INTRODUCTION
Lung cancer is the third most commonly diagnosed cancer, after breast and prostate cancers, but it is the most common cause of cancer-related death (1). Every year, 1.5 million patients die of lung cancer worldwide (1). About 70% of patients will be diagnosed with advanced stages that are not amenable to curative therapies. Only 15% of all patients diagnosed with lung cancer are alive 5 years after diagnosis.
Lung cancer is broadly divided into small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC). Approximately 85% of lung cancer is NSCLC. This chapter briefly describes the epidemiology, etiology, histology, prevention, and molecular biology of NSCLC. The major focus will be clinical presentation, diagnosis, staging, and treatment based on current clinical knowledge, with an emphasis on our approach at the University of Texas MD Anderson Cancer Center (MDACC).
EPIDEMIOLOGY
Lung cancer is rarely diagnosed in people younger than 35 years old. Incidence and death rates rise exponentially until age 75, when a plateau is reached. Non–small cell lung cancer accounts for the greatest number of deaths from cancer in both men and women over age 60.
The geographic, social, and temporal trends of the incidence of NSCLC are closely related to tobacco consumption. In developed Western countries, the incidence of NSCLC has been declining; however, it has been increasing in Asia, Eastern Europe, and developing countries (1).
Worldwide, NSCLC is more common in men, and this difference has been attributed to higher tobacco consumption. In some regions, like Eastern Europe and South America, there was an uptake of smoking by women in the 1980s, and these areas are currently experiencing a rise in NSCLC cases in women. In the United States, the incidence has been declining for both men and women as tobacco use declines; the male-to-female ratio from 2007 to 2011 was 1.4:1 (2).
There is some evidence that African Americans might be more susceptible to the carcinogenic effects of tobacco smoke (3); however, smoking behaviors might also account for socioeconomic and racial differences in lung cancer incidence.
ETIOLOGY
The causal relationship between tobacco smoke and lung cancer was established in the 1950s in case-control and cohort studies. This led to the 1964 report of the US Surgeon General, concluding that smoking can cause lung cancer. Currently, it is estimated that 85% to 90% of lung cancers are due to smoking. Nonsmokers who are exposed to secondhand smoke are also at an increased risk. There is a dose-response relationship between smoking and lung cancer risk, and smoking cessation leads to a significant risk reduction (Table 18-1) (4).
| Age (years) | Duration of Smoking | |||||
|---|---|---|---|---|---|---|
| 25 Years | 40 Years | 50 Years | ||||
| Quit (%) | Still Smoking (%) | Quit (%) | Still Smoking (%) | Quit (%) | Still Smoking (%) | |
| One-pack-per-day smokers | ||||||
| 55 | <1 | 1 | 3 | 5 | NA | NA |
| 65 | <1 | 2 | 4 | 7 | 7 | 10 |
| 75 | 1 | 2 | 5 | 8 | 8 | 11 |
| Two-packs-per-day smokers | ||||||
| 55 | <1 | 2 | 4 | 7 | NA | NA |
| 65 | 1 | 3 | 6 | 9 | 10 | 14 |
| 75 | 2 | 3 | 7 | 10 | 11 | 15 |
Tobacco smoke is a complex mixture of chemicals that includes multiple carcinogens, most importantly the N-nitrosamines (nicotine-derived nitrosamino ketone [NNK] and N’-nitrosonornicotine [NNN]) and polycyclic aromatic hydrocarbons [benzo(a)pyrene and dimethylbenz(a)anthracene]. They are activated through hydroxylation by the P450 enzyme system and exert their action through the formation of DNA adducts.
Smokeless tobacco is frequently advocated as a safer alternative. There has not been a clear association between smokeless tobacco and lung cancer; however, it increases the risk of head and neck, pancreatic, and gastric cancer. E-cigarettes deliver water vapor with scents and different amounts of nicotine containing lower amounts of nitrosamines. However, they still contain high levels of propylene glycol and glycerin, and their long-term effects on health are unknown. Their use should not be recommended to nonsmokers, and use as part of a smoking cessation approach needs further study.
Approximately 10% to 15% of cases of NSCLC occur in never smokers, corresponding to approximately 20,000 deaths annually. In addition to secondhand smoke exposure, several other agents have also been linked to the development of lung cancer (Table 18-2).
Asbestos exists in many natural forms. The silicate fiber has been implicated in carcinogenesis, is chemically inert, and can remain in a person’s lungs for a lifetime. Epidemiologic studies have confirmed the association between asbestos exposure and certain lung diseases, such as pulmonary fibrosis, mesothelioma, and lung cancer (5). Most exposure occurs in the workplace. When smoking is combined with asbestos exposure, the relative risk of lung cancer is strikingly increased (5).
Radon is a naturally occurring decay product of uranium. It is a colorless, odorless, chemically inert gas that can penetrate the earth’s crust and accumulates in buildings. It emits heavy ionizing alpha particles, which may damage DNA. It was shown that many households in Europe, Canada, and the United States have some degree of radon radiation that may increase NSCLC risk among smokers and nonsmokers (6).
The majority of studies that have examined vegetable consumption in relation to lung cancer have shown a protective effect (reviewed in Alberg and Samet [7]), but there are inherent biases in these population studies. There is also epidemiologic evidence that dietary intake of certain vitamins decreases lung cancer risk; however, trials of vitamin supplementation for cancer prevention have ultimately been unsuccessful (see later “Chemoprevention” section).
Environmental or industrial exposure to arsenic, chromium, chloromethyl ether, vinyl chloride, and polycyclic aromatic hydrocarbons increases lung cancer risk (reviewed by Field and Withers [8]). Preexisting lung disease such as tuberculosis, silicosis, pulmonary fibrosis, and chronic obstructive pulmonary disease are also associated with an increased lung cancer incidence even when correcting for the degree of cigarette consumption. This suggests that common pathways to these conditions, such as chronic inflammation, may drive the tumorigenic process.
Family history of lung cancer is associated with a two- to three-fold increase in lung cancer risk, even after correction for smoking, and this risk seems to be inherited in a Mendelian co-dominant fashion (9).
Many studies describe weak but consistent associations between some polymorphisms and lung cancer risk. The cytochrome P450 (CYP) family is responsible for the metabolism of tobacco smoke, and the polymorphism CYP1A1 Ile462Val is associated with a higher risk in Asians (odds ratio [OR], 1.61) (10). The glutathione S-transferase (GST) enzyme prevents oxidative damage, and the polymorphism GSTM1 increases risk in Asians (OR, 1.17) (11). Finally, individuals with impaired DNA repair capacity are at higher risk for developing lung cancer, even if they are nonsmokers, and the polymorphism Lys751Gln in the DNA-repairing enzyme ERCC2 was associated with lung cancer (OR, 1.15) (12).
Large genome-wide association studies (GWAS) (13) have identified three loci strongly associated with lung cancer risk in different populations: 15q25, 5p15, and 6p21. The 15q25 susceptibility region encodes three cholinergic nicotine receptor genes (CHRNA3, CHRNA5, CHRNB4), and alterations in those regions have been associated with a higher risk for nicotine dependence and higher smoking burden. In nonsmokers, they have also been associated with impaired healing of the respiratory mucosa, suggesting a higher sensitivity to toxin-induced airway damage. The 5p15 region encodes the TERT gene, responsible for telomerase function, which is frequently altered in many cancers.
PREVENTION OF LUNG CANCER
The most effective method of preventing lung cancer is reducing tobacco exposure, either through encouraging smoking cessation or preventing young people from starting to smoke. In the United States, campaigns to reduce smoking rates have been successful. The estimated percentage of Americans who actively smoke decreased from 42.4% in 1965 to 25% in 1990 and to 18.1% in 2012. However, former smokers retain an increased risk of lung cancer, and there are still a considerable number of smokers, highlighting the need for better education and prevention strategies, especially those targeted to youths.
Previous studies examined the role of chest x-ray, with or without cytologic analysis of sputum, to screen for lung cancer, and none showed a clear benefit. Most of them were confounded by lead time, length time, and overdiagnosis biases.
Spiral computed tomography (CT)—also called helical CT or low-dose CT—is much more sensitive to detect early NSCLC than chest x-ray. In 2011, the results of the National Lung Screening Trial (NLST) were published (14). In this trial, 53,454 current or former smokers age 55 to 74 years old with at least a 30-pack-year smoking history and no symptoms that could be related to lung cancer were randomized to undergo yearly chest x-rays or low-dose CT for 3 years. After a median follow-up of 6.5 years, the low-dose CT group had a 20% reduction in the rate of lung cancer–specific mortality, from 309 to 247 per 100,000 person-years, as well as a 6.7% reduction in overall mortality. In the CT group, 39% of patients had suspicious lung nodules (vs 16% in the control arm), and the majority of these (96%) were considered false-positive results. Sensitivity and specificity of low-dose CT were 93.8% and 73.4%, respectively, compared to 73.5% and 91.3% for chest radiography. Cost-effectiveness analysis showed a median cost of $43,000 per quality-adjusted life-year gained for current smokers assigned to the low-dose CT group. Based on these results, the US Preventive Services Task Force currently recommends lung cancer screening with yearly low-dose CT for current or past smokers, age 55 to 80, with a smoking history of at least 30 pack-years. Screening should be discontinued once a person has not smoked for 15 years. Another trial is currently being conducted in Belgium and the Netherlands (the NELSON study) looking at the 10-year impact of lung cancer screening using low-dose CT scan.
Despite recent developments, low-dose CT screening still has significant limitations, including high false-positive ratios and the development of interval lung cancers, which can be aggressive. Positron emission tomography (PET)-CT scan, epigenetic markers, and cell-free tumor DNA are additional approaches under investigation, which are only recommended in the setting of a clinical trial.
Cigarette smoking has an effect of field cancerization, with the accumulation of genetic mutations and other premalignant changes throughout the lungs and the aerodigestive tract. Patients treated and cured of early-stage lung cancer have a high risk of developing second primary tumors. Therefore, a series of chemoprevention trials has focused on smokers and survivors of lung and head and neck cancers.
Multiple studies evaluated supplementation with vitamins and oligo-elements, but none showed a benefit to supplementation, and several studies showed risk, with increased lung cancer incidence and mortality (Table 18-3) (15,16,17,18,19,20,21).
| Study | Intervention | Population | Size | End Point | Outcome |
|---|---|---|---|---|---|
| ATBC (15) | β-Carotene; α-tocopherol | Male smokers | 29,133 | Lung cancer incidence | Harmful |
| CARET (16) | β-Carotene; retinol | Current and former smokers | 18,314 | Lung cancer incidence | Harmful |
| Intergroup Lung Trial (17) | Isotretinoin | Resected NSCLC | 1,166 | Second malignancies | Harmful |
| Euroscan (18) | Retinol, N-acetylcysteine | Resected NSCLC and head and neck cancer | 2,592 | Second malignancies | Negative |
| ECOG 5597 (19) | Selenium | Resected NSCLC | 1,772 | Second malignancies | Negative |
| Physicians’ Health Study II (20) | Vitamin C and vitamin E | Healthy male physicians | 14,641 | Cancer incidence | Negative |
| NORVIT and WENBIT (21) | Vitamin B12 and folic acid | Patients with ischemic heart disease | 6,845 | Cancer incidence | Harmful |
Given the potential connection between inflammation and tumorigenesis, phase II studies have used anti-inflammatory agents, such as celecoxib, and prostaglandin analogues, such as iloprost. These agents have been able to reduce proliferation and dysplasia of oral and bronchial epithelium in smokers and former smokers, but their benefit in cancer prevention remains to be proven. There are currently no proven agents for the chemoprevention of lung cancer.
HISTOLOGY AND MOLECULAR PATHOLOGY
Most lung tumors arise from epithelial cells and are called bronchogenic carcinomas. Neuroendocrine tumors also arise in the lung and can appear as SCLC, carcinoids, or large cell neuroendocrine carcinomas. Bronchogenic carcinomas include NSCLCs, a category comprising three major types: adenocarcinoma, squamous cell carcinoma (SCC), and large cell carcinoma.
The most current histologic classification of NSCLC was proposed by the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society in 2011 (22) and uses immunohistochemistry to identify subgroups with different molecular profiles and clinical behaviors (Tables 18-4 and 18-5). Adenocarcinomas usually stain positive for cytokeratin 7, thyroid transcription factor 1 (TTF-1), and surfactant apoprotein A and are negative for cytokeratin 20. Metastatic adenocarcinomas from other sites except the thyroid stain negative for TTF-1. All carcinoids and most SCLCs stain positive for chromogranin and synaptophysin, whereas NSCLC is usually negative for these two markers. Mesothelioma is distinguished from adenocarcinoma by the presence of calretinin and cytokeratin 5/6 and the absence of carcinoembryonic antigen (CEA), B72.3, Ber-EP4, and MOC-31.
| Squamous cell carcinoma |
| Small cell carcinoma |
Adenocarcinoma Lepidic predominant (formerly nonmucinous bronchoalveolar carcinoma pattern, with >5 mm invasion) Acinar predominant Papillary predominant Micropapillary predominant Solid predominant with mucin production |
Variants of invasive adenocarcinoma Invasive mucinous adenocarcinoma (formerly mucinous bronchoalveolar carcinoma) Colloid Fetal (low and high grade) Enteric |
Large cell carcinoma Variants: large cell neuroendocrine (NSCLC NOS); large cell neuroendocrine (NE) carcinoma (positive NE markers); large cell carcinoma with NE morphology (morphology suggestive of NE carcinoma, but negative stains) |
| Adenosquamous carcinoma/NSCLC with squamous cell and adenocarcinoma patterns |
Carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements Carcinoma with spindle and/or giant cells Pleomorphic carcinoma Spindle cell carcinoma Giant cell carcinoma |
Carcinosarcoma Blastoma (pulmonary blastoma) Others |
Carcinoid tumor Typical carcinoid Atypical carcinoid |
Carcinomas of salivary gland type Mucoepidermoid carcinoma Adenocystic carcinoma, others |
| Unclassified carcinoma |
| Predominant Histologic Subtype | Molecular Features | Computed Tomography Scan Appearance | Relative Risk of Recurrence After Resection |
|---|---|---|---|
| Lepidic | TTF-1 positive: 100% EGFR amplification: 20%-50% EGFR mutation (nonsmokers): 10%-30% KRAS mutations (smokers): 10% BRAF mutations: 5% | Ground glass or solid nodule | 1.0 |
| Papillary | TTF-1 positive: 90%-100% EGFR amplification: 20%-60% EGFR mutation: 10%-30% KRAS mutations: 3% BRAF mutations: 5% ERBB2 mutations: 3% P53 mutations: 30% | Solid nodule | 2.7 (95% CI, 1.1-6.8) |
| Acinar | TTF-1 positive or negative EGFR amplification: 10% EGFR mutation (non-smokers): <10% KRAS mutation (smokers): 20% P53 mutation: 40% EML4/ALK translocation: >5% | Solid nodule | 2.3 (95% CI, 0.9-5.7) |
| Micropapillary | EGFR mutation: 20% KRAS mutation: 33% BRAF mutation: 20% | Unknown | 4.4 (95% CI, 1.8-11.2) |
| Solid | TTF-1 positive: 70% MUC1 positive EGFR amplification: 20%-50% EGFR mutation (nonsmokers): 10%-30% KRAS mutation (smokers): 10%-30% EML4/ALK translocations: >5% P53 mutations: 50% LRP1B mutations INHBA mutations | Solid nodule | 5.7 (95% CI, 2.2-14.7) |
| Invasive mucinous adenocarcinoma | TTF-1 negative (0%-33% positive) No EGFR mutation KRAS mutation: 80%-100% MUC5, MUC6, MUC2, CK20 positive | Consolidation; air bronchograms | Unknown |
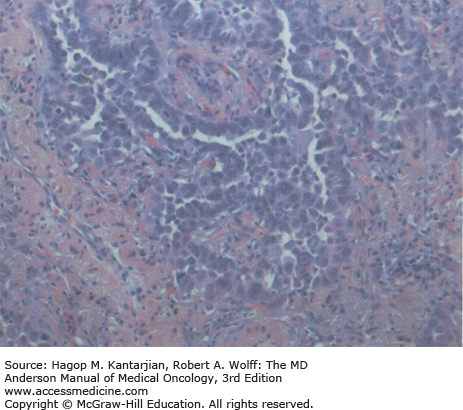
Adenocarcinoma is the most common subtype of NSCLC in the United States, representing 40% of cases in men and 50% in women. It is predominant in nonsmokers, and its incidence has been rising. These tumors are classically peripheral and, on histologic examination, demonstrate gland formation, papillary structures, or mucin production (Fig. 18-1).
Non–small cell lung cancer is one of the cancer types with the highest mutation burden, with an average of 360 exonic mutations per sample (23). However, the patterns of mutations are different for adenocarcinomas and SCCs.
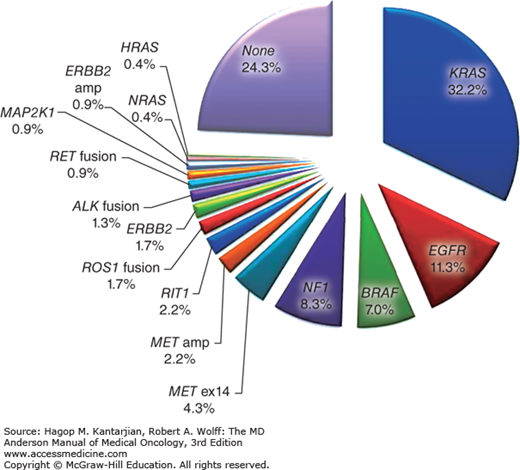
About 75% of lung adenocarcinomas have alterations in driver genes that activate intracellular pathways leading to proliferation, cell survival, and oxidative stress response (24) (Fig. 18-2). Up to 79% of those alterations are in the RTK/RAS/RAF pathway, including mutations in the ErbB family member EGFR (ErbB1).
The prevalence of EGFR mutations ranges from 5% to 10% in smokers to 40% to 50% in nonsmokers in the Western population (25). The alterations are usually in the tyrosine kinase domain of the gene within exons 18 to 24, most specifically in the intracellular adenosine triphosphate (ATP)-binding domain (Table 18-6) (25,26,27).
| Mutation | Prevalence in Treatment-Naïve EGFR-Mutant Tumors | Sensitivity to Erlotinib/Gefitinib |
|---|---|---|
| Exon 19 deletion (del19) | 45%-50% (25) | Sensitive |
| Exon 21 Leu858Arg insertion (L858R) | 40%-45% (25) | Sensitive |
| Exon 20 insertions | 5% (26) | Resistant |
| Exon 12 and 18 insertions | 2%-3% (26) | Unknown |
| Exon 20 T790M | 1%-5% (27) | Resistant |
Frequencies of EGFR, KRAS, ALK, ROS1, and other driver genetic alterations commonly found in lung adenocarcinoma samples are listed in Table 18-7 (24,25,28,29,30,31,31,32). This is a rapidly changing landscape that is defining the many molecular subsets of this heterogeneous disease.
| Name | Alteration | Main Effects | Incidence |
|---|---|---|---|
| KRAS mutations | Multiple, usually in codons 12 and 13; most common G12D (nonsmokers) and G12C (smokers) | Activation of RAS/RAF/MEK/ERK pathway | 30%-35% (mostly in smokers) (25) |
| EGFR mutations | Several alterations in the ATP-binding domain of the receptor | Activation of RAS/RAF/MEK and PI3K/AKT/mTOR pathways | 10%-15% (40%-50% in nonsmokers) (28) |
| BRAF mutations | About 50% are the V600E mutation also described in other cancers | Activation of RAS/RAF/MEK/ERK pathway | 7%-10% (24) |
| MET mutations | Exon 14 skipping; can occur with or without MET amplification | Activation of MET | 7% (24) |
| PIK3CA | Many activating mutations | AKT, TSC, and mTOR | 7% (24) |
| EML4/ALK and other ALK translocations | Inversion within chromosome 2p | Constitutively activates the anaplastic lymphoma kinase (ALK) gene leading to downstream activation of RAS and PI3K | 1%-4% (29,30) |
| ROS1 fusions | Multiple rearrangements of the ROS1 gene with different genes | Related to the ALK protein, also activates RAS and PIK3CA | 1%-2% (29) |
| RET fusions | Multiple rearrangements of the RET gene with different genes | Activation of the RET proto-oncogene and RAS pathway | 1% (31) |
| ERBB2 (HER2) mutations | Various mutations; in about 50% of cases co-occurrence with HER2 amplification | Activation of RAS/RAF/MEK and PI3K/AKT/mTOR pathways | 2%-4% (32) |
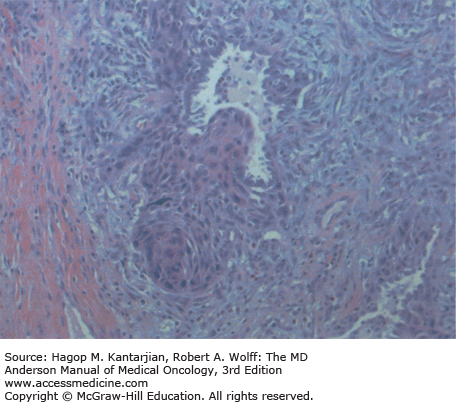
Squamous cell carcinoma is now the second most frequent histology, accounting for 30% of NSCLC in men and 20% in women. This tumor arises most frequently in the proximal bronchi and has the strongest association with smoking. Pathologically, it is characterized by visible keratinization, with prominent desmosomes and intercellular bridges (Fig. 18-3).
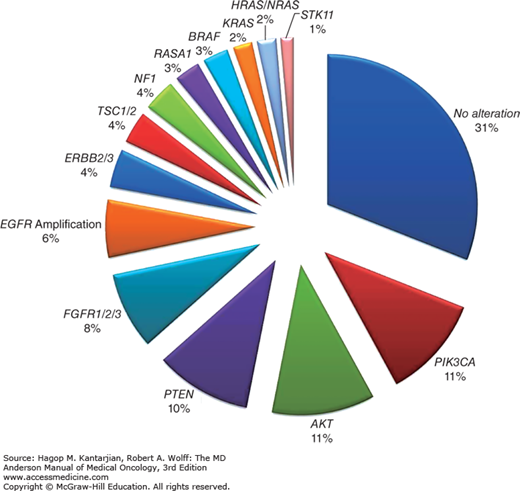
Squamous cell carcinomas frequently have amplifications in chromosome 3q, which contains genes involved in squamous differentiation (SOX2 and TP53) and cell proliferation (PI3K). About 81% to 90% of patients with SCCs have TP53 mutations, and 47% have alterations in the PI3K/AKT/mTOR pathway, whereas only 26% of SCCs have activations of the RTK/RAS/RAF pathway (23). The prevalence of EGFR mutations is 1% to 3%, 6% of patients have EGFR amplifications, and 1% to 6% have KRAS mutations (23) (Fig. 18-4).
The least common subtype of NSCLC, large cell carcinoma, accounts for approximately 8% of all NSCLCs (Fig. 18-5). Refinements in histopathologic techniques have led to the diagnosis of adenocarcinoma or SCC in cases previously diagnosed as undifferentiated large cell carcinoma.
CLINICAL PRESENTATION
Non–small cell lung cancer is often asymptomatic at diagnosis and may be found incidentally on imaging performed for other reasons. If symptoms are present, they are often related to the specific locations of tumor masses and the occurrence of paraneoplastic syndromes. The symptoms of centrally located lesions include cough, hemoptysis, wheezing, stridor, dyspnea, and postobstructive pneumonia. Peripheral lesions can cause pain due to pleural or chest wall invasion, cough, or restrictive dyspnea.
The involvement of thoracic and cervical structures can also lead to classical clinical presentations:
Pancoast syndrome: Shoulder pain radiating to the arm in an ulnar distribution caused by invasion of the eighth cervical and first thoracic nerves in the superior sulcus.
Horner syndrome: Enophthalmos, ptosis, miosis, and ipsilateral dyshidrosis caused by involvement of the paravertebral sympathetic nerves.
Hoarseness: Involvement of the left recurrent laryngeal nerve as it passes through the aortopulmonary window.
Elevation of the hemidiaphragm: Involvement of phrenic nerve at the mediastinum.
Superior vena cava syndrome: Swelling of the face and arm and superficial venous engorgement caused by compression of the superior vena cava.
The production of hormones or hormone-like substances can lead to paraneoplastic syndromes:
Cancer cachexia: The most common paraneoplastic syndrome, characterized by weight loss, impaired immune function, and weakness that are not completely explained by poor oral intake. The exact mechanism for development of cachexia is unknown, but tumor-elaborated cytokines have been implicated.
Hypercalcemia: The second most common paraneoplastic syndrome in NSCLC, hypercalcemia is caused by ectopic production of a parathyroid hormone-related protein (PTHrP) or bone metastases. It is more common in the squamous cell subtype.
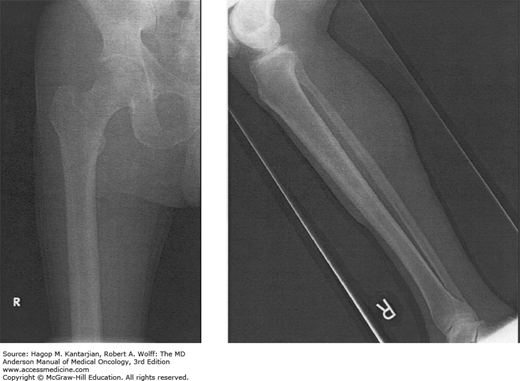
Hypertrophic pulmonary osteoarthropathy: Arthropathy and clubbing of the fingers and toes with evidence of periostitis of the long bones (Fig. 18-6). Its etiology is unknown, and it is more common in adenocarcinomas and large cell carcinoma.
FIGURE 18-6
Hypertrophic pulmonary osteoarthropathy (HPO). This 62-year-old man with non–small cell lung cancer reported a 1-month history of finger clubbing and arthritic lower extremity pain. The plain radiographs of the lower extremities show periosteal reaction in both femora as well as in bilateral tibias/fibulas that is consistent with HPO.
Non–small cell lung cancer is frequently metastatic, and symptoms secondary to metastases are common. The most common sites for metastases are intrathoracic nodes, pleura, contralateral lung, liver, adrenal glands, bone, and brain.
DIAGNOSIS
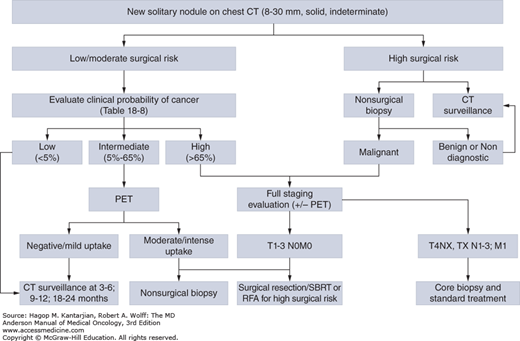
A solitary pulmonary nodule is a single asymptomatic mass that is surrounded by lung tissue, is well circumscribed, measures less than 3 cm, and does not show evidence of mediastinal or hilar adenopathy. The differential diagnosis includes primary cancer, metastatic cancer, infection, benign tumors (eg, hamartomas), vascular abnormalities, and inflammation (eg, granulomatous disease). The American College of Chest Physicians has issued guidelines on the evaluation of solitary lung nodules, based on their size (≤ or >8 mm), the clinical probability of malignancy, and the patient’s surgical risk (33) (Table 18-8 and Fig. 18-7).
| Low Probability (<5%) | Intermediate Probability (6-65%) | High Probability (>65%) | |
|---|---|---|---|
| Patient | Young, low smoking burden, no previous history of cancer | Mixture of low- and high-probability features | Older, heavy smoker, previous cancer |
| Nodule (CT scan) | Small, regular margins, non–upper lobe location, enhancement <15 HU in the contrast phase | Mixture of low- and high-probability features | Large, irregular/spiculated, upper lobe location; enhancement >15 HU in the contrast phase |
| FDG-PET results | Low or moderate clinical probability with low PET probability | Weak or moderate PET activity | Intense hypermetabolic nodule |
| Nonsurgical biopsy results (bronchoscopy or transthoracic biopsy) | Specific benign diagnostic | Nondiagnostic | Suspicious for malignancy |
| Behavior on CT surveillance | Progressive decrease in size or resolution; no growth over ≥2 years (solid nodules) or over ≥3 years (semi-solid nodules) | NA | Clear evidence of growth |
FIGURE 18-7
Management algorithm for individuals with solitary nodules (8-30 mm). CT, computed tomography; PET, positron emission tomography; RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy. [Adapted with permission from Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines, Chest 2013 May;143(5 Suppl):e93S-120S.]
The management of small nodules (<8 mm) is less clear. Lesions that are too small to be biopsied (<5 mm) should be followed with CT scans in 6 months (any risk factors for lung cancer) or 12 months (no risk factors for lung cancer). For lesions 5 to 8 mm, some recommend close follow-up with CT scans in 3, 6, and 12 months, and others recommend nonsurgical biopsies (33).
Lesions that are large (>3 cm), multiple, or with enlarged hilar, mediastinal, and/or supraclavicular lymph nodes should undergo complete lung cancer staging and a nonsurgical biopsy. Fiberoptic bronchoscopy is appropriate for central lesions and is able to establish the diagnosis in 97% of cases. For peripheral lesions, the diagnostic yield of bronchoscopy is only 55%, and percutaneous transthoracic biopsies can be considered. Because of the tissue requirements for immunohistochemistry and molecular marker testing of lung cancers, at MDACC, we usually perform an image-guided core-needle biopsy. Mediastinoscopy or endobronchial ultrasound (EBUS) can be used to obtain biopsy samples from mediastinal nodes.
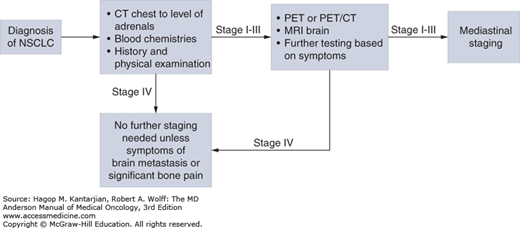
Once the histologic diagnosis of NSCLC has been established, the extent of disease must be determined (Fig. 18-8).
The stage of disease will dictate therapy. All patients must undergo a complete history and physical examination, chest x-ray, CT scan of the chest and upper abdomen (to include the adrenal glands), a complete blood count, and blood chemistry tests that include electrolyte and liver enzyme studies. All patients with stage II to IV disease should have evaluation of the brain, with magnetic resonance imaging (MRI) if possible. Central nervous system (CNS) imaging may be considered for patients with stage I disease. For stage I to III NSCLC, 18F-fluorodeoxyglucose (FDG)-PET should be performed to evaluate mediastinal nodes and for distant metastases. Because the involvement of mediastinal lymph nodes will influence surgical decisions (see section “Treatment”), the presence of FDG-avid mediastinal lymph nodes in a PET-CT scan should be confirmed with either mediastinoscopy or EBUS (see “Commentary”). Routine use of mediastinoscopy or EBUS in patients with a normal-appearing mediastinum on PET-CT scans is controversial.
Commentary: Mediastinal Lymph Node Sampling Using Endobronchial Ultrasound and Transbronchial Needle Aspiration
Accurate mediastinal lymph node staging is a critical aspect of non–small cell lung cancer (NSCLC) management. Non- invasive staging typically relies on a combination of computed tomography (CT) and 18F-fluorodeoxyglucose (FDG)– positron emission tomography (PET) data to detect mediastinal metastases. Lymph nodes are considered abnormal by CT criteria if the short-axis diameter is >10 mm; however, both false negatives and false positives are possible. FluorodeoxyglucoseFDG-PET scanning has been a welcome addition to the staging armamentarium and increases diagnostic accuracy. However, limitations of non-invasive staging modalities remain, and current guidelines recommend tissue sampling by invasive means to improve diagnostic accuracy among patients whose subsequent therapy is contingent on mediastinal involvement.
Multiple modalities are available for sampling mediastinal and hilar lymph nodes. These range from minimally invasive approaches such as endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA), endoscopic ultrasound guided–fine-needle aspiration (EUS-FNA), and CT-guided needle aspiration to surgical approaches such as mediastinoscopy and thoracotomy.
Endobronchial ultrasound–guided transbronchial needle aspiration EBUS-TBNA is a technology that ustilizes an integrated ultrasonic bronchoscope to obtain real- time image–guided transbronchial needle aspiration biopsy of lymph nodes in proximity to the central airways and. EBUS-TBNA is currently our preferred method for assessing mediastinal lymph node involvement. It is a minimally invasive procedure that is typically performed on an outpatient basis and often ustilizes only moderate sedation. Biopsies can be taken at all lymph node stations adjacent to a major airway, and as such, EBUS-TBNA can sample more lymph nodes in a single procedure than any other invasive mediastinal tissue sampling technique. Furthermore, rather than merely confirming malignancy in an enlarged or FDG-avid lymph node, true lymph node mapping can be carried out using this technology. It can be safely performed in most patients, including those that who have undergone prior surgical or radiation therapy to the thorax. The risk profile is minimal and similar to the risks of standard bronchoscopy. Numerous studies have documented the safety and diagnostic accuracy of EBUS-TBNA in staging lung cancer. Samples obtained have also been shown to be adequate for molecular testing. Importantly, EBUS-TBNA has shown significant utility even in patients with radiologically normal mediastinum.
AlthoughWhile cervical mediastinoscopy was traditionally considered the reference standard for invasive mediastinal lymph node sampling, it is difficult to repeat, is more invasive, and has higher associated morbidity and mortality than EBUS-TBNA, and increasing evidence suggests diagnostic equivalence. Current American College of Clinical Pharmacy ACCP guidelines recommend a needle biopsy technique such as EBUS- TBNA as the initial invasive mediastinal staging technique of choice for lung cancer with the caveat that negative biopsies may need surgical confirmation. In summary, EBUS-TBNA provides a safe, minimally invasive, and highly accurate method of mediastinal lymph node sampling and has rapidly become part of our standard practice in the mediastinal staging of NSCLC.
George A. Eapen
After the completion of staging evaluation, the disease is assigned a TNM stage (Figs. 18-9,18-10,18-11,18-12,18-13,18-14,18-15,18-16,18-17; Tables 18-10 and 18-11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree