Physicians in the United Kingdom in 196210 and the Surgeon General of the United States in 196411 endorsed the conclusion that cigarette smoking is the major cause of lung cancer. Population trends of lung cancer incidence mirror smoking behavior, with a typical lag time of approximately 20 years (see Figs. 41.1 and 41.2). Both the number of cigarettes smoked per day and the duration of smoking correlate with lung cancer risk, with longer duration in particular being associated with a much higher risk.12
Cigarette smoke is a complex aerosol, with nicotine being the primary determinant of addiction and tar being the particulate residue left when nicotine and water are removed from tobacco smoke. More than 50 carcinogens in tobacco smoke have been identified, including N-nitrosoamines formed by nitrosation of nicotine during smoking, and polycyclic aromatic hydrocarbines.13–15 The N-nitrosoamine 4-(methylnitrosamino)-1(3-pyridyl)-1-butanone is associated with DNA adduct formation and DNA mutations that result in the activation of KRAS oncogenes.16,17 Both N-nitrosoamines and polycyclic aromatic hydrocarbines may be metabolically activated to become carcinogenic, or alternatively may be metabolically detoxified; the balance of these processes will determine exposure. Other carcinogens in tobacco smoke carcinogens do not require activation (e.g., benzene, vinyl chloride, or radon). The dose of carcinogen(s) received from smoking will vary with the composition of the cigarette itself, including whether a filter is present, and the intensity of inhalation. Because of regulatory mandates, cigarettes contain less nicotine and tar than in years past. However, smokers may compensate by smoking more intensively (more puffs per minute and deeper, longer inhalations) to satisfy nicotine addiction. Mentholation of cigarettes may also facilitate more intense smoking, as it changes the aroma of the smoke and decreases the irritant effect of the smoke, thereby facilitating more intense inhalation.18,19
Genetic Predisposition
The cumulative lifetime risk for lifelong smokers in their eighth decade of life is approximately 16%.20 The host factors that confer “protection” from lung cancer in the majority of individuals who smoke and never develop lung cancer, or determine increased susceptibility in those individuals who do, are not clear. An estimated 300,000 lung cancer deaths occur in nonsmokers annually in the world; in many of these cases, no specific risk or exposure can be identified.21,22 It is widely accepted that genetic (inherited) factors as well as epigenetic (acquired) DNA changes contribute to the development of malignancy. The association of lung cancer with rare Mendelian cancer syndromes or in family aggregates supports the contribution of high-penetrance, low-frequency genes.23,24 Mutations in low-penetrance, high-frequency genes that encode enzymes involved in activation or detoxification of carcinogens, or enzymes involved in DNA repair, also influence lung cancer susceptibility.25–27
A large European case control study and meta-analysis of 41 studies demonstrated that the risk of lung cancer increased if there was a first-degree family member with lung cancer (odds ratio = 1.63) and increased further if two or more family members had lung cancer (odds ratio = 3.6).28 The Liverpool Lung Project reported that a family history of early onset lung cancer (age <60 years) was associated with an odds ratio for lung cancer of 2.02.29 While some polymorphisms in a few candidate genes have been identified, the broad field of host genetic factors determining lung cancer susceptibility is still poorly understood and is an area of intense investigation. We do not yet have the tools to identify which individuals might be intrinsically more likely to develop lung cancer or more vulnerable to the carcinogenic effects of cigarettes. Such knowledge eventually may help define specific populations who should be targeted for lung cancer screening.
Occupational/Environmental
Though smoking is clearly the most important modifiable risk factor, many other factors influence the development of lung cancer. It is estimated that approximately 10% of lung cancer cases are at least in part related to occupational exposures.30 Many workplace materials have been identified as carcinogens, including, among others, arsenic, asbestos, beryllium, cadmium, chromium, nickel, radon, and vinyl chloride. Of these, asbestos is the most common, having been used widely for its insulating properties and recognized as a lung carcinogen as early as the 1940s.31 There has been controversy as to whether asbestos exposure per se confers lung cancer risk or whether asbestosis, the associated interstitial lung disease, is required. A recent long-term follow-up of a large group of North American insulators originally studied in the early 1980s provided further data on asbestos exposure, asbestosis, and cigarette smoking.32 In the nonsmoking cohort, lung cancer risk was increased with asbestos exposure alone, and further increased if asbestosis was also present. In the smoking cohort, there was an additive risk of smoking and asbestos exposure, with a supra-additive increase in risk if both smoking and asbestosis were present. Thus it appears that while asbestosis clearly increases lung cancer risk, particularly in smokers, asbestos exposure alone also increases risk, albeit to a lesser degree.
Radon gas was first implicated as increasing lung cancer risk in workers in underground uranium mines. The awareness of radon as a carcinogen has focused attention on domestic radon gas as a common indoor pollutant.33–36 Radon is felt to contribute to an estimated 15,000 to 20,000 lung cancer deaths in the United States annually.36 Other indoor air pollutant carcinogens include environmental tobacco smoke and byproducts of biomass fuels used for heating and cooking. Outdoor air pollution contains carcinogens generated by fossil fuel combustion and diesel exhaust, which can be adsorbed to fine particulates small enough to be inhaled into the airways. These exposures are of particular concern in countries such as China, where indoor domestic use of coal and wood, intense urban outdoor pollution, and an epidemic of tobacco smoking are likely all contributing to an alarming rise in lung cancer incidence.
Cancer risk related to medical imaging has been an area of increasing public health concern, particularly with the escalation of use of high technology studies such as computed tomography (CT) and positron emission tomography (PET). Radiation exposure acquired in this way is cumulative, with risk extrapolated on a linear model based on known cancer risk in survivors of the atomic bomb in Japan, and after therapeutic radiation given in the past for medical diseases including tuberculosis and ankylosing spondylitis.37–40 Based on this model, even lower cumulative doses incur some risk. Recognition of the potential harms of radiation from medical imaging makes more urgent the need to utilize these tests judiciously, develop methods to track cumulative radiation exposure to specific organs, and improve technologies that will minimize patient exposure. This need is particularly cogent anticipating implementation of lung cancer screening with low-dose chest CT scanning.
Lung cancer is typically a disease of older individuals; ironically, as overall health status improves in developing countries and those populations acquire longer life expectancy, their lung cancer risk may increase. Whether gender is a factor has been a topic of considerable debate. At present, the body of evidence does not support any significant sex-related difference in susceptibility to smoking-associated lung cancer.41 African Americans have consistently been observed to have higher lung cancer rates as well as worse 5-year survival than Caucasian Americans.2 In developed nations, socioeconomic factors including education and income are inversely associated with risk. Lifestyle factors, including diet and physical activity, likely influence risk. An inverse association of diets higher in fruit and vegetable (cruciferous and carotenoid-rich) consumption with lung cancer risk has been consistently described.42,43 The evidence for physical activity is less clear, though higher levels of activity have been described as associated with lower cancer risk.44
The presence of underlying pulmonary disease is increasingly recognized to influence lung cancer risk. Cigarette smoking is the major etiologic factor in both lung cancer and chronic obstructive pulmonary disease (COPD). COPD per se is an independent risk factor after controlling for smoking.45,46 The mechanism(s) by which fixed airways disease may etiologically be implicated in carcinogenesis are not defined, but potentially include mutagenesis through activation of the NF-kappaB inflammatory pathway, protease-antiprotease imbalance, or chronic inflammation itself.47–49 The presence of interstitial lung disease has also been associated with an increase in lung cancer risk. In particular, patients with idiopathic pulmonary fibrosis have been reported to have an odds ratio for lung cancer of 8.25 compared to controls.50,51 In contrast to COPD, cigarette smoking is usually not etiologically implicated in interstitial diseases, although chronic inflammation typically is felt to contribute to the pathologic process.
Stage Classification
The stage classification for non–small-cell lung cancer (NSCLC) follows the tumor, node, metastasis (TNM) paradigm used for most solid tumors. Staging ensures a uniform standardized nomenclature to describe the anatomic extent of disease, namely the primary tumor site (T component) as well as cancer spread to nodes (N component) or distant metastatic sites (M component). The most recent (seventh edition) of the lung cancer stage classification is based on an unprecedented effort directed by the International Association for the Study of Lung Cancer (IASLC).52–55 This involved an international database of over 100,000 patients, identified over the period 1990 to 2000 from 46 sources in 19 countries in North America, Asia, Australia, and Europe, and a sophisticated statistical analysis with extensive internal and external validation.56 This stage classification applies to small cell and NSCLCs as well as carcinoid tumors.57,58
Several characteristics of the IASLC database are important to recognize in order to appropriately interpret some of the results. This was a retrospective database, with limitations in the level of details available. For example, some of the definitions of the TNM descriptors contained too few patients to allow statistical analysis of the impact of that tumor characteristic. Because the database consisted of patients diagnosed between 1990 and 2000, advances in technology (e.g., PET imaging) and treatment modalities that are routine today are not reflected. The analysis did not account for what treatment (if any) was given. In fact, there was a great deal of variation in outcomes depending on the type of source data and the geographic region. Thus, while the outcomes created a phenomenal tool to define where to draw distinctions between TNM categories and groupings, the outcomes themselves represent a global cross-section from the past with less clear relevance to specific patients today.
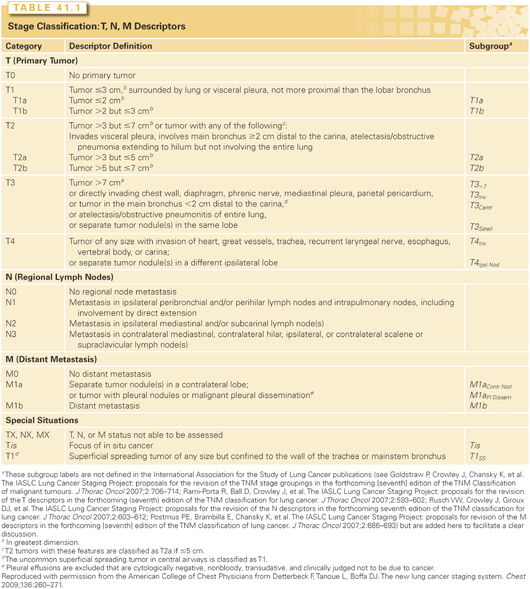
The definitions of the T, N, and M categories for both NSCLC and small-cell lung cancer (SCLC) are outlined in detail in Table 41.1.59 The T, N, and M categories are then grouped to define particular stages of lung cancer (both NSCLC and SCLC), as outlined in Fig. 41.4. While ideally each stage would represent a homogeneous group of patients, this is not necessarily the case, as is evident in the range of T, N, and M combinations that comprise stage IIB or IIIA, for example. Nevertheless, the system provides a practical way of managing the complexity of a large number of T, N and M categories into a more limited number of stage groups.
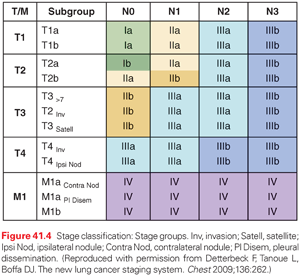
It is worth emphasizing what the stage classification system does and cannot accomplish. It is designed to be a consistent, stable nomenclature for the anatomic extent of disease. Although this is a major component in a patient’s prognosis, the prognosis depends on many other factors, including age, comorbidities, environmental factors, and treatment factors. Particular tumor or patient factors (e.g., genetic mutations, central/peripheral lung location) may have a major impact in certain settings (e.g., a particular treatment strategy) but not in others, and at certain times during the course of the disease. Therefore, stage classification is not sufficient to capture the complexity of prognostic prediction for specific patients in specific clinical settings. Furthermore, although the anatomic extent of disease is an important component of selecting a treatment approach, this is also determined by other factors (e.g., histologic type, molecular information, patient-specific factors such as comorbidities). In addition, stage classification must remain relatively static and consistent to be useful, while treatment should continually evolve and improve. Thus, treatment must be determined by the results of clinical trials and cannot be determined merely by a stage classification nomenclature.
Histologic Classification
The histologic spectrum of lung cancer has clearly changed over the past several decades. SCLC has decreased from about 25% to <15% of all lung cancers.60 Adenocarcinoma is now the most dominant histologic type, replacing squamous carcinoma. With the advent of more frequent CT imaging, an increased proportion of more indolent small tumors is being recognized.61
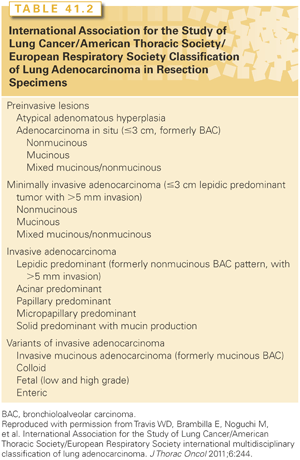
The histologic classification of lung cancer has become much more nuanced. In the past, the major NSCLC histologic types (adenocarcinoma, squamous cell carcinoma, and large cell carcinoma) were simply lumped together. It is critical to differentiate these because they respond differently to certain chemotherapeutic agents. Immunohistochemical stains and genetic characterization can facilitate the distinction between subtypes.62,63 It is recognized that the vast majority of adenocarcinomas are mixed subtypes; these are now classified according to the predominant subtype with the percent of each noted in 10% increments.63 Furthermore, the adenocarcinoma subtypes have been defined according to preinvasive, minimally invasive, and invasive groups (Table 41.2),63 reflecting the increasingly recognized spectrum of aggressiveness of lung cancers encountered in the Western world today. Finally, bronchopulmonary carcinoid and salivary gland tumors are less common types that are also included among lung cancers.64
Genetic Characterization
A fairly detailed understanding of cellular and genetic characteristics of cancer has emerged. A way to organize this is the concept of “hallmarks of cancer.” Initially 6 hallmarks were defined; by 2011, this had increased to 10 (resisting cell death, sustaining proliferative signaling, evading growth suppressors, enabling replicative immortality, activating invasion and metastasis, inducing angiogenesis, deregulating cellular energetics, avoiding immune destruction, genome instability and mutation, tumor-promoting inflammation).65,66 A potential 11th hallmark has recently been suggested (epigenetic or RNA deregulation).67 The ability to identify mutations in genes that play a role in various of these mechanisms has substantiated the importance of these hallmarks as being fundamental to malignant behavior.
Genetic characterization allows better classification of lung cancers. A systematic characterization of genetic alterations in 1,255 patients with lung cancer reveals that most histologic types have a characteristic pattern of genetic alteration.62 Large-cell lung cancer is the exception, exhibiting alterations associated with each of the other major histologic types. Using genetic characterization, the vast majority of cases initially classified simply as large-cell lung cancer could be assigned to another histologic group (e.g., adenocarcinoma, squamous carcinoma, SCLC), which was corroborated by prognostic and other similarities among the genetically grouped cohorts.62
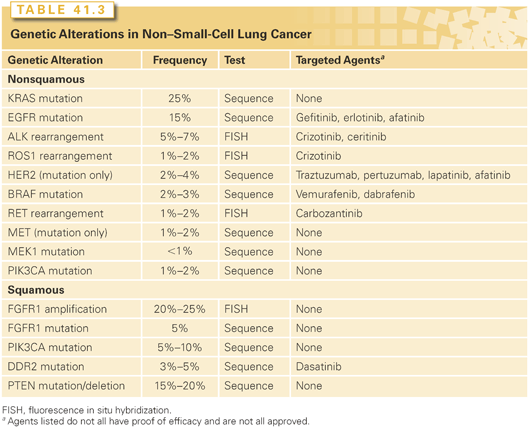
Particular mutations have sparked a great deal of attention, primarily because they have led to dramatic therapeutic breakthroughs (Table 41.3). Mostly these involve driver mutations for sustained proliferative signaling. Mutations in the epidermal growth factor receptor (EGFR) gene are the best-known examples. Such driver mutations have received much attention because targeted treatment can yield dramatic results, although the overall proportion of patients with these mutations may be quite small. Particularly because identification of these genetic alterations drives the choice of treatment, some have suggested that classification based on targetable mutations may be a better way to classify lung cancers than by histologic features.
Classification by genetic alterations is thwarted by the complexity of these changes. Whole genome and whole exome sequencing of 183 lung adenocarcinomas revealed greater complexity in genetic alterations than what is seen in most other cancers.67 Although many mutated genes were found, 15% did not have a single hallmark alteration, and almost 40% have fewer than four hallmark alterations.67 Focusing on mutations in which a targeted therapy is available or being developed may be practical, but belies the complexity of cancer biology. Even in those instances where dramatic responses have been achieved, in most cases resistance eventually develops. Furthermore, while driver mutations can sometimes clearly play a major role in cancer growth, alterations in many of the other hallmarks of cancer are less amenable to treatment, at least at this time. Finally, the well-documented finding that some EGFR-mutated adenocarcinomas that have become resistant to treatment with a tyrosine kinase inhibitor (TKI) have been transformed into a SCLC underscores that our understanding of the fundamental nature of lung cancer is still rudimentary.
Prognostic Factors
Prediction of prognosis is fervently desired, but remains an elusive goal. Developing a system to do this is complex and must solve a number of inherent conflicts. Hence the state of affairs is that we have identified a few factors that have prognostic value (at least in some clinical settings), but it is spotty and explains only a small amount of the actual observed outcomes.
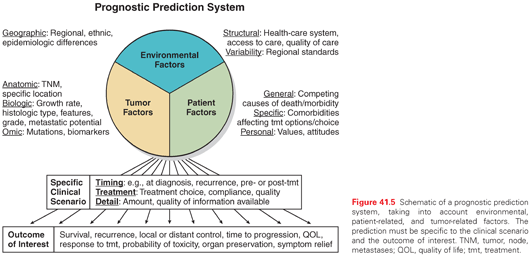
Prediction of prognosis is inherently complex.68 There are many factors that contribute; these can be grouped roughly into environmental factors, tumor-related factors, and patient-related factors (Fig. 41.5). The prognosis is inherently linked to a specific clinical scenario and to the outcome of interest. The scenario includes treatment-related factors and timing (e.g., the prognostic significance of an EGFR mutation is different if the patient is to undergo treatment with an EGFR inhibitor, regular chemotherapy, or neither, and different prior to treatment than upon developing resistance).
There are inherent conflicts in prognostication.68,69 We derive our knowledge from studying groups of patients with a particular characteristic, but we seek individualized prognostic prediction, tailored to a particular person. The ability to individualize is limited because we do not have sufficient detail of all of the other factors of the group to assess how well the group fits with the individual. Even if we had more and more detail, the more specific we get, the more limited the dataset available to derive the prediction becomes, and thus the greater the uncertainty about the prediction. Finally, the data we base the prediction on is inherently based on past observation, yet the prediction is for the future, and thus cannot take into account new developments that inevitably occur.
Prognostic prediction is inherently different than stage classification.69 Stage classification is a nomenclature to describe the anatomic extent of disease. It must remain relatively static and be used in a consistent uniform manner; the classification we assign to a particular extent of tumor today must be the same as what we assign to the same extent next year or else it is a useless nomenclature. However, prognostication is inherently fluid and constantly changing as advances occur and the setting changes. Stage classification must be consistent and definitive while prognostic prediction is inherently speculative and uncertain.
As part of the stage classification project, the international staging committee undertook a review of data on prognostic factors in lung cancer among surgically treated patients in the worldwide IASLC database.70 Pathologic TNM class was most important, but age and gender also had independent prognostic significance. Histologic cell type (particularly bronchioloalveolar carcinoma [BAC]) may have some prognostic value; however, this is linked to other variables and is probably best understood when we can take into account the newer subclassification of adenocarcinoma, which was not available at the time of the analysis.63 Many other potential factors, such as genetic features or PET intensity, were also not available for analysis. In sum, development of a prognostic model is very complex, situation specific, and constantly changing. We have only a rudimentary understanding at this point, and no overarching system of how to develop a universal prognostic prediction model.
Prevention
Tobacco Control
The global epidemic of lung cancer is linked most strongly to population engagement in cigarette smoking. While other modifiable risk factors (e.g., exposure to occupational or domestic carcinogens) are clearly identifiable, it is tobacco smoking that has driven the steep rises in lung cancer incidence and mortality witnessed over the last century in developed and developing nations. Primary prevention has the greatest overall potential to minimize lung cancer risk, and smoking cessation is still the most powerful intervention to diminish lung cancer risk in persons who smoke. Smoking cessation even into the seventh decade of life results in a decrease in lung cancer incidence, and so it is never too late to quit.20,71 While smoking rates have decreased dramatically since the publication of the first surgeon general’s report on the health consequences of smoking in 1964, approximately 20% of adult Americans still habitually smoke.
Important public health interventions have included stricter control of tobacco products by government regulatory agencies, limitations on cigarette advertising, particularly those geared toward children, the use of text and graphic warnings on cigarette packaging, and global initiatives to inform the public of the health hazards of smoking.72–75
Smoking Cessation
There are many physiologic and psychological factors that contribute to make smoking a difficult addiction to overcome. However, there have been major advances in understanding these, and solid scientific data regarding which interventions work best and in which individuals. Nicotine acts in an area of the brain associated with a sense of “safety” and “survival functions.”76 This appears to explain the paradox of difficulty in giving up smoking even when faced with a life-threatening disease that is a consequence of smoking. This struggle and the illogical nature of it, combined with the stigmatism associated with smoking, lead to feelings of shame, helplessness, and depression that further aggravate the problem.76 It is easy to succumb to a fatalistic defense that “it is too late anyhow.” However, data clearly shows that patients with lung cancer who continue to smoke approximately double their risk of dying.76 Quitting smoking is associated with better response to treatment, better quality of life (QOL), better long-term survival, and a lower risk of second primary cancers.76–79 Furthermore, data shows that a diagnosis of lung cancer represents a particularly opportune moment to intervene.76
Smoking cessation intervention should be included in the health care of any individual smoking cigarettes. It is important to use a sophisticated, evidence-based approach and an organized smoking cessation program to achieve the best results.76 A simple recommendation to stop smoking or provision of self-help materials is largely ineffective. Several points are important. Tobacco dependence is best managed in a chronic disease model with repeated intervention over time. Intensive behavioral therapy and counseling (e.g., weekly) is of significant benefit in several randomized controlled trials (RCT). In addition, seven first-line medications reliably increase long-term smoking abstinence rates (bupropion, varenicline, nicotine patch, gum, lozenges, inhaler, and nasal spray).76 These increase effectiveness two- or three-fold and result in abstinence rates of ~25%. Often, combinations of these may be more effective than a single intervention. These medications have been shown to be safe in most patients, including those who are about to undergo either surgery, RT, or chemotherapy. It is best to initiate the cessation interventions at the outset. Specifically, it is safe (and beneficial) for patients to stop smoking even a short time (e.g., 1 to 2 weeks) before undergoing surgery; pharmacologic interventions are safe to continue in the perioperative period as well.76 A well-organized thoracic oncology program, therefore, should include an evidence-based smoking cessation program that is fully integrated with the diagnostic and treatment components of patient care.
Chemoprevention
The concept of chemoprevention is based on the data that lung cancer is the end result of a multistep accumulation of carcinogen-driven genetic and epigenetic changes. In theory, chemical agents might prevent these changes by a variety of proposed mechanisms, such as detoxifying carcinogens and modifying pathways that influence cell growth and behavior. Epidemiologic and animal studies had suggested that derivatives of the antioxidant vitamins A and E might be protective against lung cancer. However, large clinical trials of alpha-tocopherol and beta carotene in subjects at risk of developing lung cancer failed to demonstrate any benefit, and two studies suggested that beta carotene was actually associated with an increased incidence of lung cancer as well as cardiovascular disease.80–83 To date, no chemopreventative intervention has been demonstrated to be of benefit for lung cancer. The focus has shifted from large RCTs to studies to better define the underlying biology and appropriate surrogate end points.84
Screening
At present, the majority of patients with lung cancer have advanced disease at the time of diagnosis. This preponderance of advanced disease, where prognosis is poor even with treatment, is a major contributor to the dismal overall 5-year survival rate of 18%; this contrasts starkly with breast, colon, and prostate cancers, the next three leading causes of cancer death, whose 5-year survival rates over the past several decades have increased to 90%, 65%, and nearly 100%, respectively.2 These improved survival rates are arguably attributable at least in part to early detection resulting from widely available and broadly accepted, albeit still controversial, screening interventions.
An effective screening tool for early detection of lung cancer has been an elusive goal for decades. Several large randomized trials performed during the 1960s and 1970s evaluating lung cancer screening with chest radiography with or without sputum analysis at varying time intervals failed to demonstrate any mortality benefit,85–89 and a Cochrane meta-analysis90 concluded that there was no evidence to support the use of chest radiography or sputum cytology as a lung cancer screening modality. More recently, chest radiography was re-examined as a lung cancer screening tool in the Prostate, Lung, Colorectal and Ovarian trial, which enrolled 154,901 participants aged 55 to 74 years from 1993 to 2001.91 No difference in lung cancer mortality was seen between those randomized to screening with annual chest radiography versus no screening, regardless of the degree of smoking. Together, these studies clearly demonstrate that there is no mortality benefit associated with serial chest radiography as a screening tool.
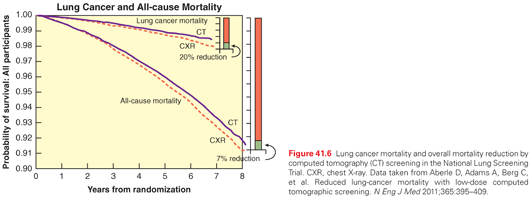
In the 1990s, intense interest was generated by the results of a number of observational studies evaluating low-dose CT (LDCT) as a lung cancer screening modality. Eventually, a number of RCTs were performed in various sites around the world. The largest of the RCTs was the National Lung Screening Trial (NLST), which included 53,454 subjects, ages 55 to 74 with at least 30 pack-years of cigarette smoking, who were either currently smoking or had quit smoking within the prior 15 years.92 NLST subjects underwent three rounds of annual screening, randomized to either chest radiograph or LDCT. Approximately 1% of subjects had lung cancer over the duration of the trials. At a median follow-up of 6.5 years, there was a 20% relative reduction in lung cancer mortality observed in the LDCT arm (Fig. 41.6).92 None of the other RCTs evaluating lung cancer screening with LDCT is of the scale of the NLST; in the trials in which the data has become available, the mortality benefit has been nonsignificant, and without a trend toward a benefit.93–95 The subjects in all of these studies had smoked and were of middle to older age. A multisociety systematic review of lung cancer screening with LDCT analyzed the evidence from 8 RCTs and 13 prospective cohort studies.96 This review found a composite significant benefit for LSCT screening, with few ensuing harms, when LDCT screening was conducted in the setting of an organized, structured program. Consistent with this, many organizations (American College of Chest Physicians [ACCP], American Cancer Society, Society of Thoracic Surgeons, American Association of Thoracic Surgery, National Comprehensive Cancer Network [NCCN], US Preventative Services Task Force) have recommended that healthy smokers or former smokers (quit <15 years ago, ≥30 pack years of smoking) age 55 to 74 years or 80 years be considered for LDCT screening.96–101
Teasing out further details on who to consider for screening will rely heavily on modeling studies as the effort needed for further RCTs is too great. To explore this, the Cancer Intervention and Surveillance Modeling Network developed five independent models based on a US cohort born in 1950.102 All five models included dose-response information relating to cigarette exposure; 26 scenarios varying in age, screening frequency, and smoking exposure were modeled with respect to the percentage of cancers detected at an early stage, the number of lung cancer deaths prevented, and life-years gained were balanced against outcome measurements of harm, including the number of CT screenings required, the number of follow-up imaging exams, the number of overdiagnosed lung cancers, and radiation-related lung cancer deaths. Based on the models, a range of different screening scenarios are arguably valid, with different balances of benefits and harms, with the most efficient screening projected in a population similar to the NLST. On the basis of the Cancer Intervention and Surveillance Modeling Network analysis, the US Preventative Services Task Force now recommends “annual screening for lung cancer with low-dose computed tomography in adults ages 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years.”101
There are potential downsides to screening with LDCT that must be considered in the decision to pursue screening for any population or for a given patient. The most obvious of these is the high rate (20% to 50%) of finding small nodules, which are benign and inconsequential in about 97%.96 These findings can create unnecessary anxiety, lead to further more intense imaging with significant radiation exposure, and sometimes invasive biopsies with potential complications. In the highly organized LDCT screening studies, only 1.2% to 5.6% of subjects underwent a surgical biopsy or procedure, about 25% of these were for what turned out to be benign nodules.97 Finally, it is clear that screening detects a higher proportion of slow-growing, less aggressive cancer, some of which may even be inconsequential.103,104 Appropriate management of these issues that are inextricably linked to screening is essential in order to minimize harms and achieve the greatest benefits.
The complexities of the identification of the “right” population or individuals to screen, combined with the potential harms associated with LDCT screening, argue strongly that screening for lung cancer is a multifaceted process, involving much more than the simple ordering of a scan. The process for most individuals should include an individualized risk assessment for lung cancer29,105–108; a discussion about the potential harms of screening, including false-positive results; the potential for cumulative diagnostic radiation; and the possibility of invasive evaluation to diagnose nonmalignant disease. Smoking cessation intervention should be an integral part of this process for individuals still smoking or at risk for relapse. This process should be performed in a multidisciplinary setting. There is data to suggest that if lung cancer screening is done in an unstructured way, the ratio of benefits to harms may be significantly altered. Because implementation of lung screening is just beginning, careful consideration and monitoring is needed to see if the benefit seen in the NLST can be achieved with broader application.
Clinical Presentation
Approximately a quarter of patients with lung cancer are diagnosed at early stage. These patients are typically free of symptoms; their cancers are identified incidentally during evaluation of unrelated issues. While the proportion of asymptomatic early stage lung cancers may increase with the implementation of screening, at present more than half of patients have advanced lung cancer at the time of diagnosis. These patients typically come to attention because of symptoms related to the primary tumor, metastasis to distant sites, or paraneoplastic syndromes.109
The most common pulmonary symptoms are cough, hemoptysis, and dyspnea.109 Cough may represent the effects of the primary tumor on the airways, with endobronchial or extrinsic airway obstruction, postobstructive atelectasis or infection, or airway inflammation with secretions. Hemoptysis may also result from airway inflammation or necrosis, but may also be related to parenchymal tumor necrosis and cavitation. Dyspnea may arise from a wide variety of tumor-related issues, including mechanical compromise of the airways, lymphangitic spread, pleural effusion, hypercoagulability with pulmonary thromboembolism, or even pericardial effusion. Direct extension of the primary tumor to adjacent structures may cause pain from invasion of the chest wall or brachial plexus, hoarseness from impingement of the recurrent laryngeal nerve, superior vena cava (SVC) syndrome, Horner syndrome (ptosis, miosis, anhidrosis) from invasion of the sympathetic chain and stellate ganglion, or pericardial tamponade.109
Symptoms related to metastatic disease may be constitutional or organ related. The most common sites of NSCLC metastases are the brain, bone, liver, adrenals, and lung, though any organ can be affected. Focal neurologic symptoms, persistent headache, bony pain, or unexplained weight loss, anorexia, or fatigue should raise the suspicion for metastatic disease. Likewise, laboratory abnormalities such as anemia, liver function test abnormalities, or hypercalcemia should raise concern for distant spread.109
Paraneoplastic syndromes are well described with lung cancer; it is important to recognize that these syndromes are unrelated to metastatic disease and do not preclude curative intent therapy. Hyponatremia related to syndrome of inappropriate secretion of antidiuretic hormone is seen most commonly with SCLC but can occur with other malignant or benign lung processes. Hypercalcemia related to ectopic production of parathyroid hormone–related peptide is more common than hypercalcemia related to bony metastases and is most commonly associated with squamous cell carcinomas. Ectopic corticotrophin (Cushing syndrome) is usually associated with SCLC or early stage carcinoid tumors. Hypertrophic pulmonary osteoarthropathy typically manifests as symmetric and painful arthropathy of the limbs associated with characteristic findings of new periosteal bone formation of the long bones. Neurologic syndromes are less common but include the Lambert-Eaton myasthenic syndrome and encephalomyelitis-subacute sensory neuropathy, both of which are typically associated with SCLC.109
Diagnostic Approach
In most patients, an experienced clinician can make a clinical diagnosis of lung cancer with a high degree of reliability (>95%).110 The main factors that contribute to this are the risk factors for development of lung cancer (e.g., age, smoking history, family history, presence of significant COPD), the clinical presentation, and the radiographic appearance of the lesion on CT (e.g., spiculated, upper lobe, node enlargement). If needed, algorithms are available that can predict the likelihood of lung cancer,108,112–115 but the judgment of experienced clinicians is just as good.112
If the probability of lung cancer is high (e.g., >80%), it is generally more efficient to proceed with evaluation of the stage than confirmation of the diagnosis.110,116,117 Frequently, this will identify a necessary procedure that will serve both to confirm the stage as well as the diagnosis. For example, biopsy of a potential solitary metastasis or of a suspicious mediastinal node can confirm both the stage and diagnosis. Those situations that require tissue confirmation of the stage are discussed in the next section. In other situations, the stage is reliably defined by imaging alone; in this case, confirmation of the diagnosis is achieved from whatever site is easiest. Nevertheless, establishing a presumptive clinical stage is an important step that defines how best to proceed to confirm the diagnosis.
There are also situations in which the reliability of the clinical diagnosis is less certain; this occurs most frequently in the case of a localized, solitary pulmonary nodule (SPN). An SPN is defined as a solitary lesion <3 cm in diameter, surrounded by normal lung, and not associated with other abnormalities in the thorax, such as lymphadenopathy or pleural effusion. These nodules are usually found as incidental findings on imaging studies done for other reasons. When the probability of cancer is intermediate (i.e., about 5% to 65%), PET imaging can be helpful in defining a management algorithm.118,119 PET does not definitively establish the diagnosis; therefore, it is only helpful when it alters the probability of lung cancer sufficiently to justify either proceeding with a biopsy or observation. False-positive PET results may occur with any inflammatory or infectious process: tuberculosis, fungal infections, rheumatoid nodules, and sarcoidosis—when such a process is suspected, PET is generally not helpful (it does not differentiate between such a process and cancer). PET also carries a high false-negative rate with ground glass opacities (GGO), carcinoid tumors, or small lesions; in these situations, PET should also generally be avoided. Specifically, the false-negative rate of PET in a GGO is approximately 90%.118 The false-negative rate in solid lesions <1.5 cm is ~15% and in lesions <1 cm ~30% to 50%.118 Although the “resolution” of modern PET scanners is ~6 mm, this is a technical and not a clinical term—a lesion must have a diameter of four times the resolution in order to detect >90% of the 18-fluorodeoxyglucose activity that is present. This fact also explains why the detected standard uptake value of a lesion falls linearly for lesions smaller than ~2 cm, even if the actual amount of 18-fluorodeoxyglucose activity remains the same.118,120
A nonsurgical biopsy can be accomplished by transthoracic needle aspiration (usually guided by CT) or via bronchoscopy. This is most useful when a benign diagnosis is suspected, but this is rather uncommon.121 In patients with a high suspicion of lung cancer, a biopsy can confirm the diagnosis, but in this situation the false-negative rate of a nonspecific diagnosis is ~20%116; therefore, a surgical biopsy should be pursued unless the patient is too high risk.119 When doing a tissue biopsy, it is crucial to obtain enough tissue for histologic and molecular characterization.116
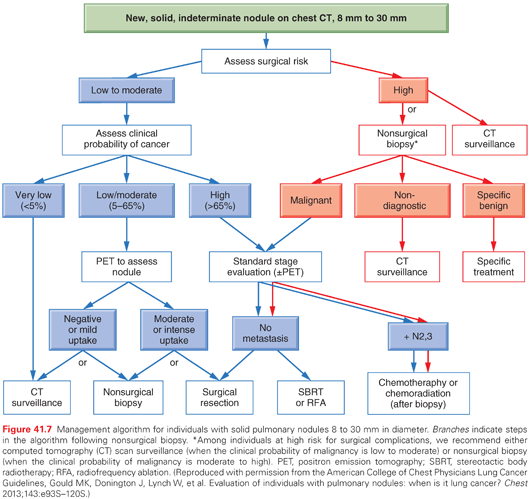
To summarize, PET is most useful when there is an intermediate probability of lung cancer; PET should not be used for lesions that are <1 cm or for a GGO. A biopsy can be important in certain instances when there is doubt about the diagnosis. With a high probability of cancer, it is best to complete the stage evaluation and obtain tissue in a manner that is suited to confirm the stage as well. A detailed management approach for SPN is available for the ACCP lung cancer guidelines, as summarized in Fig. 41.7.119
General Approach
Staging is a critical aspect of the evaluation of all patients known or suspected of having lung cancer. Clinical stage (identified by a “c” prior to the stage group) is determined by all information available before any definitive treatment. This may involve merely a simple history and physical examination, may include imaging studies, or may involve invasive biopsies or surgical procedures with sampling the primary tumor, intrathoracic lymph nodes, pleural fluid, or extrathoracic sites. Pathologic staging (identified by a “p” prior to the stage group) is determined only if surgical resection with intent to cure is performed. All patients undergo clinical staging; the subset of patients who have pathologic staging all first had a clinical stage defined that determined that a surgical resection was indicated. Pathologic staging is inherently more accurate than clinical staging; comparison of survival typically demonstrates better survival for pathologically as compared to clinically staged patients.52 Nonetheless, it is the clinical stage that drives the initial treatment decisions, and thus it is imperative that the process of defining the clinical stage be performed rigorously.
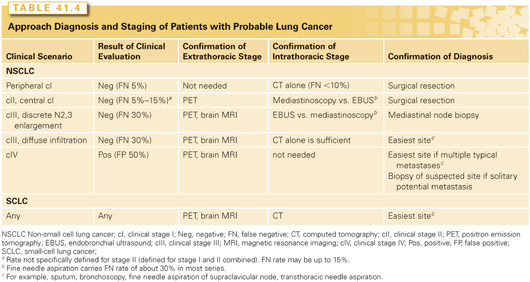
The process of stage evaluation should begin as soon as there is a strong suspicion of lung cancer, and the data and recommendations in the rest of this section apply to such patients. As noted in the previous section, in most patients a review of the CT and the patient’s symptoms and risk factors for lung cancer will establish a clinical diagnosis of lung cancer with a high degree of accuracy.110 A thoughtful approach will often establish stage and diagnosis simultaneously and efficiently (Table 41.4). The approach to confirming the clinical diagnosis and stage is different for patients with a negative clinical evaluation and clinical stage I lesion on CT, a positive clinical evaluation suggestive of distant metastases, or those with a negative clinical evaluation but a CT suggestive of hilar or mediastinal node involvement.110
Extrathoracic Staging
As noted, the initial comprehensive clinical evaluation is an important first step in determining whether a primary tumor has spread. The clinical evaluation consists of a comprehensive history, physical examination, and simple laboratory testing. Constitutional symptoms, focal symptoms, abnormalities on the physical examination, or unexplained liver function abnormalities, anemia, or hypercalcemia may suggest distant metastases. Because the false-positive rate of this evaluation is around 50%, this suspicion must be confirmed. If the clinical evaluation points strongly to a particular site, further imaging or biopsy should be targeted to the observed abnormalities. For example, needle aspiration of enlarged supraclavicular nodes identified on physical examination may efficiently establish both diagnosis and stage. If the clinical evaluation is positive but less focal, imaging for distant metastases is needed (PET and brain magnetic resonance imaging [MRI] or CT).122
If the patient has a typical presentation with multiple sites identified on imaging that are typical of metastases, there is no need to confirm the stage (stage IVb) histologically (although there is still a need to get tissue to document the type of cancer, which should be obtained from whatever site is easiest). However, if there is a solitary site that is typical of metastatic disease, in general this should be confirmed by biopsy (see Table 41.4). Several studies have found in such scenarios that the suspected solitary metastasis is actually benign in 10% to 50%.123,124 Confirmation should also be obtained if the presentation is unusual or the appearance of the possible metastases is unusual.
If the clinical evaluation is negative, the incidence of finding occult distant metastases detectable by imaging varies according to the clinical intrathoracic stage. For cI by CT with a negative clinical evaluation, the incidence of finding true distant metastases is approximately 5%,110,124–132 and the incidence of detecting a false-positive finding of a distant metastasis is actually higher. In patients with cIII (N2) the incidence of finding occult disease is 25% to 30%.110,125,127,128,130,133–136 For stage cII, there is much less data but the incidence appears to be 15% to 20%.110,128 Therefore, there is good reason in cII or cIII patients to pursue imaging for distant metastases: PET and brain MRI with and without contrast are optimal, although an abdominal/pelvic CT, bone scan, and brain CT with contrast is reasonable if PET is not available or brain MRI is not possible.
The value of PET imaging for the detection of distant metastases in larger cI tumors is controversial. There no clear data specifically for this group. RCTs of the value of PET in general have shown varying results depending on the clinical setting and the likelihood of metastases in the patient population included.131,137–140 Those studies showing a benefit have included patients selected by minimal criteria (i.e., a general practitioner’s suspicion of lung cancer based on a chest X-ray alone) or patients with clinical findings suggestive of metastases and little conventional imaging,138,139 whereas those that have found no difference involved patients that had a low suspicion of metastases based on the clinical evaluation by a lung cancer specialist and who had already undergone conventional imaging.131,137 The ACCP and NCCN guidelines are relatively generic in recommending PET in essentially all patients with a suspected or diagnosed lung cancer, without accounting for details of the clinical stage before PET or the clinical setting (only cIa and GGO lesions are excluded from the PET recommendation by the ACCP).110,141
Mediastinal Staging
If there are no distant metastases, the status of the mediastinal nodes becomes critical in determining the right treatment strategy. Much information is already available from the CT scan. However, while there are situations in which it is reliable, there are also many in which it is notoriously unreliable. One can distinguish four groups of patients based on the CT findings: (A) tumors with mediastinal infiltration, meaning that discrete lymph nodes can no longer be distinguished or measured; (B) tumors with enlargement of discrete mediastinal node(s) (≥1 cm in short axis diameter on an axial image); (C) normal mediastinal nodes but either N1 node enlargement or a central (hilar) tumor; and (D) peripheral tumors with no evidence of N1 or N2,3 node enlargement.110
In group A, clinical experience shows the false-positive rate is essentially 0; thus, invasive biopsy is not needed to confirm mediastinal involvement (Fig. 41.8). The rate of a false positive of discrete node enlargement in category B is ~40%, making invasive staging necessary. For central tumors (category C), the false-negative rate of the lack of N2,3 enlargement is ~25%, again making invasive biopsy important. In category D (peripheral tumor with no N1-3 enlargement), the false-negative rate of CT is ~10% (<10% for T1 and ~12% for T2); thus, many feel that invasive confirmation of a lack of N2,3 involvement is not needed (especially if PET is also negative; see the following), whereas some feel this rate is high enough to justify invasive biopsy.110
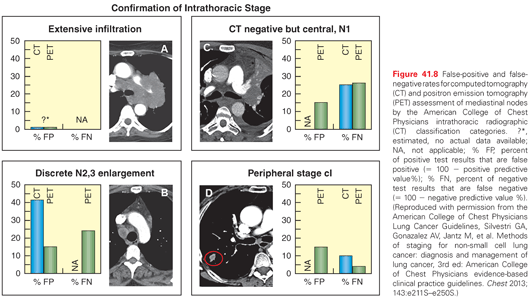
PET scanning adds information, but does not alter the indications for invasive staging significantly.110 A positive PET in an enlarged node (group B) carries a 15% to 20% false-positive rate, whereas a negative PET in an enlarged node carries a 20% false-negative rate, making invasive biopsy necessary either way.110 For central tumors (group C), a negative PET still has a 20% false-negative rate, and a positive PET in the mediastinum has a 20% chance of being a false positive. For a peripheral tumor with no node enlargement, a negative PET has a false-negative rate of ~3% (but a positive PET may be false positive). This data makes it clear that in groups A and D imaging can generally be considered adequate, but in groups B and C invasive biopsy is needed regardless of the PET results (unless compelling data for distant metastases is present).110
There are multiple techniques to invasively confirm the presence or absence of N2,3 involvement. These include traditional mediastinoscopy, video-mediastinoscopy, so-called super mediastinoscopies that involve a complete mediastinal lymphadenectomy performed as an outpatient via a cervical incision, endobronchial ultrasound (EBUS) and needle aspiration, esophageal ultrasound and needle aspiration, simple “blind” transbronchial needle aspiration, video-assisted thoracic surgery (VATS), and rarely CT-guided transthoracic needle aspiration. There are differences in which nodes are accessible, whether these procedures are applicable to multiple node sampling, and how feasible they are for sampling normal sized versus enlarged nodes (Table 41.5). The technique and thoroughness of how the procedure is done probably has a major impact on the reliability of the results.110,142
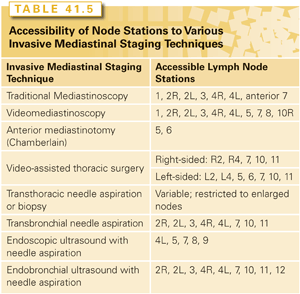
The choice of which invasive staging technique to use is probably best tailored to specifics of the institution and the patient. In trained hands, all can be performed with low morbidity and mortality. There is RCT data to suggest that if good quality EBUS is available, this may be the best first step in most patients.110,143 However, the institutional availability of expertise is an important factor. In many reports, the false-negative rate of EBUS has been ~20%, although in expert hands this appears to be lower (particularly if lymphocytes are obtained). Similarly, a video-assisted lymphadenectomy via mediastinoscopy has a false-negative rate of ~2%, but is not available in most institutions. What is critical is an interest in thorough staging and experience with at least some of the various techniques available.
Overview of Lung Cancer Treatment Modalities
Structural Aspects of Patient Care
The field of lung cancer has grown to encompass a huge body of knowledge, more than what any one person can stay abreast of. Furthermore, it has become complex, with many different specialties that contribute to the evaluation and management of the patient. This makes it important to provide care in a multidisciplinary manner. The key aspect is to have a system of care delivery that is developed together with all relevant disciplines, and to have a forum for discussion so that major patient management decisions can be made with involvement of relevant specialties. It is important to provide an opportunity for different specialties to bring up points that one is unaware of, not merely to involve another specialty when one knows that they have something to offer. Unfortunately, many people think that simply having patients referred to another specialty from time to time constitutes multidisciplinary care; however, it is the system of care developed by the entire team, the ability to introduce salient points that are not being considered, and the collaborative decision making that really defines multidisciplinary care.
It is recommended and even mandated in many countries that patients with lung cancer receive care in a multidisciplinary fashion.109,144 This is recommended by the ACCP lung cancer guidelines, based on a review of literature; specifically, the staging evaluation, the physiologic evaluation of the ability to undergo treatment, and the treatment planning should be done in a multidisciplinary setting.144 Multidisciplinary evaluation is also recommended by the NCCN guidelines.141 It has been difficult, however, to quantitate the impact of multidisciplinary care, because it is difficult to separate this aspect from other structural, thoroughness, and quality of care issues. A growing body of literature is showing benefits in terms of timeliness of care, more frequent use of various treatment modalities, and survival.109,144 In the end, it may not be important to disentangle the impact of multidisciplinary care from other quality of care issues. The key point is to recognize that organization of care, which involves multiple disciplines, has a major impact on outcomes of patients with NSCLC.
There is extensive data that the care for a large proportion of patients with lung cancer is suboptimal. A study of the US National Cancer Database revealed that the proportion of patients with NSCLC and no comorbidities that received suboptimal care (defined as deviating from guidelines that were in place at the time) was approximately 33% for stage I, 40% for stage II, 46% for stage III, and 47% for stage IV.145 Other studies show that there are major regional differences (more than three-fold) in the United States in the frequency of use of different treatment modalities, implying that these are not related to patient factors or what is appropriate, but likely related to regional differences in the quality of care delivery.146,147 Several studies have shown that despite the existence of clinical guidelines for many years recommending careful mediastinal staging with biopsy confirmation, the large majority of patients are staged using CT alone (which is notoriously inaccurate).148–150 Furthermore, such limited clinical staging was associated with dramatically worse outcomes, stage for stage (adjusted hazard ratio [HR] for death of ~1.7 to 2.3 for overall and cancer-specific survival).148
There is extensive evidence that structural aspects of care (e.g., case volume, specialization) have a marked effect on outcomes.151,152 A comprehensive literature review on effect of volume/specialization in quality of cancer care concluded that “An extensive, consistent literature that supports a volume-outcome relationship was found for cancers treated with technologically-complex surgical procedures. . . . Across studies, the benefit from care at high-volume centers exceeds the benefits from break-through treatments.”153 In lung cancer, most of these studies have focused on surgical treatment. With very few exceptions, these studies have found lower perioperative mortality and better long-term survival in high-volume versus low-volume centers, teaching versus nonteaching facilities, and when treatment is delivered by thoracic versus general surgeons (Fig. 41.9).151,152
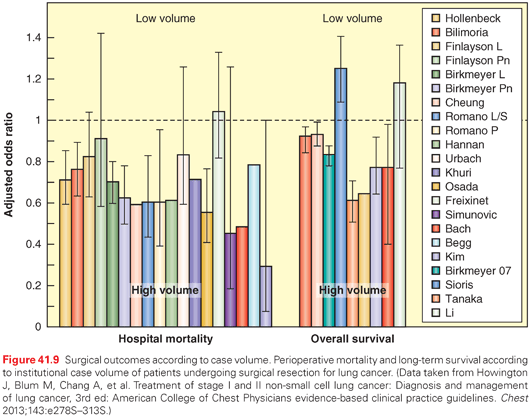
However, the relationship between volume and outcomes is not consistent on an individual institution level; there are low-volume institutions with excellent outcomes and vice versa. This suggests that volume is only a marker for other aspects of care. It is possible that low-volume centers have less availability of expertise or cutting-edge treatment interventions. However, it is much more likely that it is a marker for how organized the care delivery is. In most high-volume institutions, care will become more organized simply out of necessity. Although it has not been directly studied, there is indirect data to support that the organization of care is the critical component.154 At any rate, developing a locally appropriate, organized, multidisciplinary process of care together with tracking of outcomes certainly represents an opportunity for many institutions, and appears likely to have a significant impact on outcomes. The impact of such organized care appears to be much greater than many treatment modality advances that are heralded as major breakthroughs.148,155,156
In constructing a review of lung cancer for international use, one must recognize there are regional differences (e.g., the proportion of specific cell types, use of specific treatment modalities, penetration of screening). Although some of these have been identified, their impact on the management of patients has not received much study. Nevertheless, a recognition that they exist is important. Furthermore, general conclusions and recommendations must always be tailored to the local setting. Policies may sometimes not apply because of differences in the patient population, the availability of particular interventions, and what is easily accepted in the local culture.
There are major regional differences in outcomes, perhaps best highlighted by the IASLC database results. There was a more than two-fold difference in survival rates between regions (e.g., median survival cNO 29 months versus 70 months; cN2 12 months versus 20 months for Asia versus Europe; cT1a 74 months versus 64 months, cT1b 67 months versus 46 months for Asia versus Europe, respectively).157 The results were inconsistent, with different regions being better or worse depending on the TNM subgroup in question, although there was a slight trend toward better outcomes in Asia.158 It is not clear what the reasons for these differences are. It is well known that the incidence of EGFR mutation is higher in the Asian lung cancer population. There are differences in the proportion of subtypes of NSCLC. There are societal differences regarding how aggressive and which treatment modalities are generally accepted. There are differences in the availability of various technologies and treatment methods (e.g., EBUS, PET, VATS, four-dimensional RT, stereotactic body RT [SBRT], genetic testing). The impact of these differences is not clear, but it does emphasize that general recommendations need to be thoughtfully considered in light of differences as compared with the population and setting in which the data was derived.
Surgery
There have been major advances in surgical treatment of lung cancer in the past two decades, just as there have been in RT and chemotherapy. Most prominent is perhaps the use of minimally invasive resection (VATS), which makes a lung cancer resection a very different experience for the patient. The perioperative morbidity is cut in half, the hospital stay reduced to 3 to 4 days in the United States, and the return to normal functioning is dramatically quicker. This is well documented in several meta-analyses,159–165 large-scale outcomes studies,166–170 propensity matched studies,168,171–182 and small randomized studies.183–185
While the data makes VATS the recommended treatment in the ACCP lung cancer guidelines,151 it is used variably in different centers. Those that have embraced it perform about 80% of resections by VATS, but there remain many institutions that are not comfortable and use VATS relatively sparingly. It is likely this will change, given the amount of data supporting the VATS approach. Furthermore, robotic-assisted thoracic surgery is now being practiced at some centers. It is clear this is also a safe and effective approach. Whether there are benefits for the patient with respect to perioperative or long-term outcomes for robotic versus VATS resections is unclear.
Even open thoracotomy has changed. Smaller, muscle-sparing incisions are now commonly used. There are many newer ways of pain management that allow greater patient mobility and avoidance of complications. The management of postoperative air leaks has changed; quantitation using digital devices allows earlier chest tube removal and earlier hospital discharge.186 Such postoperative management approaches have changed the experience for patients undergoing thoracotomy from what it typically was 20 years ago.
The value of care delivered by dedicated thoracic surgeons, who have a focused interest in noncardiac thoracic surgery and lung cancer in particular, is increasingly recognized. The ACCP lung cancer guidelines recommend that surgical care be delivered by such an individual, on the basis of a review of the literature that demonstrates better outcomes.151 Intraoperative lymph node staging was previously often spotty; there is now a much better appreciation for the necessity to do this for both the surgeon and pathologist, and acceptance of substandard intraoperative staging is diminishing.151
There has also been a major advance in the ability to safely carry out extensive resections, for example involving cancers invading the thoracic inlet, vertebral column, and SVC. This makes it feasible to consider surgery as part of the treatment approach for tumors that appear to be only locally invasive. In the past, many of these tumors were simply considered unresectable; however, the modern techniques and ways of reducing morbidity make surgery a reasonable option.
The advances in surgical therapy have changed patient selection for surgery. The improved perioperative outcomes allow older and more frail patients to undergo surgery (e.g., a VATS resection or a segmentectomy).151,166,187 Patient selection is also altered by the recognition that previous criteria had been very conservative. These had primarily defined thresholds above which there was little concern; however, they ignored the fact that risk is a continuous variable and that many patients below previous thresholds could undergo resection with quite acceptable low risk of perioperative mortality. Other previous contraindications for surgery such as a history of myocardial infarction are rendered moot if a successful intervention for the coronary artery disease has been accomplished and overall cardiac function has been preserved. Thus, evaluation of which patients can safely undergo surgery, including VATS and sublobar resection, has become much more nuanced and is best approached in a multidisciplinary fashion, which should include a dedicated thoracic surgeon, a pulmonologist with knowledge about lung cancer treatment options, and a radiation oncologist as well.
Radiation Therapy
Advances in Radiation Technique
Three-Dimensional Conformal Radiation Therapy. Before the widespread use of three-dimensional conformal RT (3DCRT) in the 1990s, the predominant RT technique for treating locally advanced lung cancer was two-dimensional. Radiation beams would be designed using bony anatomic landmarks visible on fluoroscopic imaging. 3DCRT uses CT datasets, using beams from multiple angles to conform to contoured target volumes and similarly avoid contoured normal tissue. Inherent in 3DCRT is the use of dose-volume histograms to compare the normal tissue dose among different beam arrangements. 3DCRT provides a significant advantage over two-dimensional radiation: conformal beam design and the ability to manipulate beam geometry and weighting through the planning process improves coverage of the tumor target, and decreases the dose to normal tissue. The use of CT datasets in RT planning also enables the fusion of complementary imaging modalities, such as PET or MRI.
Intensity-Modulated Radiation Therapy. The use of intensity-modulated RT (IMRT) has been increasing in frequency over the past two decades. In contrast to 3DCRT, IMRT is inverse planned. The radiation oncologist specifies the dose to tumor targets, dose limits to organs at risk, and assigns their relative priority. Beam geometry is selected, and a computer algorithm is used to determine the optimal beam intensity and fluence pattern to meet the planning constraints. While 3DCRT beams are static, in IMRT the beam intensity is not constant—the use of a dynamic multileaf collimator varies the intensity across the beam aperture during treatment. The use of IMRT results in a more conformal plan than 3DCRT, thus reducing the exposure of surrounding normal tissue to high doses of radiation.188,189 In patients with bulky NSCLC, the use of IMRT decreased the volume of lung receiving >20 Gy by 10%, corresponding to a decrease of >10% in the calculated risk of radiation pneumonitis (RP). An example of 3DCRT and IMRT plans for a patient with locally advanced NSCLC is shown in Fig. 41.10.
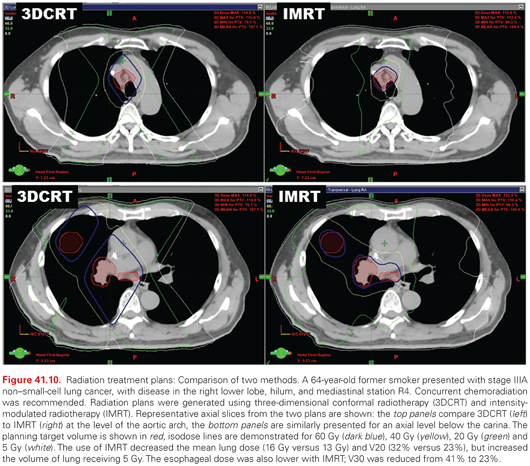
Image-Guided Radiotherapy and Tumor Motion Considerations. Image-guided RT implies the presence of radiographic imaging incorporated into the radiation treatment device. Commonly used imaging devices include kilovoltage orthogonal planar imaging or cone-beam CT scanning. This allows direct visualization of the tumor target and/or critical organs immediately before treatment is delivered, while the patient is immobilized, as frequently as every treatment fraction. The use of image-guided RT allows the reduction of the expansion margin around the clinical target volume, since interfraction variations in patient positioning are reduced.190 This can, in turn, reduce the exposure of the surrounding normal lung tissue.
A large component of the expansion margin for lung cancer is designed to allow for positional uncertainty of the tumor that corresponds with the respiratory excursion of the diaphragm. Allowing for intrafraction respiratory motion is a required part of radiation treatment planning for lung cancer, and can be accomplished in several ways. The use of four-dimensional CT scans in treatment planning allows the accurate measurement of tumor motion for an individual patient,191 and the correlation of tumor position with an external fiducial. After the acquisition of a four-dimensional CT scan, various motion management techniques can be employed: expansion of the intended treatment volume based on the measured motion, gated treatment in which the patient is only treated during a portion of the respiratory cycle, or abdominal compression where the diaphragmatic motion is damped using external compression devices. Tumor tracking, where the radiation aperture follows tumor while it moves, is an area of growing interest.
Proton Therapy. The use of charged particle therapy such as protons is an area of active investigation in lung cancer. Protons have distinct physical characteristics that suggest they can be used to deliver thoracic radiation with a lower risk of side effects, when compared to standard photon therapy. The deposition of proton energy in tissue can be modulated by changing the beam energy, leading to much lower entry doses than photons, and an even more significant in the drop-off of the exit dose. Proton radiation beam arrangements do not need to enter and exit through lung tissue to avoid critical structures such as the spinal cord, which should decrease the risk of RP and radiation fibrosis.192 Phase 2 trials of fractionated proton therapy with concurrent chemotherapy in locally advanced lung cancer have demonstrated excellent median survival, with relatively low toxicity.193,194 A large cooperative group randomized trial is ongoing.
Radiation Toxicity. The risk and severity of radiation toxicity are related to the dose and volume of normal tissue that are exposed, to the presence or absence of underlying comorbidities, as well as the functional organization of the particular organ at risk. Emami et al.195 published a comprehensive review of partial-volume organ tolerances for normal tissue that served for more than a decade as the standard source for radiation dose limits, and risk of toxicity. These parameters were based predominantly on clinical data from older, two-dimensional RT planning. Recently, a large multidisciplinary effort was undertaken, the Quantitative Analysis of Normal Tissue Effects in the Clinic, to summarize the published three-dimensional dose-volume/toxicity data in the literature, review normal tissue complication probability modeling, and provide practical guidance for organ dose limits.196
Radiation Pneumonitis and Pulmonary Fibrosis. Clinically significant pneumonitis occurs in somewhere between 5% and 50% of patients receiving definitive fractionated radiation for locally advanced lung cancer, and is often the dose-limiting factor in radiation planning. RP may occur during fractionated treatment or up to 18 months afterward, with a peak incidence at approximately 2 months. The most common clinical presentation is a persistent, nonproductive cough; dyspnea; low-grade fever; and fatigue. Chest X-ray or CT scan may be normal, or depending on the time course there may be GGO (within 2 to 6 months), patchy consolidation (4 to 12 months), or fibrosis (≥10 months). The earliest radiographic changes occur within the medium- to high-dose radiation volumes, though later changes may extend into unirradiated lung. Pulmonary function testing shows reduced lung volumes, tidal volumes, and diffusion capacity. A variety of dose-volume models have been evaluated as predictive metrics of RP, including threshold volumes (i.e., Vdose), mean lung dose (MLD), and normal tissue complication probability models. Accumulated data from the Quantitative Analysis of Normal Tissue Effects in the Clinic effort suggest that while there is gradually increasing risk with increasing exposure, with no safe threshold dose below which the risk of RP is zero,196 the risk of grade 2 or greater RP was <20% when the MLD was held to <20 Gy during conventional radiation fractionation. Commonly used thresholds include a V20 <30% to 35%, or V5 <60%, corresponding to a risk of RP of <20%.
RP occurs less commonly after SBRT in comparison to conventionally fractionated radiation.197 The risk of symptomatic RP does seem to follow a similar relationship to dose and volume irradiated as seen in conventionally fractionated treatment,198 though specific dose thresholds are evolving as experience with SBRT grows. Takeda et al.199 retrospectively examined 265 patients, and with a median follow-up of 19 months the incidence of grade 2 to 5 pneumonitis was 18.5%, 4.5%, 0%, and 0.4%, respectively. Predictors of grade 2 or higher RP on multivariate analysis included V20. A large series from Indiana University examined dosimetric predictors of pneumonitis200 in a group of 143 patients treated with SBRT. RP (grade 2 to 4) occurred in 9.4% of cases. Pneumonitis was noted in 4.3% of patients with a MLD of ≤4 Gy, compared with 17.6% of patients with MLD of >4 Gy (p = 0.02), and in 4.3% of patients with a V20 ≤4% compared with 16.4% of patients with V20 of ≥4% (p = 0.03).
RT-induced dyspnea may have several contributing causes, including not only RP but also RT to other thoracic organs at risk. Emerging evidence suggests an interaction between cardiac dose and RP,201 and there may be additive dyspnea due to pleural and pericardial effusions, cardiomyopathy, and bronchial stenosis or bronchiectasis. Bronchial fibrosis and stenosis has been reported with radiation dose escalation beyond 70 Gy.202
Several patient- and treatment-related factors also impact the risk of RP, independent of dose and volume. In a large dataset derived from patients treated on Radiation Therapy Oncology Group (RTOG) trials, the risk of RP was significantly higher for tumors in the lower lung fields.203 Older age may increase the risk of RP, and patients who continue to smoke through treatment may be at decreased risk.196 Several chemotherapy agents that are commonly administered to patients with lung cancer concurrently are associated with an increased risk of RP, including docetaxel, gemcitabine, and particularly the commonly used carboplatin and paclitaxel combination.204
Glucocorticoids are commonly used to treat RP in patients who have moderate to severe symptoms, although the efficacy and appropriate starting dose and tapering schedule have not been defined in a prospective fashion. Prophylactic antibiotics or anticoagulants do not appear to effect the development of RP, although they are frequently given. Pulmonary parenchymal fibrosis is the underlying cause of long-term dyspnea after thoracic radiation, likely the result of chronic treatment-induced inflammation. No standard clinical approach has been definitively demonstrated to reverse or even slow the progression of pulmonary fibrosis, though several therapies have shown signs of activity, including pentoxifylline.205 Amifostine is a radioprotector that has been tested in several randomized trials, with mixed results.206,207 Captopril is an angiotensin-converting enzyme inhibitor that has been shown to reduce the development of radiation-induced fibrosis in animal models.208
Esophagitis and Esophageal Stenosis. Acute esophagitis is typically the dose-limiting side effect during fractionated RT for thoracic malignancies. Grade 3 or greater acute symptoms (i.e., severely altered eating/swallowing, tube feeding, parenteral nutrition, or hospitalization indicated) occurred 18% of the time, in a series of >1,000 patients undergoing chemoradiation.204 Acute esophagitis may coexist with, and be exacerbated by, comorbid conditions such as candidiasis or reflux disease. Other factors identified as increasing the risk or severity of acute esophagitis include the use of accelerated fractionation and older patient age.209 The use of concurrent chemotherapy is associated with an increased risk.210 The use of concurrent bevacizumab with RT has led to case reports of fistulae.211,212 Clinically significant late toxicity, such as stenosis or fistula formation, is less common after conventionally fractionated radiation, occurring in <5% of patients.213,214
Treatment of acute esophagitis is primarily supportive, frequently requiring topical agents, dietary changes, and narcotic pain medication. It is often prudent to either evaluate or treat empirically for viral or candida esophagitis. Patients may benefit from proton pump inhibitors for comorbid reflux disease, and topical agents for mucosal irritation such as sucralfate or local anesthetic agents. The ability of the radioprotectant amifostine to reduce the risk and severity of acute esophagitis has been evaluated in several prospective trials, but no benefit was noted in a large randomized, cooperative group study.207
Heart. For thoracic malignancies, the dose and volume of radiation to the heart and great vessels varies considerably, depending upon the anatomic distribution of the target and the treatment technique. The overall excess risk of cardiac mortality after thoracic RT is low,215 but moderate toxicities have been reported more commonly. Acutely, this can include pericarditis, which can develop during treatment or within several months afterward. Months after radiation, there can be pericardial effusion, and over years progressive fibrosis may rarely lead to constrictive pericarditis. Ischemic changes in the cardiac muscle can manifest with a long latency and may lead clinically to congestive heart failure or ultimately a higher cardiac mortality.216 Valvular abnormalities have been reported, presumably due to late fibrotic changes. In the great vessels, there can accelerated atherosclerosis that worsens over years to decades, leading to an increased risk of structural damage such as aneurysm. Comorbid clinical conditions may increase the risk of RT-induced cardiac toxicity,217 including hypertension, diabetes, obesity, and genetic predisposition. The risk of cardiac mortality from RT has been specifically demonstrated to be increased in patients over 60 years old, and by tobacco use.218,219 There are reports that concurrent paclitaxel may also increase this risk.220,221
Brachial Plexus. Radiation brachial plexopathy is a rare but serious complication of fractionated radiation to conventional doses. There are case reports of early, transient neuropathy that may occur during or shortly after radiation, and may resolve spontaneously.222 Late radiation plexopathy is more clinically significant; it manifests years after radiation to the supraclavicular area and may manifest as hypesthesia, paresthesia, and weakness of the affected arm and shoulder. It may progress to total paralysis of the affected arm, and severe pain. The dose tolerance of the brachial plexus is less defined than other thoracic organs, partly due to the difficulty in defining the plexus radiographically during radiation treatment planning. A contouring atlas has been adopted, so that more robust clinical data can be collected.223 Late plexopathy is rare in patients who receive conventional doses of fractionated radiation.224 For SBRT, brachial plexopathy is a more significant concern, because the biologically effective dose prescribed to the target exceeds the tolerance of the plexus.
Chemotherapy
There has been a long evolution in the treatment of NSCLC since the early editions of this textbook. It was not until the late 1980s that the benefits of chemotherapy with respect of overall survival (OS) and QOL versus supportive care were appreciated in patients with advanced disease.225–227 In the early 1990s, a new generation of chemotherapeutic agents, used in combination with platinum analogs, consolidated the benefits of treatment and established chemotherapy.
In the mid to late 1990s, the combination of chemotherapy and RT in patients with stage III disease showed superior outcomes compared to single modality and created a new paradigm of combined modality therapy, still considered the standard to this date.225 During the 2000s, chemotherapy was tested in the adjuvant setting and became a new standard of care by improving the cure rates in selected patients with stage IB through IIIA disease.228 Remarkably, over the course of two decades, or five editions of this textbook, chemotherapy has become an established treatment modality for the majority of patients with NSCLC.
The discovery of somatic mutations in a subset of lung tumors is the most significant paradigm shift in the treatment of lung cancer in the last decade. Since the initial report on EGFR,229 the understanding that specific molecular alterations serve as oncogenic drivers, and that blockade of these pathways by specific agents can lead to robust and prolonged responses, has revolutionized the approach to patients with lung cancer. This discovery has launched the era of personalized medicine, in which patients are managed based on specific characteristics of their tumors.
The evolution of chemotherapeutic management of NSCLC has ushered in many more fundamental changes in how we think about stage IV NSCLC. At one time, it was questionable whether the treatment was worse than the disease, now this is a vibrant, successful, fast-paced field with new discoveries that challenge the ability to keep pace. There are many effective cytotoxic agents, targeted therapy, and immunotherapy, and which are used in first-, second-, and third-line settings. The treatments can be dramatically effective, and it is no longer surprising when a patient with incurable NSCLC can be successfully managed over many years. We are clearly moving toward management of stage IV NSCLC as a chronic disease.
The management of stage IV NSCLC is complex. It is not merely a question of which drug to use, but which sequence, how to plan the first course of therapy taking into account later options, and when to initiate a new treatment. Treatment is viewed less as a defined course of therapy and more as a continuum over time. The lines between treatment courses are blurred: when does a course of therapy become maintenance, when is maintenance therapy early institution of second-line treatment? More aggressive palliation of specific symptoms or conditions (brain, spine, bone metastases, airway obstruction, etc.) has far-reaching effects, including a better QOL and a more positive attitude, but also a better ability to tolerate active treatment.
The evolution of chemotherapy treatment of NSCLC has led to different end points in clinical trials. OS, once considered a hard end point, has become problematic. Because OS reflects the entire continuum of management and a combination of interventions, it is often difficult to discern what effect a particular portion of the treatment strategy has on OS. Progression-free survival (PFS) has become more prominent as it can assess a specific portion of the continuum of treatment. However, it is controversial whether and under what circumstances it is an appropriate measure.230 More subtle measures such as toxicity and QOL are also used as primary end points. Thus, end points of clinical research have become more difficult to measure and attribute to a particular intervention. At the same time, the standards of what is considered clinically relevant and sufficient to call a trial positive has decreased.231 The combination of these trends raises issues for clinical research moving forward.
The treatment has become much more specific; having evolved from a one-size-fits-all NSCLC approach to being histology based, and even more specifically directed to small cohorts identified by particular genetic mutations. These markers have an impact not only on response but also on susceptibility to toxicity in some cases. This radically changes the economics of treatment, research, and drug development. The specific cohorts are smaller and more difficult to identify. The need to screen a larger population to identify a few individuals can be costly and inefficient. Cost is determined not just by the cost of therapy but also cost of and ability to find the right cohort.232–234 The dramatic response that can be seen with such targeted therapy can lead to approval of a therapy based on nonrandomized data alone, reducing the costs of drug development. However, the more limited cohort for whom it is applicable also limits the potential return. The pace of new discoveries in specific tumor subgroups creates challenges in how to conduct appropriate clinical research in a timely enough manner. New approaches to trial design, such as an adaptive Bayesian structure, may offer innovative, more flexible options than the traditional phase 1, 2, and 3 research schema.235 There are many forces in play in the dynamic, rapidly changing field.
Preinvasive and Minimally Invasive Disease (Bronchial Intraepithelial Neoplasia, Adenocarcinoma in Situ, Ground Glass Nodules)
Bronchial intraepithelial neoplasia appears to be a precursor to central airway squamous cell carcinomas. These lesions are infrequently encountered, usually in patients with abnormal sputum cytology or during surveillance of the central airways. Although techniques have been developed (autofluorescence bronchoscopy, narrow-band imaging) that are more sensitive in detecting such lesions, it is unclear how these should be implemented.236 Among high-risk patients who participated in a screening program, about 30% of high-grade dysplasia and 50% of carcinoma in situ lesions were observed to progress; however, the rates were highly variable among studies (with follow-up periods of ~1 to 10 years).236 A substantial proportion of lesions regresses during follow-up. One of the problems is that there is moderate interobserver variability in the classification of such biopsies. Photodynamic therapy seems to be fairly effective at treating lesions deemed worthy of intervention, but it is not well established which lesions need intervention, as many do not progress.236
In 2011, a new subclassification of adenocarcinoma was introduced, covering a spectrum from atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), lepidic predominant adenocarcinoma, and various types of invasive adenocarcinoma (acinar, papillary, micropapillary, etc.).63 Although not conclusively established, this carries the implication that the earlier lesions (e.g., AAH, AIS) may be precursors to invasive adenocarcinoma. It must be emphasized that these entities can only be classified after resection (not on the basis of a limited biopsy).63 However, there is a strong correlation between the histologic classification and the radiographic appearance, although this has not been quantitatively defined. AAH and AIS lesions generally appear as pure ground glass nodules (GGN) on CT, and MIA and lepidic predominant adenocarcinoma are often part-solid GGNs, while other types of adenocarcinoma are generally mostly solid-appearing lesions on CT. The increased use of CT has led to more frequent recognition of these lesions and creates a need to define how to manage them.
Pure GGNs are focal lesions seen incidentally on a chest CT; this is distinct from more diffuse or patchy ground glass appearance of the lung, which generally signifies benign interstitial or inflammatory lung disease. A number of studies have reported outcomes of such lesions during a period of observation of 1 to 3 years.237 It is difficult to be precise because of slight differences in definitions and inclusion criteria and duration of follow-up. Approximately 10% to 20% of pure GGNs disappear, usually within 3 months. About 10% decrease in size, but it should be noted that approximately 20% of adenocarcinomas that were eventually resected after a period of observation were observed to have decreased in size.237 Most remain stable, around 20% increase in size, and ~10% (range, 0% to 30%) develop a solid component.237
Other papers have focused on diagnoses made during observation of a GGN, which may skew the data toward the more suspicious lesions that motivated intervention. These studies have found that approximately 75% of pure GGNs are benign (and still followed), ~10% are AAH, 10% to 20% are BAC, and ~5% are adenocarcinoma.237 It is hard to translate the older terms BAC and adenocarcinoma used in these studies into the new subclassification of adenocarcinoma. Among studies that focused on lesions that were actually resected, ~30% of pure GGNs were benign (including AAH), the majority was BAC, and ~10% were diagnosed as adenocarcinoma. The incidence of adenocarcinoma was <5% in pure GGNs <10 mm in size, and ~25% if >10 mm.237
The rate of invasive cancer is substantially higher in part-solid GGNs (although these are by definition still >50% ground glass). A diagnosis of adenocarcinoma is made in ~10% during a period of observation. Of those coming to actual resection, ~30% are adenocarcinoma and most of the rest were classified as BAC; ~10% were adenocarcinoma among part-solid GGN <10 mm versus ~40% if >10 mm.237
Triggers for intervention are still evolving. There is a growing impression that pure GGNs may be simply observed, while a part-solid GGN usually warrants intervention, especially if the solid component has recently developed. The rate of growth should also be factored in—a lesion that has grown 2 mm in 5 years may not warrant intervention. Significant growth over the course of a year or development of a solid component should generally trigger resection.119,237,238 Exact definition of recommendations and specific documentation of results is hampered by the short periods of follow-up in studies so far, the loose correlation between radiographic appearance and histologic results, the inability to establish a diagnosis of a preinvasive lesion before resection, and the uncertainty about the malignant potential of AAH, AIS, MIA, and even lepidic predominant adenocarcinoma.
Multiple studies suggest that a limited resection of a pure or >50% GGN results in excellent outcomes (5-year survival >95% with no recurrences).237 One can question whether all of these lesions actually need to be resected in the first place. Nevertheless, one prospective carefully done study of limited resection found no recurrence at 5 years, but by 10 years 13% developed a staple line recurrence of a genetically apparently identical tumor, despite a reasonably wide negative margin originally.239 The ACCP lung cancer guidelines suggest a sublobar resection for a pure GGN <2 cm with careful attention to achieving an adequate margin.151
Patients with GGNs often have several lesions. These lesions should be approached conceptually as independent lesions. Each lesion should be managed individually, intervening when necessary and observing other when there is no change and a low suspicion of invasive cancer.119,237,238 There is no reason to give chemotherapy to these patients if these are independent early cancers or preinvasive lesions; chemotherapy has no role as a chemopreventative agent.
Stage I (T1,2 N0)
The standard of care for healthy patients with a clinical stage I NSCLC is surgical resection.141,151 This is based on decades of experience showing good long-term survival rates with few recurrences after resection. Examination of the 2010-11 US National Cancer Database shows that surgery was the dominant treatment strategy (surgery in 66%, RT in 23%, and no treatment in 9%). In the US Surveillance Epidemiology and End Results (SEER)-Medicare database (i.e., patients age >65 years), the treatment given to stage cI patients in 2007 was surgery in 67%, RT in 16%, and no treatment in 18%.240 The ACCP Lung Cancer Guideline recommends that patients with stage I NSCLC being considered for curative therapy be evaluated by a thoracic surgeon or a multidisciplinary team (even if nonsurgical therapy is being considered). Those undergoing surgery should be treated by a thoracic surgeon with a demonstrated focus on lung cancer (grade 1B).151 There is a growing body of literature that demonstrates that outcomes are demonstrably better with specialty training and at higher volume centers (HR = 0.7 for perioperative mortality and 0.9 for long-term outcome in high-volume centers in a recent meta-analysis).152
Across the world, for NSCLC cases diagnosed between 1990 and 2000, staged and treated according to the local routines at the time the survival as reported by the huge IASLC database is 50% for cIa and 43% for cIb, and 73% for pIa and 58% for pIb.52 More recent Japanese national cancer statistics demonstrate 5-year survival rates of 77% and 60% for cIa and cIb, and 84% and 66% for pIa and pIb.241 It is important to note that only about half of the deaths within 5 years in stage I NSCLC are due to recurrences; the 5-year recurrence rate is only about 20%.242–244 The majority (60%) of recurrences are identified within the first 2 years.244,245 Late recurrences beyond 5 years are seen in 10% to 20% of patients, but it is unclear how many of these “recurrences” are actually new primary cancers.246,247
The ACCP Lung Cancer Guideline suggests that a minimally invasive (e.g., thoracoscopic [VATS] or robotically assisted) resection is preferred over an open thoracotomy for stage I NSCLC (grade 2C).151 This is based on consistent data from small RCTs, matched case-control studies, propensity matched cohort studies, meta-analyses, and large outcomes studies, which demonstrate lower perioperative mortality, fewer complications, and shorter hospitalization as well as at least equivalent long-term stage-matched survival.151,159,160,168,171,176,248–250 The penetration of VATS for major lung resection has been growing annually at US academic medical centers, but is performed almost exclusively by thoracic surgeons (not cardiac, general, or oncology surgeons).251 RCTs and large database outcome studies show no difference in the ability to biopsy or resect mediastinal nodes by VATS,159,176,183,184,252,253 although there may be a less thorough assessment of N1 nodes.252 The ability of patients to receive adjuvant chemotherapy is improved following VATS lobectomy.254,255
A lobectomy remains the treatment of choice for good-risk patients with stage I NSCLC in general (ACCP Lung Cancer Guideline grade 1B).151 This is a general conclusion, which is a different issue than asking whether an alternative resection is acceptable in patients that cannot tolerate a lobectomy, or whether there are particular cohorts for which the general statement does not apply. The recommendation for lobectomy over a more limited resection is based on better survival and lower local recurrence rates after lobectomy. Unfortunately, the data is not of high quality. This result is demonstrated by a RCT with only minor flaws,256,257 but because it stems from the late 1980s, the relevance to the current era can be questioned. Other uncontrolled comparative studies corroborate these results, but the patients undergoing sublobar resection were clearly not the same (often not able to tolerate lobectomy or with smaller tumors). Furthermore, the sublobar resections often involved a mixture of segmentectomy and wedge resection, although segmentectomy generally results in better survival and less local recurrence than a wedge.
Specific groups that have been considered to be potentially suitable for a sublobar resection include patients with tumors <2 cm, <1 cm, pure GGO lesions, and elderly patients. For solid smaller lesions and older patients, the data is unclear; comparisons have yielded conflicting results, probably because of the inability to account for selection and confounding factors. Comparisons involving primarily wedge resection tend to show worse results, whereas those involving segmentectomy are more similar to lobectomy.151 Two RCTs are under way to address the value of segmentectomy for solid tumors <2 cm. Increased perioperative mortality or competing causes of death such as might be present in older patients are reasonable to consider; however, clear guidance on when this tips the balance away from lobectomy is not possible from the currently available data.151 On the other hand, there is fairly extensive data that patients with predominantly GGO lesions <2 cm consistently experience excellent 5-year outcomes after sublobar resection (predominantly segmentectomy), with the implication that it would be difficult for lobectomy to yield even better results. This led the ACCP guideline committee to recommend sublobar resection for predominantly GGO lesions <2 cm (grade 2C).151 However, it is important to have an adequate margin, and there is data from a carefully done prospective study that segmentectomy may be associated with late recurrences (between 5 and 10 years).239
At the time of surgical resection, at least a systematic lymph node sampling should be performed. This involves systematically looking for and sampling a representative node from N1 and ipsilateral mediastinal node stations. Data supports that this results in more accurate staging than selective sampling.258–261 However, there appears to be no survival benefit to a complete lymph node dissection in RCTs, at least not in clinical stage I patients.151,260–262
Adjuvant chemotherapy or RT is not indicated after complete resection of stage I NSCLC. Although the majority of recurrences after resection of stage I NSCLC are distant, multiple RCTs that have included stage I patients have suggested that there is no benefit to adjuvant chemotherapy.263–265 A meta-analysis that addressed stage-specific subgroups did not find a benefit for either stage Ia or Ib.155 An unplanned subgroup analysis in one study suggested that there may be a benefit for tumors ≥4 cm,266 but this has not been borne out by further follow-up or other analyses. Other negative prognostic factors have been suggested (i.e., tumor differentiation, vascular invasion, histologic features), but the argument that chemotherapy will have an impact is purely speculative. The ACCP NSCLC guideline, which is more evidence based, takes the position that adjuvant chemotherapy should not be given for stage Ia or Ib with the stance that speculative arguments should not overpower RCT data.151 The NCCN guideline, however, which is primarily consensus based, suggests considering adjuvant chemotherapy for high-risk stage Ib patients, by taking into account potential negative prognostic factors.141 On the other hand, tegafur (an oral 5-fluorouracil agent available in Japan) has been found to improve survival in two RCTs when given orally for 2 years.267,268
Adjuvant RT is also not indicated after complete resection of a stage I NSCLC; in fact, the RCT data suggest it is likely to be harmful.151,269 A more recent RCT and an outcomes analysis of the SEER database confirm this result.270,271
While lobectomy remains the standard of care for stage I NSCLC, there are patients who are considered poor candidates for this approach. However, poor risk for lobectomy is difficult to define. VATS resection has decreased the perioperative mortality,272,273 data shows that lung function is reduced much less or even improved in patients with severe COPD,272,274–276 and the majority of data mixes lobectomy and various forms of sublobar resection together (often including good-risk patients with low-risk tumors together with poor-risk patients with high-risk tumors). Furthermore, data suggest that limited pulmonary reserve correlates primarily with perioperative mortality, whereas long-term functional limitations are often due to random unpredictable events. The ACCP guideline recommends caution about undertaking lobectomy if the predicted postoperative FEV1 or predicted postoperative DLCO is <30% or <22 m on a stairclimbing test.272 Such patients should undergo a formal exercise test and a careful multidisciplinary evaluation. While lobectomy may still be found to be appropriate, other options such as a segmentectomy, wedge, SBRT, or radiofrequency ablation (RFA) should also be considered.272 The multidisciplinary decision making is crucial, because there are many aspects that must be taken into account (e.g., how functional the portion of lung is that contains the cancer, technical issues with doing either surgery, SBRT or RFA, and issues related to confirming the stage of the patient).
Sublobar resection undertaken as a compromise due to poor pulmonary reserve cannot be compared to sublobar resection undertaken electively because of favorable tumor characteristics. Furthermore, the outcomes for a segmentectomy cannot be compared to a wedge resection, although frequently these are combined. The available studies show that the perioperative mortality for a sublobar resection in compromised patients is around 5%, even when the resection involves a thoracotomy (not VATS).151 The issue is one of long-term results. In the ACCP guideline review, 5-year survival after segmentectomy/wedge for stage I NSCLC in compromised patients ranged from 40% to 70%; there was a trend to worse outcomes than for lobectomy in case-matched comparisons, although the differences were not statistically significant in most studies. The recurrence rate after sublobar resection was 0% to 18%.151 In one of the few series to specifically compare (retrospectively) compromised patients undergoing segmentectomy versus wedge, the 5-year survival was better for segmentectomy (80% versus 48%; p = 0.005) and the recurrence rate was lower (16% versus 55%; p = 0.001).277 In conclusion, when surgery (usually open thoracotomy) was judged to be reasonable in compromised patients, a sublobar resection could be accomplished quite safely (mortality ~5%) and the long-term outcomes were encouraging, especially for segmentectomy, which appears to be better than a wedge resection.
Nonsurgical treatment options have also emerged for compromised patients. SBRT has received the most attention. In contrast to conventionally fractionated radiation therapy, which uses small daily fractions over the course of 6 or more weeks, an SBRT treatment plan typically delivers a much higher biologically effective dose over three to five treatment visits. This requires a high degree of precision to avoid delivering the increased dose to the surrounding tissue. For lung cancer, this means highly reproducible immobilization, management of respiratory motion, and accurate localization during treatment delivery. The result is markedly higher rate of local control and survival and markedly lower toxicity when compared to conventional RT.
Because SBRT is well tolerated, does not require general anesthesia, and results in minimal damage to surrounding lung, it has been used primarily in the management of early stage lung cancers in patients who could not tolerate surgical resection of any type. In general, these appear to have been rather severely compromised patients in reported studies. The diagnosis of lung cancer was confirmed in about 75% of patients (range, 30% to 100%). In many of the studies, the cases not confirmed by biopsy were clinically diagnosed as lung cancer by a multidisciplinary tumor board on the basis of growth and/or PET activity. The lesions treated appear to have been primarily solid typical lung cancers, not indolent, ground glass tumors or screening detected cancers. The median tumor size has generally been 2 to 3 cm.
Many of the studies involving SBRT for NSCLC have reported 2-year outcomes. Survival is approximately 60% at 2 years (45% to 79%) and 40% at 5 years (24% to 50%).151 Cancer-specific survival is approximately 75% at 2 years (50% to 90%) and 60% at 5 years (34% to 73%).151 Disease control at the primary tumor site is approximately 90% at 2 years (83% to 100%).
Early prospective trials suggested that SBRT is associated with a higher risk of moderate to severe adverse events in a central or perihilar tumors. However, there are several published examples of safe SBRT schedules for such patients, all delivering a somewhat lower biologically effective radiation dose than that described in the RTOG 0236.278–281 Taken together, the cumulative experience in SBRT for central lung tumors suggests that the use of more protracted fractionation, lower dose, and prioritizing normal tissue dose constraints over target volume coverage appear to reduce the risk, but there is a suggestion that this comes at the cost of somewhat lower local control. The RTOG 0813 was a phase 1/2 dose escalation study of SBRT in central tumors that has completed accrual, and the results will inform treatment of these tumors.
There is much less data for RFA, but reported OS is approximately 80% at 2 years and 30% at 5 years (20% to 61%), with a control at the primary tumor site being achieved in approximately 60% (58% to 92%). It is very difficult to compare outcomes of patients treated surgically to those treated with SBRT or RFA—the degree of comorbidity is different, the confirmation of stage and diagnosis is different, and the definition of local control outcomes are different. Randomized studies have not been able to be conducted.
In summary, it can be said that surgical resection remains the standard for stage cI NSCLC. This should involve a lobectomy with at least a systematic mediastinal node sampling and should be performed by VATS in an experienced center. Tumors that are amenable to a sublobar resection (with attention to adequate margins!) are primarily GGO lesions <2 cm; the data for small solid tumors or in elderly patients is not clear. In patients with limited pulmonary reserve who are considered poor risk for lobectomy, segmentectomy can be accomplished safely in selected patients with good long-term outcomes. A wedge resection is probably oncologically similar to nonsurgical treatment. Although survival results appear slightly better for wedge resection than SBRT, any comparison is confounded because the patients, tumors, and workup are quite different. However, the results of such interventions appear to be good enough to justify their use and appear to be better than that of completely untreated, conventionally detected stage I NSCLC.
Stage II (T1,2 N1)
The recommended treatment for clinical stage II NSCLC without major comorbidities is surgical resection followed by adjuvant chemotherapy.141,151 Surgery has been the mainstay of treatment for many decades; an additional benefit to adjuvant chemotherapy has been demonstrated more recently (discussed subsequently). The ACCP recommends that patients be evaluated by a thoracic surgeon with a focus on lung cancer and be discussed in a multidisciplinary forum (grade Ib).151 This is the standard in many European countries as well.
In the IASLC database, the 5-year survival was 36% for cIIa and 25% for cIIb (representing frequent stage misclassification). Survival was 46% for pIIa and 36% for pIIb.52
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree