Chapter 13 Nevirapine
Nevirapine (NVP) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) of HIV-1. It inhibits HIV-1 replication by binding directly to reverse transcriptase (RT) in a pocket adjacent to the catalytic site of the enzyme.1 Once NVP has bound to RT, it causes a conformational change that inactivates the enzyme, thereby preventing the polymerization of viral RNA to DNA. NVP is highly specific for HIV-1 RT and does not interfere with human DNA polymerases. NVP freely enters the cell and is active in many different cell lines, including T-lymphocytes and macrophages, well-known targets of HIV; this is in contrast to the nucleoside analogs (NRTIs), which have variable activity in different cell lines.2,3 Unlike the NRTIs, NVP does not need to be phosphorylated intracellularly to become active; as a result, drug exposure is very consistent within and between cell lines. NVP can also bind to extracellular virion RT in the plasma. This means that the amount of cell-free viral RT is decreased, leading to a reduction in the infectivity.4 NVP is a potent inhibitor of HIV-1, with a 90% inhibitory concentration (IC90) of 60 nM.5 The 90% inhibitory quotient for NVP (the concentration of free unbound drug in plasma relative to the IC90) at trough is 113. NVP resistant HIV-1 is usually resistant to the other licensed NNRTIs (efavirenz (EFV) and delavirdine). Similarly to other NNRTIs, NVP is not active against HIV-2 isolates. NVP has no cross-resistance with any of the protease inhibitors (PIs) or NRTIs, including multinucleoside-resistant strains. NVP is available both as a tablet (200 mg) and as an oral suspension (10 mg/mL).
HUMAN PHARMACOKINETICS
Distribution
NVP is lipophilic and is nonionized at physiologic pH. Following intravenous administration to healthy adults, the apparent volume of distribution (Vdss) of NVP was 1.21 ± 0.09 L/kg, suggesting that NVP is readily distributed in humans. A review by Pacifici showed that neonates have a greater volume of NVP distribution in comparison with adults.6 The t½ rates were comparable for the neonate and adult, but the tmax of 14.8 ± 8.9 h for neonates was substantially lower than 2–4 h for adults, suggesting a lower absorption rate in neonates.7 NVP readily crosses the placenta and is found in breast milk, and NVP has been detected in semen.7–10 NVP is ∼60% bound to plasma proteins in the plasma concentration range of 1–10 μg/mL. In contrast, intracellular NVP does not correlate with protein binding; concentrations are relatively low and remain constant over a 0–12 h interval.11 This lower concentration of NVP in cells can be monitored by a higher P-glycoprotein expression. Moreover, penetration of NVP into the central nervous system has been extensively documented in experimental models and in animal models. NVP concentration in human cerebrospinal fluid (CSF) is 45% (±5%) of that in plasma; this ratio is approximately equal to the fraction not bound to plasma protein.12 However, it is unknown to what extent CSF drug levels correlate to brain tissue drug levels.13 Reduced CSF viral load has been demonstrated in patients taking NVP based regimens.12
Plasma Pharmacokinetics
NVP is readily absorbed (>90%) after oral administration in healthy volunteers and in HIV-1-infected adults. Absolute bioavailability in 12 healthy adults following single dose administration was >90%, whether administered as a tablet or in oral solution.14,15 Pooled data from several studies indicate that peak plasma concentrations of 2 ± 0.4 mg/mL (7.5 mM) were reached within 4 h following a single 200 mg oral dose of NVP.16 Following multiple doses, NVP peak concentrations appear to increase linearly in the dose range of 200–400 mg/day.17–19 Steady-state trough NVP concentrations of 4.5 ± 1.9 mg/mL (or 17 ± 7 mM) were attained at 400 mg/day. These parameters were not altered substantially whether the drug was given with or without food or with alkaline buffers (such as antacids).18
It is currently recommended that NVP be administered twice a day; however, because of its long half-life at steady state (25–30 h), once-daily dosing is frequently used in clinical practice. An open-label, randomized, crossover study by van Heeswijk et al demonstrated that there was no significant difference in overall exposure to NVP (AUC) when dosed as 400 mg once daily versus 200 mg twice daily.19 The trough level of NVP when dosed qd was ∼25% lower than if dosed bid; this was still substantially above the IC50. The SCAN study found that once-daily NVP was as effective and well tolerated as twice-daily NVP in patients in the early stages of HIV infection.20 Similarly, the Atlantic study showed that a triple drug combination including two nucleosides (didanosine (ddI) and stavudine) plus NVP dosed qd, had a similar antiviral and CD4+ effect to a triple combination regimen including the same two nucleosides and a protease inhibitor (indinavir).23
Metabolism/Elimination
NVP is extensively metabolized via cytochrome P450 (CYP450) to several hydroxylated metabolites in vivo and in vitro. In vitro studies with human liver microsomes suggest that oxidative metabolism of NVP is mediated primarily by CYP450 isozymes from the CYP3A family, although other isozymes may also play a secondary role. CYP450 metabolism, glucuronide conjugation, and urinary excretion of glucuronidated metabolites represent the primary routes of NVP metabolism and elimination in humans.16,17,21
NVP has also been shown to be an inducer of CYP450 metabolic enzymes. As a result, the apparent oral clearance of NVP increases by approximately 1.5-fold to twofold from 2 to 4 weeks of dosing. Autoinduction also results in a corresponding change in the terminal-phase half-life of NVP in plasma from ∼45 h (single dose) to ∼25–30 h following multiple dosing.17,21 The pharmacokinetics of NVP in patients with renal impairment is unchanged. During renal dialysis NVP is cleared from the body and repeated dosing of NVP after dialysis should be considered; patients with moderate or severe hepatic impairment (Child–Pugh score >8 ascites) are at risk of accumulating NVP, and more importantly, NVP induced liver toxicity. It is not recommended to use NVP in this setting. If NVP needs to be used in this setting, a decreased dose should be considered.22
A study by Hong-Brown et al has also demonstrated a decrease in protein synthesis in myocytes due to the presence of NVP in these cells. This decrease in protein synthesis is correlated with a decrease in phosphorylation levels of translation initiating proteins.26
Gender/Race/Age
No substantial gender or race differences have been reported in NVP pharmacokinetics across several clinical trials. In one Phase I study involving healthy volunteers (15 women, 15 men), the weight-adjusted Vdss of NVP was higher in women (1.54 L/kg) than in men (1.38 L/kg), suggesting that NVP was distributed more extensively in women. However, this difference was offset by a slightly shorter terminal-phase half-life in women, resulting in no significant gender difference in NVP oral clearance or plasma concentrations following either single- or multiple-dose administrations.17 In adults, NVP pharmacokinetics do not change substantially with age (range 18–68 years). The apparent clearance rate of NVP in children reaches a maximum by the age of 1–2 years, and subsequently decreases over time. As a result, the recommended dose for children 2 months to 8 years of age is 4 mg/kg once daily for the first 14 days followed by 7 mg/kg twice daily thereafter, compared with 4 mg/kg once daily for the first 14 days followed by 4 mg/kg twice daily in children 8 years of age and older.
Human Dose-Ranging Studies
Daily doses of 12.5, 50, 200, and 400 mg were studied in adult patients with CD4+ T-lymphocyte counts <400 cells/mm3. Dose-proportional effects were found, with the 400 mg/day dose being superior in terms of magnitude and duration of effect.17,21 In a separate study, daily doses of 600 mg were associated with increased toxicity and no substantial gain in antiretroviral activity. The main dose-limiting toxicity observed was rash. In addition, some liver enzyme elevations and occasional somnolence were described. As a result, a dose of 400 mg daily was selected for clinical development.
TOXICITY
The most frequent drug-related adverse events reported as possibly related to NVP treatment in clinical trials include rash, fever, fatigue, headache, somnolence, nausea, liver enzyme elevations and chemical hepatitis. Rash is the most prevalent adverse event that has been attributed to NVP therapy. In four controlled, combination therapy trials with NVP, the overall incidence of rash, regardless of causal association assigned by the investigator, was 35% in the NVP group and 19% in the control group. The difference was statistically significant, for a 16% overall incidence of rash attributable to NVP.24,25,28,29 Rashes with NVP are generally mild and self-limited, and the risk of rash is greatest within the first 6 weeks of therapy. Among NVP-treated patients in four controlled clinical trials, a total of 6.5% experienced grade 3 or 4 rash as their most severe rash event, compared to 1.3% in the controls.27 Only 7% of patients in the NVP group discontinued because of rash, compared to 1% in the control groups, and most of them experienced a grade 3 or 4 rash. Based on 2861 NVP-treated patients in various clinical trials, nine patients were confirmed to have Stevens–Johnson syndrome (SJS), including two reported to have SJS/toxic epidermal necrolysis (TEN). This represents an overall SJS incidence of 0.3%. Although fatal cases have been reported in postmarketing surveillance, most cases are managed with supportive therapy, including fluid replacement and pain control. The incidence of rash in pediatric patients is no different from that in adults.27
To date, no factors have been identified that might predispose a patient to the development of rash. Also, the mechanism of NVP-induced rashes remains to be determined. Plasma concentrations of the drug do not correlate with the occurrence of rash, nor has a proven relationship been identified with gender, race, concomitant medications, rash history, or disease stage. The currently recommended NVP dosing regimen is 200 mg qd for 2 weeks, followed by 200 mg bid thereafter. This is based on retrospective analyses suggesting that this regimen is less frequently associated with rash. Nevertheless, patients need to be carefully warned about this potentially serious drug-related effect. In a NVP compassionate use program in the Netherlands, no rashes occurred in patients who had been pretreated with other antiretroviral agents and had plasma HIV-1 levels below the limit of detection.30 Table 13-1 provides a set of empiric rash management guidelines developed with input from an expert panel. These guidelines provide appropriate direction for the continued use or discontinuation of NVP in the event that a patient experiences rash. A number of protocols for rash prevention, including the use of corticosteroids, antihistamines, and slower dose escalation, have been investigated. Corticosteroids do not appear to prevent NVP-related rash, and may even cause an increase in its incidence.31,32,34 The roles of antihistamines or slower dose escalation for the prevention of NVP-related rash require further evaluation in prospective controlled trials. Whether corticosteroids or antihistamines may play a role in the treatment of NVP-related rash is also dependent on further investigations.36
Table 13-1 Guidelines for the Management of Rash
| Rash Description | Action with Nevirapine |
|---|---|
| Mild/moderate rash (may include pruritus) | Can continue dosing without interruption |
| Erythema | If rash or other suspected nevirapine toxicity occurs during lead-in, the dose should not be escalated until the rash resolves |
| Diffuse erythematous macular or maculopapular cutaneous eruption | If nevirapine is interrupted for >7 days, reintroduce with 200 mg/day lead-in |
| Urticaria | As above; however, if nevirapine is interrupted, do not reintroduce |
| Any rash with associated constitutional findings such as fever >39°C, myalgia/arthralgia, blistering, facial edema, oral lesions, general malaise, conjunctivitis, severely increased, liver function tests | Permanent discontinuation; no reintroduction |
| Severe rash | Immediate discontinuation; no reintroduction |
| Extensive erythematous or maculopapular rash or moist desquamation | |
| Angioedema | |
| Serum sickness-like reactions | |
| Stevens–Johnson syndrome | |
| Toxic epidermal necrolysis |
From Pollard RB, Robinson P, Dransfield K. Safety profile of nevirapine, a nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus infection. Clin Ther 20:1071, 1998. Copyright Excerpta Medica, Inc.
Elevations of liver enzymes, including hepatitis, have also been reported in NVP-treated patients. Isolated gamma-glutamyl transpeptidase increases are relatively frequent; however, these are of no clinical consequence and have been attributed to liver enzyme autoinduction of NVP metabolism. Drug-induced hepatitis was reported in nine of 906 (1%) patients chronically treated with NVP in controlled clinical trials.27 A similar result was obtained in the BI 1090 trial.35 This was a randomized, placebo-controlled study comparing 1121 patients taking NVP plus two NRTIs with 1128 patients taking placebo plus two NRTIs over 2 years. Using an expanded hepatitis definition (including viral hepatitis), 2.8% of patients in the NVP group and 1.4% in the placebo group had hepatitis over the course of the study (P = 0.026). No significant difference in the incidence of serious hepatic events was observed between the two groups. The overall rate of withdrawal as a result of hepatitis in adult NVP trials has been 0.8%, but a limited number of fatal hepatitis cases associated with NVP use have been reported. Data which have aroused some concern have been reported from the blinded FTC-302 study,37 a phase III study comparing the NRTIs emtricitabine and lamivudine, in which all patients also received stavudine and were stratified to receive either NVP or EFV, according to their baseline viral load. A total of 87% of the study subjects were black, and 59% were women. There was a high incidence of grade 3 and 4 hepatotoxicity, affecting 58 (15%) of 468 subjects, all of whom were receiving NVP, and two cases were fatal. The vast majority of cases occurred within the first 8 weeks of therapy, with a temporal association with the dose escalation of NVP from 200 to 400 mg at day 14 of treatment. A statistically significant twofold greater incidence of hepatotoxicity was noted in women. The gender-adjusted incidence of hepatotoxicity was ∼11%, not dissimilar to that reported in the NVP package insert.38 Thus current recommendations advise against initiating NVP in women with CD4+ T-lymphocyte counts >250 cells/mm3 or men with CD4+ T-lymphocyte counts >400 cells/mm3 to reduce the effect of adverse hepatic events.39 Furthermore, hepatotoxicity increases with longer therapy duration and is associated with polymorphisms in the MDRI gene for the drug pump, P-glycoprotein.40,41
While further study is required to identify risk factors that predispose patients to develop hepatitis, it has been suggested that patients with hepatitis C co-infection may be at higher risk for developing clinical hepatitis when receiving NVP based therapy.41,42 In turn, HBV and HCV co-infection are associated with a higher NVP trough concentration.44 In a recent analysis of a clinical cohort (Athena study), covering ∼70% of Dutch patients receiving HAART, independent risk factors associated with development of grade IV transaminase elevations were: higher baseline ALT levels (hazard ratio (HR) 1.06 per 10 units increase), chronic hepatitis B virus infection (HR 9.3), chronic hepatitis C virus infection (HR 5.2), recent start of NVP (HR 8.5) or ritonavir (HR 3.7), and female gender (HR 2.6). Furthermore, in patients chronically co-infected with hepatitis B virus, discontinuing the use of lamivudine (3TC) was associated with the development of grade 4 liver enzyme elevations (HR 4.8).42
To date, no previously unrecognized adverse events have been identified when using NVP in combination with different antiretrovirals, including NRTIs and PIs. Furthermore, the rate of known adverse events has not increased when NVP was used in combination with these agents. Importantly, untoward lipid abnormalities, including hyperlipidemia and lipodystrophy, have not been associated with NVP. Indeed, it has been suggested that lipid abnormalities at least partially revert following a switch from a PI to NVP.46 In the Atlantic study, NVP-containing HAART resulted in an antiatherogenic lipid profile, with a striking increase in high-density lipoprotein (HDL) cholesterol (49%) and a significant reduction in the total over HDL cholesterol ratio (14%).43 Recently several cases were reported where severe liver toxicity developed when NVP was given as part of a postexposure prophylaxis regimen. Although a number of potential confounders were noted in each case, it is at least likely that NVP may have contributed to the development of this serious toxicity. Until this issue is further clarified, NVP use in this setting is not recommended.41,42,45
CLINICAL TRIALS
Treatment-Naive Patients
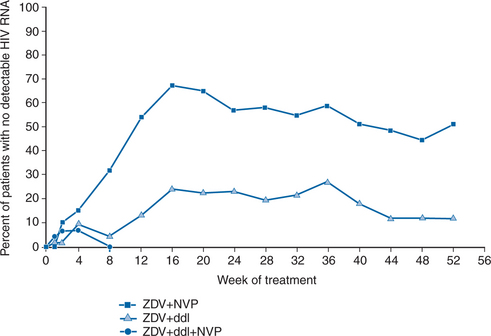
The INCAS trial was among the first to illustrate that triple combination therapy was required to achieve a durable treatment response.29 This study examined the activity, safety, and tolerance of three regimens (NVP + ZDV + ddI, NVP + ZDV, and ZDV + ddI) in 151 antiretroviral-naive HIV-1-infected patients with 200–600 CD4+ cells/mm3. At baseline, mean plasma HIV RNA was 4.41 log10 copies/mL and mean CD4+ T-lymphocyte count was 374 cells/mm3. The mean maximum viral load decrease from baseline for patients in the triple arm of the study was 2.94 log10 copies/mL. As shown in Figure 13-1, NVP + ZDV was associated with substantial but transient effects on virologic markers. However, NVP + ZDV + ddI was consistently superior to ZDV + ddI, providing a durable response in both virologic and immunologic markers, with 51% maintaining viral loads <20 copies/mL at 52 weeks. Raboud et al demonstrated that patients who achieve HIV-1 RNA levels <20 copies/mL were able to maintain their antiretroviral response.47 Indeed, long-term follow-up of patients from the INCAS study demonstrated that viral suppression could be sustained with NVP-based triple-combination therapy for up to 4 years.51 The INCAS study also demonstrated the need for high levels of adherence to the therapeutic regimen to achieve a sustained response. Adherence to the triple therapy regimen was associated with decreased recoverable virus and lessened the likelihood of developing resistance, whereas nonadherence was associated with virologic failure. Although the study was not designed to evaluate clinical events, fewer NVP + ZDV + ddI-treated patients (12%) had HIV progression events, compared with patients who took ZDV + ddI (25%) or NVP + ZDV (23%).29
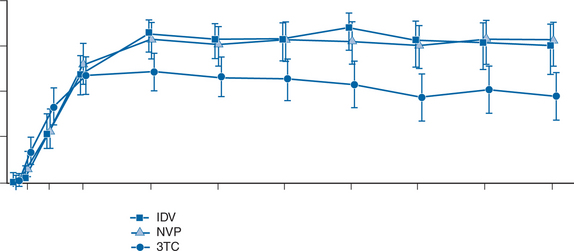
The INCAS trial was the first to demonstrate the powerful potential of NVP. A cross-study evaluation, using an intent-to-treat analysis, showed that the results of the INCAS trial were consistent with those of similar triple combination regimens including PIs or the NNRTI, EFV. Two recent studies have further revealed the efficacy of NVP relative to PIs in comparative trials.23,48 The Atlantic study, an international, multicenter, open-label, randomized trial, compared NVP + d4T + ddI with indinavir (IDV) + d4T + ddI and 3TC + d4T + ddI in antiretroviral-naive patients. This trial enrolled 298 patients with median baseline viral loads of 4.25 log10 copies/mL, and baseline CD4+ T-lymphocyte counts of 406 cells/mm3. After 48 weeks, 58.4%, 57%, and 58.7% of patients in the NVP, IDV and 3TC-arms had <500 plasma HIV-1 RNA copies/mL in an intent-to-treat analysis (Not significant). After 96 weeks, these figures were 59.6%, 50.0% and 45.0%, respectively (Not significant). Looking at plasma HIV-1 RNA <50 copies/mL, figures (% undetectable) at 96 weeks in the intent-to-treat analysis were 81.8% for NVP, 79.0% for IDV and 50.9% for 3TC; the 3TC arm being inferior to the other arms (P = 0.001). The Atlantic trial showed comparable efficacy for a triple combination including NVP or a PI (IDV) (Fig. 13-2)
More recently, preliminary results from the COMBINE study have been presented. This open-label randomized study directly compared a simple twice-daily regimen of NVP + Combivir to twice-daily nelfinavir + Combivir.48 The trial enrolled 142 HIV-infected, antiretroviral therapy-naive patients. The median baseline viral load was 59 698 copies/ml in the NVP + Combivir arm and 65 806 copies/mL in the nelfinavir + Combivir arm. The median baseline CD4+ T-lymphocyte count was also similar in the two arms, with 361 cells/mm3 and 351 cells/mm3 in the NVP- and nelfinavir-containing arms, respectively. After 9 months, there was no significant difference in outcome between the two arms, with 84% in the NVP + Combivir arm and 78% in the nelfinavir + Combivir arm having viral loads less than 200 copies/mL. The results remain consistent whether an on-treatment or intent-to-treat approach was used. However, using the more sensitive viral load assay with a limit of detection of 20 copies/mL, 80% of patients in the NVP arm had undetectable viral loads, significantly more than the 56% who achieved this level in the nelfinavir arm (P = 0.02). The difference between the NVP and nelfinavir arms may be explained in part, by greater adherence in the NVP arm. In summary, these two studies demonstrate that NVP-based triple-drug therapy is at least as effective as PI-based therapy in treatment-naive patients.
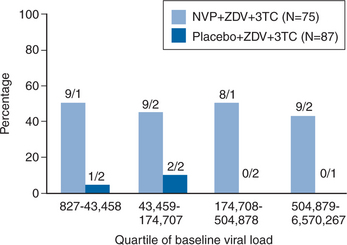
A substudy of the 1090 trial focused on the treatment response among treatment-naive patients with very high plasma viral load and very low baseline CD4+ T-lymphocyte count.55 Patients were randomized to take NVP + ZDV + 3TC or placebo + ZDV + 3TC. 77 patients in the NVP + ZDV + 3TC arm had a mean baseline viral load of 138 986 copies/mL and a mean baseline CD4+ T-lymphocyte count of 101 cells/mm3. There were 94 patients in the placebo arm, which had a mean baseline viral load of 146 332 copies/mL and a mean baseline CD4+ T-lymphocyte count of 93 cells/mm3. Figure 13-3 demonstrates that, although response in the placebo arm of this study was very limited at 48 weeks in all viral load strata, NVP + ZDV + 3TC was as effective in patients with viral loads >500 000 copies/mL as in those with lower plasma viral load levels. Overall, 45% of patients in the NVP group had viral loads <50 copies/mL at 48 weeks using an intent-to-treat analysis, comparable to that observed in the INCAS and Atlantic trials thereby confirming similar efficacy of NVP even in patients with advanced disease.49 Of note, patients with very low CD4+ T-lymphocyte counts at baseline (<38 cells/mm3) had a very poor response to therapy compared with those with higher CD4+ T-lymphocyte counts. Overall, these results suggest that in this particular group of patients (with very high baseline plasma viral load and very low baseline CD4+ T-lymphocyte count) the antiviral effect of the regimen was dependent on the baseline CD4+ count but independent of plasma viral load.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree