References
1. Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178(6):2314-2330.
2. Nieder AM, Brausi M, Lamm D, et al. Management of stage T1 tumors of the bladder: International Consensus Panel. Urology 2005;66(6 Suppl 1):108-125.
3. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: bladder cancer including upper tract tumours and urothelial carcinoma of the prostate. Version 1. NCCN 2007.
4. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49(3):466-465; discussion 475-467.
5. Fernandez-Gomez J, Madero R, Solsona E, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol 2009;182(5): 2195-2203.
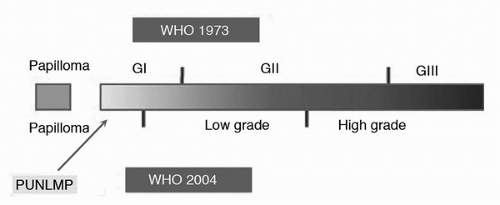
6. Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998;22(12):1435-1448.
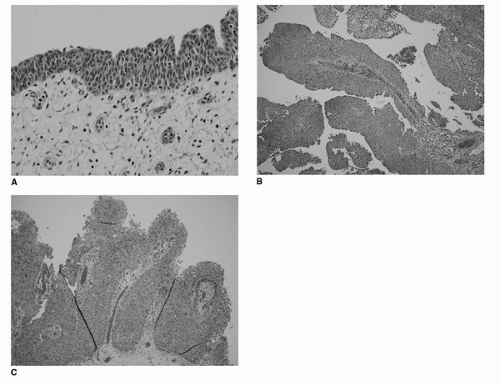
7. MacLennan GT, Kirkali Z, Cheng L. Histologic grading of noninvasive papillary urothelial neoplasms. Eur Urol 2007;51(4):889-897; discussion 897-888.
8. Bostwick DG, Mikuz G. Urothelial papillary (exophytic) neoplasms. Virchows Arch 2002;441(2):109-116.
9. Murphy WM, Takezawa K, Maruniak NA. Interobserver discrepancy using the 1998 World Health Organization/International Society of Urologic Pathology classification of urothelial neoplasms: practical choices for patient care. J Urol 2002;168(3):968-972.
10. Mamoon N, Iqbal MA, Jamal S, et al. Urothelial neoplasia of the urinary bladder—comparison of interobserver variability for WHO Classification 1972 with WHO/ISUP Consensus Classification 1998. J Ayub Med Coll Abbottabad 2006;18(2):4-8.
11. Witjes JA, Kiemeney LA, Schaafsma HE, et al. The influence of review pathology on study outcome of a randomized multicentre superficial bladder cancer trial. Members of the Dutch South East Cooperative Urological Group. Br J Urol 1994;73(2):172-176.
12. Witjes JA, Moonen PM, van der Heijden AG. Review pathology in a diagnostic bladder cancer trial: effect of patient risk category. Urology 2006;67(4):751-755.
13. Van Der Meijden A, Sylvester R, Collette L, et al. The role and impact of pathology review on stage and grade assessment of stages Ta and T1 bladder tumors: a combined analysis of 5 European Organization for Research and Treatment of Cancer Trials. J Urol 2000;164(5):1533-1537.
14. van Rhijn BW, Vis AN, van der Kwast TH, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol 2003;21(10):1912-1921.
15. Gomez-Roman JJ, Saenz P, Molina M, et al. Fibroblast growth factor receptor 3 is overexpressed in urinary tract carcinomas and modulates the neoplastic cell growth. Clin Cancer Res 2005;11(2 Pt 1):459-465.
16. van Oers JM, Wild PJ, Burger M, et al. FGFR3 mutations and a normal CK20 staining pattern define low-grade noninvasive urothelial bladder tumours. Eur Urol 2007;52(3):760-768.
17. Kompier LC, van der Aa MN, Lurkin I, et al. The development of multiple bladder tumour recurrences in relation to the FGFR3 mutation status of the primary tumour. J Pathol 2009;218(1):104-112.
18. Palou J, Rodriguez-Rubio F, Huguet J, et al. Multivariate analysis of clinical parameters of synchronous primary superficial bladder cancer and upper urinary tract tumor. J Urol 2005;174(3):859-861; discussion 861.
19. Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2008;54(2):303-314.
20. Vrooman OP, Witjes JA. Urinary markers in bladder cancer. Eur Urol 2008;53(5):909-916.
21. Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66(6 Suppl 1):4-34.
22. Brausi M, Collette L, Kurth K, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol 2002;41(5):523-531.
23. van der Meijden A, Oosterlinck W, Brausi M, et al. Significance of bladder biopsies in Ta,T1 bladder tumors: a report from the EORTC Genito-Urinary Tract Cancer Cooperative Group. EORTC-GU Group Superficial Bladder Committee. Eur Urol 1999;35(4):267-271.
24. Babjuk M, Soukup V, Petrik R, et al. 5-aminolaevulinic acid-induced fluorescence cystoscopy during transurethral resection reduces the risk of recurrence in stage Ta/T1 bladder cancer. BJU Int 2005;96(6):798-802.
25. Jichlinski P, Jacqmin D. Photodynamic diagnosis in non-muscle-invasive bladder cancer. Eur Urol 2008;7:529-535.
26. Schmidbauer J, Witjes F, Schmeller N, et al. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol 2004;171(1):135-138.
27. Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol 2004;171(6 Pt 1):2186-2190; quiz 2435.
28. Gudjonsson S, Adell L, Merdasa F, et al. Should all patients with non-muscle-invasive bladder cancer receive early intravesical chemotherapy after transurethral resection? The results of a prospective randomised multicentre study. Eur Urol 2009;55(4):773-780.
29. Dobruch J, Herr H. Should all patients receive single chemotherapeutic agent instillation after bladder tumour resection? BJU Int 2009;104(2):170-174.
30. Kaasinen E, Rintala E, Hellstrom P, et al. Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficial bladder carcinoma. Eur Urol 2002;42(2):167-174.
31. Solsona E, Iborra I, Ricos JV, et al. Effectiveness of a single immediate mitomycin C instillation in patients with low risk superficial bladder cancer: short and long-term followup. J Urol 1999;161(4):1120-1123.
32. Berrum-Svennung I, Granfors T, Jahnson S, et al. A single instillation of epirubicin after transurethral resection of bladder tumors prevents only small recurrences. J Urol 2008;179(1):101-105; discussion 105-106.
33. Hendricksen K, Witjes WP, Idema JG, et al. Comparison of three schedules of intravesical epirubicin in patients with non-muscle-invasive bladder cancer. Eur Urol 2008;53(5):984-991.
34. Bohle A, Leyh H, Frei C, et al. Single postoperative instillation of gemcitabine in patients with non-muscle-invasive transitional cell carcinoma of the bladder: a randomised, doubleblind, placebo-controlled phase III multicentre study. Eur Urol 2009;56(3):495-503.
35. Herr HW. Does cystoscopy correlate with the histology of recurrent papillary tumours of the bladder? BJU Int 2001;88(7):683-685.
36. Hernandez V, Alvarez M, de la Pena E, et al. Safety of active surveillance program for recurrent nonmuscle-invasive bladder carcinoma. Urology 2009;73(6):1306-1310.
37. Mack D, Holtl W, Bassi P, et al. The ablative effect of quarter dose bacillus Calmette-Guerin on a papillary marker lesion of the bladder. J Urol 2001;165(2):401-403.
38. Gardmark T, Carringer M, Beckman E, et al. Randomized phase II marker lesion study evaluating effect of scheduling on response to intravesical gemcitabine in recurrent Stage Ta urothelial cell carcinoma of the bladder. Urology 2005;66(3):527-530.
39. Gontero P, Casetta G, Maso G, et al. Phase II study to investigate the ablative efficacy of intravesical administration of gemcitabine in intermediate-risk superficial bladder cancer (SBC). Eur Urol 2004;46(3):339-343.
40. van der Heijden AG, Moonen PM, Cornel EB, et al. Phase II marker lesion study with intravesical instillation of apaziquone for superficial bladder cancer: toxicity and marker response. J Urol 2006;176(4 Pt 1):1349-1353; discussion 1353.
41. Holmang S, Johansson SL. Stage Ta-T1 bladder cancer: the relationship between findings at first followup cystoscopy and subsequent recurrence and progression. J Urol 2002;167(4):1634-1637.
42. Mariappan P, Smith G. A surveillance schedule for G1Ta bladder cancer allowing efficient use of check cystoscopy and safe discharge at 5 years based on a 25-year prospective database. J Urol 2005;173(4):1108-1111.