Tania C. Sorrell, David H. Mitchell, Jonathan R. Iredell, Sharon C-A. Chen
Nocardia Species
Nocardia is a genus of aerobic actinomycetes responsible for localized or disseminated infections in animals and humans. The genus is named after Edmond Nocard, who in 1888 described the isolation of an aerobic actinomycete from cattle with bovine farcy. The first human case of nocardiosis was reported by Eppinger in 1890. Cases of human disease have increased substantially in the past 2 decades, in association with an increasing population of immunocompromised hosts and improved methods for detection and identification of Nocardia species in the clinical laboratory.
Classification
The aerobic actinomycetes are a large and diverse group of gram-positive bacteria1 that appear on microscopy as branching, filamentous cells. Members of the group are often only distantly related phylogenetically. A subgroup, classified in the suborder Corynebacterineae, is the most important cause of human and veterinary infection and includes the genera Mycobacterium, Corynebacterium, Nocardia, Rhodococcus, Gordona, and Tsukamurella.1 All members of the group have cell walls containing meso-diaminopimelic acid, arabinose, galactose (type IV cell wall1), and mycolic acids of various chain lengths. The latter are responsible for varying degrees of acid fastness on modified acid-fast staining. Acid fastness is best seen in clinical specimens and can be lost on subculture. In addition, Nocardia species are characterized by an ability to form aerial hyphae and to grow in media containing lysozyme and by an inability to grow at 50° C.1
Traditional phenotypic laboratory methods for identification of Nocardia species,1,2 which are based on simple biochemical reactions and hydrolysis tests, and by cell wall mycolic acid analysis, are limited in their ability to differentiate these organisms, especially between phylogenetically closely related species, and have been largely superseded.1,2 Instead, various molecular techniques have been developed. These are essential for accurate species determination.
The application of molecular methods2–4 has greatly expanded the spectrum of pathogenic Nocardia species and has led to significant taxonomic changes and species reassignment within the genus. This is particularly evident among isolates formerly assigned to the Nocardia asteroides complex.2 A large number of Nocardia species have been identified (National Center for Biotechnology Information taxonomy for Nocardia: www.ncbi.nlm.nih.gov/Taxonomy/), many of which have been implicated in human disease (Table 255-1).2,3 Major pathogens include those formerly classified in the N. asteroides complex. Newer names for isolates formerly in the N. asteroides complex are listed in Table 255-2 and include Nocardia cyriacigeorgica,5 Nocardia abscessus,6 the Nocardia nova complex, and the Nocardia transvalensis complexes. Other major clinically isolated species include Nocardia otitidiscaviarum, Nocardia farcinica, and Nocardia brasiliensis.2 Some recently described or reclassified species have been reported to cause human infection. They include Nocardia paucivorans (N. brevicatena/paucivorans complex2,7), Nocardia africana,8 and Nocardia veterana9 (N. nova complex2) and, most recently, Nocardia wallacei and Nocardia blacklockiae (N. transvalensis complex10). Use of the name “N. asteroides species complex” is now outdated because it comprises such a heterogenous group of organisms.
TABLE 255-1
Current Classifiable Nocardia Species Names
| NOCARDIA SPECIES* | FREQUENCY† |
| Nocardia abscessus | 22 |
| Nocardia acidovorans | |
| Nocardia africana | |
| Nocardia alba | |
| Nocardia alboflava | |
| Nocardia altamirensis | |
| Nocardia amamiensis | |
| Nocardia anaemiae | |
| Nocardia aobensis | 5 |
| Nocardia araoensis | 1 |
| Nocardia arthritidis | 4 |
| Nocardia asiatica | 21 |
| Nocardia asteroides | 2 |
| Nocardia beijingensis | 24 |
| Nocardia blacklockiae | |
| Nocardia brasiliensis | 71 |
| Nocardia brevicatena | |
| Nocardia caishijiensis | |
| Nocardia carnea | 3 |
| Nocardia caverna | |
| Nocardia cerradoensis | |
| Nocardia coeliaca | |
| Nocardia concava | 3 |
| Nocardia coubleae | |
| Nocardia crassostreae | |
| Nocardia cummidelens | |
| Nocardia cyriacigeorgica | 60 |
| Nocardia devorans | |
| Nocardia elegans | 12 |
| Nocardia exalbida | 4 |
| Nocardia farcinica | 160 |
| Nocardia flavorosea | |
| Nocardia fluminea | |
| Nocardia fusca | |
| Nocardia gamkensis | |
| Nocardia globerula | |
| Nocardia grenadensis | |
| Nocardia harenae | |
| Nocardia higoensis | 1 |
| Nocardia ignorata | |
| Nocardia inohanensis | 1 |
| Nocardia iowensis | |
| Nocardia jejuensis | |
| Nocardia jiangxiensis | |
| Nocardia jinanensis | |
| Nocardia kruczakiae | |
| Nocardia levis | |
| Nocardia lijiangensis | |
| Nocardia mexicana | |
| Nocardia mikamii | |
| Nocardia miyunensis | |
| Nocardia neocaledoniensis | |
| Nocardia niigatensis | 4 |
| Nocardia ninae | |
| Nocardia niwae | |
| Nocardia nova | 81 |
| Nocardia novocastrensa | |
| Nocardia otitidiscaviarum | 14 |
| Nocardia paucivorans | 1 |
| Nocardia pigrifrangens | |
| Nocardia pneumoniae | |
| Nocardia polyresistens | |
| Nocardia pseudobrasiliensis | 2 |
| Nocardia pseudosporangifera | |
| Nocardia pseudovaccinii | |
| Nocardia puris | 4 |
| Nocardia rhamnosiphila | |
| Nocardia roseoalba | |
| Nocardia salmonicida | |
| Nocardia salmonicolor | |
| Nocardia seriolae | |
| Nocardia shimofusensis | |
| Nocardia sienata | 1 |
| Nocardia soli | |
| Nocardia speluncae | |
| Nocardia strombolensis | |
| Nocardia takedensis | |
| Nocardia tartaricans | |
| Nocardia tenerifensis | |
| Nocardia terpenica | 1 |
| Nocardia testacea | 2 |
| Nocardia thailandica | 1 |
| Nocardia transvalensis | 13 |
| Nocardia uniformis | |
| Nocardia vaccinii | |
| Nocardia vermiculata | |
| Nocardia veterana | 3 |
| Nocardia vinacea | 3 |
| Nocardia violaceofusca | |
| Nocardia wallacei | 10 |
| Nocardia xishanensis | |
| Nocardia yamanashiensis | |
* Species names in http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi. Accessed May 24, 2013.
† Frequency of classifiable isolates at Chiba University, Medical Mycology Center, 1999-2007.
Data from Mikami U. [Recent progress in taxonomic studies on pathogenic nocardia and usefulness of the bacteria for the studies on secondary metabolites and antibiotic resistant mechanisms]. Nihon Ishinkin Gakkai Zasshi. 2010;51:179-192 [in Japanese].
TABLE 255-2
Nocardia asteroides Complex: Major Changes in Taxonomic Categories*
| FORMER SPECIES OR SPECIES GROUP ASSIGNMENT | CURRENT SPECIES GROUP DESIGNATION | CURRENT SPECIES DESIGNATION |
| N. asteroides drug pattern I | — | N. abscessus |
| N. asteroides drug pattern II | N. paucivorans/N. brevicatena complex | N. paucivorans† N. brevicatena† |
| N. asteroides drug pattern III | N. nova complex‡ | N. nova sensu stricto, N. africana N. aobensis N. elegans, N. kruczakiae, N. veterana |
| N. asteroides drug pattern IV§ | N. transvalensis complex | N. wallacei, N. transvalensis sensu stricto, N. blacklockiae |
| N. asteroides drug pattern V | N. farcinica | |
| N. asteroides drug pattern VI | N. cyriacigeorgica |
* N. brevicatena and N. paucivorans are not new species names—they have been reclassified.
† N. asteroides sensu stricto is rarely pathogenic.
‡ It is uncertain to which species the former N. asteroides drug pattern III isolates now corresponds.
§ Only N. wallacei is designated as the former “N. asteroides drug pattern IV.” The other members of the N. transvalensis complex are previously either separate as “N. asteroides complex” or are recently identified species.
Data from Conville PS, Witebsky FG. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Versalovic J, Carroll KC, Funke G, et al, eds. Manual of Clinical Microbiology, 10th ed. Washington, DC: ASM Press; 2011.
Ecology and Epidemiology
Nocardia species are ubiquitous environmental saprophytes, occurring in soil, organic matter, and water.1,2 Human infection usually arises from direct inoculation of the skin or soft tissues or by inhalation. N. brasiliensis is the commonest cause of mycetoma due to nocardial infection in immunocompetent hosts reported from tropical regions of the southern United States, Mexico, Central and South America, and Australia. Worldwide, respiratory and disseminated infections occur predominately in immunosuppressed hosts and are most often due to N. cyriacigeorgica, N. nova, and N. farcinica.1,2,11,12
Nocardia species are well-recognized causes of infection in animals, with bovine mastitis being the most common.2 There are no reports of animal-to-human transmission. Clusters of invasive nocardiosis acquired by patients in oncology and transplantation units, presumed to be associated with inhalation of contaminated dust, have been described.2 Concurrent transmission via the hands of staff or contaminated fomites appeared likely in one of these outbreaks.2 Hospital construction work may have been a risk factor in separate clusters of postsurgical wound infections due to Nocardia species.2,13 Cases of indwelling intravenous line–associated bloodstream infection, mostly due to members of the “N. asteroides species complex” or N. nova, have been reported occasionally in immunosuppressed patients.14 A community cluster of N. cyriacigeorgica infections associated with unlicensed cosmetic procedures has also been described.15 Pulsed-field gel electrophoresis,13 random amplification of polymorphic DNA (RAPD) fingerprinting,16 and multilocus sequence typing15 have been used successfully for confirming clusters and defining common sources.
Pathology and Pathogenesis
Sections of tissues infected with Nocardia species usually demonstrate an acute pyogenic inflammatory reaction. Branching, beaded, filamentous bacteria, similar to those seen in smears taken from cultures, may be demonstrated within the abscesses on Gram staining (Fig. 255-1). “Sulfur granules” (bacterial macrocolonies) similar to those seen in actinomycosis may be found in nocardial mycetomas. Nocardia species usually stain acid fast in tissue sections if a method such as that of Fite-Faraco is used, whereas Actinomyces species do not.1
Disease manifestations of nocardiosis are determined by the portal of entry, tissue tropism, growth rates in vivo, ability to survive phagocyte attack, the nature of the host immune reaction, and the characteristics of the infecting strain. After intravenous inoculation into mice, virulent nocardia are cleared from the blood within a few hours and localize in a number of organs (lung, brain, kidneys, liver, and spleen). The outcome of infection is largely determined by the ability of a given strain to resist the initial neutrophil leukocyte response and subsequent attack by activated macrophages.17
Protective immune responses to Nocardia species are primarily T cell mediated, and nocardiosis is more problematic in patients with impaired cell-mediated immunity, eliciting little in the way of an effective humoral response.17 Innate immune function is important in the initial response to infection; in an intranasal mouse model of pulmonary nocardiosis, effective clearance was dependent on early neutrophil recruitment initiated by interleukin (IL)-17 production by γδ T lymphocytes.18 Early neutrophil mobilization appears to retard the process until lymphocyte-mediated cytotoxicity and activated macrophages effect a definitive response.17,19 Healing is associated with strong, sustained rises in interferon-γ (IFN-γ) in animal models, and IFN-γ may have a therapeutic role in humans with chronic granulomatous disease.20
Specific Virulence Determinants
The nocardial envelope of the Corynebacterineae suborder, which includes Mycobacterium spp. and Nocardia spp., is an asymmetric bilayer composed of inner leaflet mycolic acids and assorted noncovalently bound outer-layer glycolipids. Up to 25% of the cell wall mass in rapidly growing organisms, and nearly twice that in stationary phase, is composed of peptidoglycan.21,22 Mycolic acid polymers are found in many actinomycetes, including Nocardia species,21 and are associated with virulence.23,24 Outer-layer lipids induce production of proinflammatory cytokines IL-1β and IL-6 in macrophages and thus appear to be responsible for the powerful granulomatous reaction that characterizes the initial pathogenesis of disease due to species such as N. brasiliensisis,25 but they are also implicated in the immunosuppressive microenvironment that becomes evident at days 30 to 60.26,27
Nocardia species contain no cell wall lipopolysaccharide, exopolysaccharide capsule, or surface fimbriae. Strain-dependent specific adhesins and invasive properties influence the outcome of infection in animal models.28,29 Specific toxins including hemolysins and proteases have been identified, but these are not thought to be widespread or particularly significant virulence factors.17,30,31 Highly pathogenic Mycobacterium tuberculosis and N. asteroides complex secrete superoxide dismutase (SOD) into growth media, whereas nonpathogenic mycobacteria and Nocardia species do not,32,33 and catalases and superoxide dismutase are common to the genomes of pathogenic Nocardia and mycobacteria.34–36 Virulent strains of N. asteroides are relatively resistant to neutrophil-mediated killing,37 and organisms in the logarithmic growth phase are more toxic to macrophages.21 Patients with specific defects in the phagocyte oxidative burst (e.g., chronic granulomatous disease)11,20 may be more vulnerable to this infection.
Bacteremia does not feature in classical descriptions of lymphocutaneous farcy38 but occurs, although rarely, in pulmonary and even cutaneous nocardiosis.39 Up to half of all pulmonary nocardiosis in immunosuppressed hosts will disseminate to sites such as the brain.40 Virulent Nocardia inhibit phagosome-lysosome fusion more successfully in vitro,41 giving rise to cell wall–deficient forms (L-forms) that can be isolated from within macrophages many days later.42,43 Small gram-negative Nocardia L-forms (“filterable Nocardia”) are readily cultured from in vitro broth filtrates, especially when supplemented with erythrocytes,44 and L-forms have been isolated from serious human and animal infections.45,46
Ciliated epithelia appear relatively resistant to invasion by Nocardia species. However, a range of susceptible lung- and airway-associated cell types has been observed in rat models of infection.29 Tropism for cerebral tissue is evident experimentally, but neuroinvasiveness and macrophage penetration vary significantly between strains. Electron microscopic studies of infected macrophage and astrocytoma-derived or astrocytoma-related cell lines suggest that the penetration competence of invasive “N. asteroides species complex” is localized to the bacterial apex.30 Specific lectins have been shown to determine site specificity in the murine brain,30 intrinsic differences in expression of which may contribute to variations in host susceptibility.47
Though intravenous catheter-associated bloodstream infections are rare in clinical practice, nocardiae promote heavy growth of biofilms, both on the surface of central venous catheter segments in vitro and in a biofilm model.14 When embedded in such a matrix, the organisms are resistant to antimicrobial drugs unless exposed to very high local concentrations, such as can be achieved with intraluminal antimicrobial lock therapy.14
Clinical Epidemiology
Members of the former “N. asteroides species complex” are responsible for about 80% of noncutaneous invasive disease and for most systemic and central nervous system (CNS) disease.17 N. farcinica is an important48 and generally more antibiotic-resistant member of this complex. There is evidence from mouse models that it may be more virulent than other Nocardia species.49 N. brasiliensis is the most often reported cause of cutaneous and lymphocutaneous disease, particularly in tropical areas. N. pseudobrasiliensis, a species now separated from N. brasiliensis, appears to be associated with disseminated, including CNS, infections.50 Pulmonary disease is the most frequent presentation of nocardiosis caused by the less common pathogens, N. transvalensis51 and N. otitidiscavarium,17 although both may cause severe cutaneous infection.52 Superficial nocardiosis following implantation is not necessarily associated with compromised cell-mediated immunity but may progress to disseminated disease in that setting.52
Immunocompromise is a well-established risk factor for nocardiosis. Nocardia species may therefore be considered as opportunistic pathogens, which cause serious and disseminated disease in settings such as organ transplantation and lymphoreticular neoplasia. The relative risk for progressive disease reflects the level of immunosuppression. A compilation of more than a thousand randomly selected cases from the literature showed that more than 60% of all reported nocardiosis cases are associated with preexisting immune compromise, ranging from alcoholism and diabetes to chronic granulomatous disease, organ transplantation, and acquired immunodeficiency syndrome (AIDS).17 In a contemporary study of nocardiosis in recipients of solid-organ transplants, significant risk factors included receipt of high-dose corticosteroids or cytomegalovirus disease within the preceding 6 months and high serum levels of calcineurin inhibitors within the preceding 30 days.12 Preexisting immunocompromise was present in more than one third of N. farcinica infections in another study.48 The use of anti–tumor necrosis factor-α (anti-TNF-α) agents has been associated with disseminated nocardial infections.53,54 Persons with pulmonary alveolar proteinosis and almost any condition requiring long-term corticosteroid use are also at risk. Although cases of nocardiosis have been described in patients with AIDS, the overall incidence is low and not fully explained by the use of sulfonamide prophylaxis against Pneumocystis jirovecii pneumonia.55
Clinical Manifestations
Superficial Infection and Mycetoma
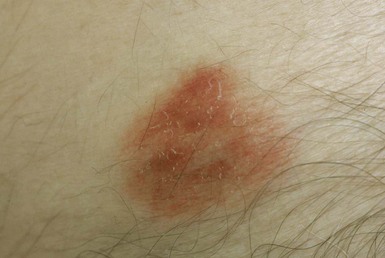
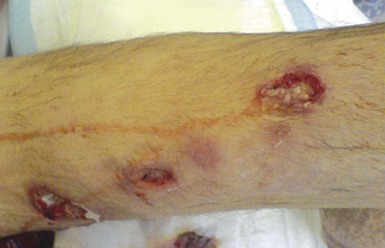
Ubiquitous in soil, nocardiae can establish superficial infection after relatively trivial inoculation injuries (Fig. 255-2), which may vary from insect and animal bites to puncture wounds and contaminated abrasions. N. brasiliensis is the most common cause of progressive cutaneous and lymphocutaneous (sporotrichoid) disease, whereas members of the former “N. asteroides complex” more commonly cause self-limited infection.11,17 Unlike other forms of nocardiosis, primary cutaneous disease usually develops in immunocompetent hosts.56 Because the initial response to Nocardia is pyogenic, self-limited skin lesions may initially be treated as staphylococcal or streptococcal in origin; however, nocardial disease is usually more indolent. In more advanced disease, a mycetoma can develop with sinus tract formation. Mycetomas are a chronically progressive, destructive disease, occurring days to months after inoculation, and are typically located distally on the limbs. Eumycetoma (of fungal etiology) and actinomycetoma (due to actinomycetes) are equally prominent in the literature, the epidemiology varying with geographic location (see Chapter 263). Overall, Streptomyces and Actinomadura species appear to be of equal or greater importance than Nocardia species among causative agents of actinomycetoma. Suppurative granulomas, progressive fibrosis and necrosis, sinus formation with destruction of adjacent structures, and macroscopically visible infective granules are regular features of nocardial mycetoma.
Pulmonary Manifestations
Pulmonary disease is the predominant clinical presentation because inhalation is the primary route of exposure (>40% of reported cases).12,17 Any species may cause lung infection, although the most common are N. cyriacigeorgica, N. nova, and N. farcinica. Pulmonary nocardiosis is usually suppurative in nature, but granulomatous or mixed responses occur. Onset of symptoms may be subacute or chronic and include one or more of productive or nonproductive cough, dyspnea, hemoptysis, and fever and other systemic symptoms. Clinical manifestations of established infection include endobronchial inflammatory masses, pneumonia, lung abscess, and cavitary disease with contiguous extension to surface and deep structures, including effusion and empyema. Radiologic manifestations include irregular nodules (usually cavitating when large), reticulonodular or diffuse pneumonic infiltrates, and pleural effusions (Fig. 255-3). The “halo sign,” considered characteristic of aspergillosis in neutropenic patients, has been described. Progressive fibrotic disease may develop in the immunocompetent host and diagnosis is often difficult. Pulmonary nocardiosis may occasionally complicate advanced human immunodeficiency virus (HIV) infection (most commonly when the CD4 count is <200/mm3); in these patients it often presents with alveolar infiltrates that progress during therapy rather than as cavitary disease.57–59 Nocardiosis should always be considered in the differential diagnosis of indolent pulmonary disease, particularly in the setting of cellular immune compromise, along with other actinomycetes (e.g., mycobacteria, Actinomyces species) and fungi (e.g., Cryptococcus neoformans, Aspergillus species). Clues to a nocardial etiology include spread to contiguous structures, especially with soft tissue swellings or external fistulas, and to the CNS. Secondary cerebral localization and clinically silent destructive infection are sufficiently common that cerebral imaging, preferably magnetic resonance imaging (MRI), should be performed in all cases of pulmonary and disseminated nocardiosis. Invasive diagnostic procedures should be considered early in the immune compromised host because disease may be rapidly progressive; in patients with severe immunodeficiency coexisting pathology with similar clinical characteristics (e.g., aspergillosis, tuberculosis, malignancy) is well documented.60
Disseminated Disease
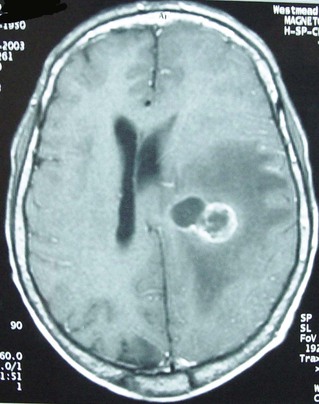
CNS involvement was recognized in more than 44% of cases of all systemic nocardiosis in one large survey.17 Cerebral nocardiosis often accompanies pulmonary disease, but isolated CNS disease is not uncommon. Indeed, up to one quarter of cases of nocardial disease (excluding mycetoma) involve the CNS. Nearly half of these affect the CNS exclusively.11,61–63 Insidious presentations are often mistaken for neoplasia because of the paucity of clinical and laboratory signs of bacterial inflammation, and silent invasion and persistence make diagnosis and management more difficult.11,17 Clinical manifestations of CNS nocardiosis usually result from local effects of granulomas or abscesses in the brain, which are commonly multiple and, less commonly, the spinal cord or meninges (Fig. 255-4). Tissue diagnosis of a cerebral mass in the setting of proven pulmonary nocardiosis is not always necessary.11 However, cerebral biopsy or aspiration should be considered early in the immunocompromised patient because of the higher incidence of serious coexisting pathology and a more aggressive course than that ascribed traditionally to cerebral nocardiosis.
Disseminated infection is characterized by widespread abscess formation. The most commonly reported sites include the CNS and eye (particularly the retina), skin and subcutaneous tissues, kidneys, joints, bone, and heart (Fig. 255-5).