Neurologic Syndromes
General
Acute infection of the central nervous system (CNS) is the most likely cause of a febrile illness with manifestations of CNS involvement (Table 9-1). Stiff neck or crying when handled suggests meningeal irritation. Bulging fontanel, headache, or vomiting suggests increased intracranial pressure. Papilledema is unusual in any of the acute neurologic infections but should be excluded before doing a lumbar puncture. If severe papilledema is present, a more chronic process may be involved.
A change in consciousness, such as confusion or disorientation, is an alarming sign that suggests a disturbance of cerebral cortical function that may have many causes, including cerebral anoxia, inflammation, or edema. Any of the findings in Table 9-1 should be regarded as suggesting a medical emergency until further evaluated.
Classification
Purulent Meningitis
Purulent meningitis is best defined by a cerebrospinal fluid (CSF) that is cloudy and contains more than 1000 neutrophils/mcL. Whether or not a bacterial etiology is proven by culture, purulent meningitis is almost always bacterial. When the term “meningitis” is not further modified, it usually means purulent meningitis (Table 9-2).
Nonpurulent Meningitis
A CSF leukocyte count of 10–500/mcL, usually predominantly lymphocytes, can be defined as nonpurulent meningitis and this usually indicates a nonbacterial process (aseptic meningitis syndrome), but not always. Patients with CSF cell counts in the intermediate range (500–1000/mcL) can usually be classified as having presumed bacterial meningitis or aseptic meningitis syndrome on the basis of the cell count and differential, glucose, protein, Gram stain, and state of consciousness. Definitions of aseptic meningitis syndrome are discussed further in that section.
Acute Encephalitis
Acute encephalitis is defined in this book as a severe and nontransient disturbance of consciousness with a CSF cell count like that of nonpurulent meningitis. Fever is usually present. Ordinarily, the number of leukocytes is less than 300 but sometimes exceeds 1000 per mcL. A disturbance of consciousness should be considered nontransient if it persists for more than 8 hours and should be distinguished from febrile delirium, which occurs only at the time of a high fever.
Acute Encephalopathy
In this book, the acute onset of severe and nontransient disturbance of consciousness and a normal CSF white cell count (fewer than 10/mcL) is defined as acute encephalopathy. Other manifestations of brain disease, such as convulsions and abnormal focal neurologic signs, are variably present. Fever is often absent. Otherwise, encephalopathy has the same clinical pattern as encephalitis except for a normal CSF white cell count. This distinction between encephalitis and encephalopathy is a useful one, as the causes of encephalitis are usually infectious or postinfectious, whereas the causes of encephalopathy are usually toxic, metabolic, or vascular. Encephalitis and encephalopathy are discussed in detail later in this chapter.
This classification is not perfect, and there is definite overlapping, but it is a helpful preliminary classification that has remained useful with years of use.1 It is also useful to search in both the encephalitis and encephalopathy sections for answers.
Rarely, we have used two preliminary problem-oriented diagnoses such as purulent meningitis or encephalitis (in a case of La Crosse encephalitis) or acute encephalitis or encephalopathy (in a case when the cause was never found). The term
“meningoencephalitis” is used too often when the patient can be classified as having nonpurulent meningitis or acute encephalitis, using the state of consciousness to distinguish them.
“meningoencephalitis” is used too often when the patient can be classified as having nonpurulent meningitis or acute encephalitis, using the state of consciousness to distinguish them.
TABLE 9-1. MANIFESTATIONS OF CENTRAL NERVOUS SYSTEM INFECTIONS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Other CNS Syndromes
Other syndromes associated with paralysis, ataxia, tetanus-like rigidity, or ventriculitis are discussed in later sections. The relative frequency of these syndromes in hospital admissions depends on the age of the child, on the season, and on whether a lumbar puncture is done before admission. In community hospitals, aseptic meningitis is more frequent than purulent meningitis, especially in the summer and fall months. Referral hospitals have more admissions for purulent meningitis than for aseptic meningitis, probably because such clinically severe illness often requires referral. All of these various syndromes occur most frequently in young infants.
TABLE 9-2. CLASSIFICATION OF MAJOR INFECTIOUS NEUROLOGIC SYNDROMES | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||
Differential Diagnosis
Meningismus
“Meningismus” is a term best used to describe a stiff neck secondary to local or reflex irritation, which may occur from streptococcal pharyngitis or pneumonia. Rheumatoid arthritis and tetanus also may be associated with nuchal rigidity and normal spinal fluid. This diagnosis should not be made unless the spinal fluid is normal.2,3 The term “meningismus” is sometimes used as a synonym for “nuchal rigidity” or “stiff neck,” a usage that should be avoided, as it spoils its meaning of “stiff neck with normal spinal fluid,” which is a cumbersome substitute for “meningismus.”
Retropharyngeal Abscess
Deep cervical adenitis and retropharyngeal abscess may produce meningismus and are discussed in Chapter 6.
Pseudotumor Cerebri
An increased pressure, manifested by a bulging fontanel or papilledema, with infection, tumor, sinus thrombosis, and obstruction of the ventricular system specifically excluded, is defined as benign intracranial hypertension or pseudotumor cerebri. Tetracycline is a possible cause. A bulging fontanel may also be produced by early congestive heart failure.
Lumbar Puncture
Indications and Risks
When meningitis is suspected, lumbar puncture is an emergency procedure. Lumbar puncture (LP) is relatively simple in children and should be done when bacterial meningitis is suspected, because the risk of undiagnosed or inadequately treated meningitis is significant. If the results are normal, but the child’s clinical condition is worsening and still suggests meningitis, then the tap should be repeated.4 Clinical suspicion should not be ignored; we have seen bacterial meningitis (confirmed by culture) in patients with a classical presentation but normal initial CSF profile.
Before lumbar puncture is done, the optic fundi should be examined to exclude papilledema. Papilledema takes time to develop, and its absence does not rule out elevated intracranial pressure (ICP). Clinical features that suggest elevated ICP include focal neurologic signs, postural or respiratory abnormalities, absent oculocephlic (doll’s eyes) reflexes, dilated or unequal pupils, ophthalmoplegia, protracted seizures, or severe obtundation or coma (Glasgow Coma Scale less than 8).5,6 Patients with these signs or symptoms should receive an emergency computed tomography (CT) scan prior to lumbar puncture in order to minimize the risk of cerebral herniation. Unfortunately, even a normal CT scan is not entirely protective; several cases of children who experienced herniation following lumbar puncture despite a normal CT scan have been reported.6,7 Spinal subdural hematomas8 and even intracranial hematomas have been reported,9 but these complications are extremely rare. Overall the procedure is safe. Although the decision regarding performance of a lumbar puncture should be made thoughtfully, in the vast majority of patients the benefits of early lumbar puncture far outweigh the small risks. The practice of routinely ordering head CT scans prior to lumbar puncture has no support in the literature and may cause unnecessary delay in institution of treatment.
Several diseases may require special caution or delay in doing a lumbar puncture. Reye syndrome is a disease in which lumbar puncture should sometimes not be done because of increased pressure.4 Papilledema is usually not present, but cerebral edema may be extreme. An elevated serum transaminase concentration and elevated blood ammonia is helpful in making the diagnosis if the clinical findings are compatible, as described in the section on acute encephalopathy. Fortunately, Reye syndrome is now quite rare. Children with posterior fossa tumors may present with fever and nuchal rigidity, but a careful history will usually indicate that the illness began several days or even weeks earlier. Suspicion of a brain abscess may be a reason for the physician to postpone a lumbar puncture if a brain CT scan can be obtained on an emergency basis. A cautious lumbar puncture 30 minutes after a mannitol infusion, as described later, with withdrawal of less than 1 mL of fluid, is probably the best way to deal with this dilemma when increased pressure is suspected but cannot be evaluated.
Patients with hemophilia A (or B) may safely undergo the procedure if they are given infusions of factor VIII (or factor IX) prior to beginning; no complication occurred in a series of 58 patients managed in this way.10
Technique
Use of a scalp vein needle without a stylet appears to be simpler for newborns. However, the possibility of the needle cutting a core of skin and injecting the dermal cells into the lumbar space has been suggested as a cause of epidermoid CNS tumors. Therefore, this method is not recommended. An alternative method, in which the stylet is removed after the needle has been advanced through the skin and subcutaneous tissues, has been suggested as a way to minimize the number of traumatic lumbar punctures without increasing the risk of spinal epidermoid tumors, but this hypothesis has not been proved. Lumbar puncture in newborn infants requires special caution. Studies have indicated the newborn should not be positioned with the neck flexed lying in the lateral position, and the sitting position is probably preferable to avoid respiratory deterioration.11,12
The measurement of pressure is not indicated when acute infection is suspected, and attempts to measure pressure with a manometer may result in a bloody tap. Pressure can be estimated by counting
the number of drops over a specified period of time. At patient temperatures lower than 40°C, using a 22-gauge 1.5 inch needle, the number of drops of CSF delivered in 21 seconds is an estimate of CSF pressure in cm of H2O. For a 22-gauge, 3.5 inch needle, the counting time is 39 seconds, while for a 20 gauge, 3.5 inch needle, the counting time is 12 seconds. If the patient’s temperature is higher than 40°C, the counting periods are shorter: 20, 37, and 11 seconds, respectively. These estimates are accurate if the patient is calm and in the lateral recumbent position.13
the number of drops over a specified period of time. At patient temperatures lower than 40°C, using a 22-gauge 1.5 inch needle, the number of drops of CSF delivered in 21 seconds is an estimate of CSF pressure in cm of H2O. For a 22-gauge, 3.5 inch needle, the counting time is 39 seconds, while for a 20 gauge, 3.5 inch needle, the counting time is 12 seconds. If the patient’s temperature is higher than 40°C, the counting periods are shorter: 20, 37, and 11 seconds, respectively. These estimates are accurate if the patient is calm and in the lateral recumbent position.13
In larger children, measurement of the opening pressure can be more easily obtained without compromising the integrity of the LP, and should be done.
If the fluid appears cloudy or purulent, an immediate rapid intravenous infusion or injection of a third-generation cephalosporin, followed by vancomycin infusion, is indicated without delay, as described later in the section on emergency treatment of meningitis.
Cell Count
A Wright stain of a smear should be examined under oil for an accurate differential count. When the tap has been traumatic, the presence of red blood cells suggests that some of the white blood cells have come from the peripheral blood. Various formulas based on experimental studies have been used to try to calculate the effect of contamination of CSF by peripheral blood, but the calculations are complicated by the decreased survival time of white blood cells (WBC), especially neutrophils.14,15
Contamination with more than 100,000 red cells/mcL occasionally can obscure the recognition of purulent bacterial meningitis, and the use of peripheral white to red blood cell ratios to interpret the CSF often underestimates the CSF white count.16,17 Many authors who have studied this problem suggest “corrected white blood cell counts of blood-contaminated fluids (should) be viewed with skepticism and not be given undue weight in clinical decisions.”17 However, a review of 92 children who had a traumatic LP, 30 of whom had bacterial meningitis, showed that all patients with bacterial meningitis had an observed to predicted ratio of WBC greater than 1. In fact, 28 (93%) of the 30 had a ratio greater than 10, and 24 (80%) had ratios greater than 100.18 Additionally, a predominance of neutrophils in the CSF (97% vs. 11%), hypoglycorrhachia, (73% vs. 3%) and positive Gram stains (80% vs. zero) were found more commonly in the patients with meningitis.
It is sometimes important to differentiate a traumatic tap from subarachnoid hemorrhage. Blood contamination of the CSF tends to decrease from the first tube to the last, and often produces a difference that can be seen by the naked eye. Additionally, centrifugation of the fluid usually produces a clear supernatant in traumatic tap, whereas xanthochromia persists in cases of subarachnoid hemorrhage. The best test for differentiating a traumatic tap from subarachnoid hemorrhage is the D-dimer assay; D-dimer assay is negative in traumatic tap.19 The CSF protein is also frequently elevated beyond that expected by calculations in the cleared tube of a “bloody tap.”
A study of the spinal fluid of 108 term neonates in whom infection was very carefully excluded showed a mean of 7.3 WBC/mcL, with a median of 4 and a range of 0 – 130.20 The patient with 130 WBC was clearly an outlier, however. Older studies defined normal as a maximum of 7/mcL with as many as 4 of these being polymorphonuclear cells.21 After 6 weeks of age, the maximum count in normal CSF can be taken as 5, of which 2 can be “polys.”21 In an earlier study, in the first week of life, normal term infants had a maximum of 32 leukocytes with a mean of 8/mcL.22 Preterm infants had a maximum of 29 leukocytes, with a mean of 9/mcL.
Glucose and Protein
In general, glucose and protein values should be determined whenever spinal fluid is obtained. The importance of these values and the mechanisms involved in creating abnormal ones are discussed in the sections on purulent meningitis and nonpurulent meningitis. The CSF glucose can be considered abnormal when it is less than 40 mg/dL or less than 40% of the blood glucose.23
Smears
Centrifugation of the spinal fluid before microscopic examination may be helpful, but it is usually not practical when small amounts of fluid have been obtained. One drop of sediment from the centrifuged CSF should be allowed to dry on each of two slides and fixed by brief gentle flaming. One specimen is Gram stained and examined for bacteria.
The other is stained with Wright stain for a differential count if necessary.
The other is stained with Wright stain for a differential count if necessary.
A Gram stain should be done on all spinal fluid specimens with an increased number of white blood cells. Bacteria are most likely to be observed in purulent fluid and are rarely seen in CSF with low leukocyte counts. The exceptions include some cases of neonatal meningitis, where there may be a poor leukocyte response, with only a few hundred leukocytes per mcL, yet many bacteria. Early infection with meningococci or pneumococci occasionally produces a positive Gram stain of the CSF, confirmed by culture, before any remarkable CSF pleocytosis occurs.24 The ability to detect bacteria on the Gram stain depends on their CSF concentration, which is correlated with the concentration of bacteria in the blood.25
Contamination of tubes or other equipment is rare. In such cases, a variety of stained bacteria are typically seen.
CSF Culture
Although the main purpose is culture of the spinal fluid for bacteria, an extra tube can be held in the laboratory until the cell count, the glucose, and the protein are determined, in case this information suggests the need for further studies such as culture for viruses or tuberculosis (TB).
The microbiology laboratory may delay reporting the species obtained from the spinal fluid until all the metabolic studies are complete. However, the physician can make clinical judgments sooner and can presume, for example, that a pure culture of a gram-negative diplococcus is going to be meningococcus, even though definitive identification may require several days.
The frequency of various bacterial contaminants of CSF cultures has been studied.26 Staphylococcus epidermidis and diphtheroids are the most common contaminants, but in special circumstances (especially CSF shunts) they can be pathogens.
Positive CSF Culture with Minimal CSF Abnormalities (Seeded Meningitis)
Sometimes, the physician finds a positive CSF culture with minimal other abnormalities. This problem was the subject of a short clinical report, but most large series of patients with meningitis include a few examples.24 Bacteremia is usually found if a blood culture has been taken and involves the organism recovered on culture of the CSF, although the cell count, Gram stain glucose, and protein are normal. Usually, patients with these findings are very sick and are suspected of having a bacteremia of unknown source. Typically, the patient is hospitalized and treated for sepsis and often gets a second lumbar puncture 12–36 hours later that reveals purulent meningitis.27 Occasionally, this pattern is observed early in endocarditis (Chapter 18) or in bacteremia in an outpatient (Chapter 10).
The working diagnosis for severely ill patients should be “probable sepsis” until objective evidence of CSF abnormalities or a positive CSF culture is found. The diagnosis of meningitis should generally not be made with normal CSF findings, but a word is needed to describe a positive CSF culture in this situation. Bacteria in the CSF can be called “bacterrhachia,” analogous to “bacteremia” and “hypoglycorrhachia.” Bacterrhachia without CSF pleocytosis is further discussed later.
The pattern of “seeding” of the CSF during bacteremia without any other CSF abnormalities is typically not associated with the complications of purulent bacterial meningitis. The prognosis depends rather on the disease causing the bacteremia.
Antigen Detection
Various methods have been developed to detect bacterial antigens in the CSF.28 It was hoped that these tests would be positive when prior antibiotics prevented a positive culture. In reality, these tests for bacterial antigens (latex agglutination, enzyme-linked immunosorbent assay [ELISA], or other) rarely help and should not be ordered routinely. Antigen detection studies can be ordered when the patient has received previous antibiotics but can be delayed until the Gram stained smear is found negative by a qualified technologist.
Other CSF Tests
Tests for bacterial endotoxin (Limulus lysate test), bacterial enzymes (transaminase, lactic acid dehydrogenase), bacterial products (lactic acid), and acute-phase reactants (C-reactive protein), among other things, have been studied in an attempt to aid in the differentiation of viral meningitis from partially-treated bacterial meningitis. Many of these substances are elevated in the CSF, but either the sensitivity and specificity are not sufficient to aid in diagnosis or the test is reflected in other, simpler
tests. For example, lactate levels increase linearly with lactate-producing cells; therefore, they correlate with leukocytosis,29 which is a lab value the clinician already has at hand. In adults, neither CSF lactate nor CSF C-reactive protein, nor a combination of the two yields anything better than 60% positive predictive value for bacterial meningitis.30 The enthusiasm for such tests has been based on their good correlation with clear-cut cases of bacterial meningitis or normals. However, the results are typically equivocal in patients with negative cultures and an equivocal conventional CSF glucose, protein, and cell count.
tests. For example, lactate levels increase linearly with lactate-producing cells; therefore, they correlate with leukocytosis,29 which is a lab value the clinician already has at hand. In adults, neither CSF lactate nor CSF C-reactive protein, nor a combination of the two yields anything better than 60% positive predictive value for bacterial meningitis.30 The enthusiasm for such tests has been based on their good correlation with clear-cut cases of bacterial meningitis or normals. However, the results are typically equivocal in patients with negative cultures and an equivocal conventional CSF glucose, protein, and cell count.
As yet, no test has been shown to correlate better with cultures than the combination of protein, glucose, and white cell count with a differential study.31,32 If available, enteroviral polymerase chain reaction (PCR) on CSF can be helpful in distinguishing viral meningitis from bacterial meningitis obscured by previous oral antibiotic therapy.33 This test is particularly cost-effective during enteroviral season (summer and fall).34
Serum Tests
Serum tests have actually fared much better than CSF tests in navigating the muddy waters. In one large study of 325 children with bacterial meningitis and 182 with proven or suspected viral meningitis, the serum C-reactive protein level (CRP) averaged 11.5 mg/dL in those with bacterial disease versus less than 20 mg/dL in those with viral CNS infections. Serum CRP was the only test that reliably discriminated gram-stain negative bacterial meningitis from meningitis of viral etiology; a serum CRP of less than 2.0 mg/dL had a negative predictive value of 99% for bacterial meningitis.35 In another study, 18 children with bacterial meningitis had a mean serum procalcitonin value of 54.5 mg/L, whereas 41 children with viral meningitis had a mean value of 0.32 mg/L. The highest value found in a child with viral meningitis was 1.7 mg/L, and the lowest found in a child with a positive CSF culture was 4.8 mg/L; thus, there was no overlap between the two groups.36 Although the preliminary data look fairly promising, these laboratory tests have not yet become commonplace in clinical practice.
Fever and Convulsions
Definitions
Several diagnostic phrases are used to describe a variety of clinical situations with fever and convulsions.37 “Fever and convulsions” is the most neutral expression and therefore the best syndrome diagnosis when no etiologic diagnosis is yet possible. “Seizures precipitated by fever” is an etiologic diagnosis indicating that the patient is known to have a convulsive disorder and now has had a convulsion precipitated by fever. “Simple febrile convulsion” is best regarded as an etiologic diagnosis that should be based on exclusion of many other possibilities. In a child with a first convulsion with fever, the following criteria should be present for the etiologic diagnosis of simple febrile convulsion:
Fever at the time of the convulsion.
Brief generalized (nonfocal) seizure, usually lasting less than 5 minutes and not longer than 15 or 20 minutes, in a child 6 months to 5 years of age. No recurrence of seizure within the first 24 hours.
Prompt recovery to normal state of consciousness without definite neurologic abnormalities, such as paralysis or weakness. If the state of consciousness does not return to normal within about 30 minutes after the convulsion, the patient should be considered to have an acute encephalopathy or a CNS infection until proven otherwise.
Family history of febrile convulsions or past convulsion with fever supports the diagnosis of simple febrile seizure but is not in itself sufficient to establish the diagnosis.
Exclusion of increased intracranial pressure by examination of the optic fundi.
Exclusion of CNS infections such as meningitis or encephalitis, when lumbar puncture is indicated.
Exclusion of metabolic causes of convulsions, such as hypoglycemia, hypocalcemia, or hyponatremia, when indicated.
Normal developmental history.
The principal advantage of the use of the diagnosis of simple febrile seizure is that it avoids the term “epilepsy,” which is often associated with much misunderstanding and fear among laypersons. The major disadvantage is that the improper use of this diagnosis may lull the physician into symptomatic therapy without searching for treatable, and sometimes urgent, specific causes.
Emergency Management
A quick history should be obtained to look for recent head injury (in which case sedation may be contraindicated)
and current or recent medications such as anticonvulsants or toxin or poison ingestion. A quick physical examination should be done to check for evidence of head injury and to clear the airway and position the child with the head turned to avoid aspiration. Fever reduction by pharmacologic means should be begun immediately if the temperature is above 40°C (104°F). Oxygen may be indicated if the patient is cyanotic, and the airway should be checked to be sure it is clear.
and current or recent medications such as anticonvulsants or toxin or poison ingestion. A quick physical examination should be done to check for evidence of head injury and to clear the airway and position the child with the head turned to avoid aspiration. Fever reduction by pharmacologic means should be begun immediately if the temperature is above 40°C (104°F). Oxygen may be indicated if the patient is cyanotic, and the airway should be checked to be sure it is clear.
Anticonvulsant drugs should be given to stop the convulsion if it has not already stopped. A short-acting anticonvulsant such as lorazepam is given in a dose of 0.05–0.10 mg/kg. This dose can be repeated if the seizure persists. On a rare occasion, phenytoin (or fosphenytoin) at a dose of 15–20 mg/kg will be required. This drug should be administered slowly to avoid hypotension and cardiac rhythm problems.
Phenobarbital can also be used, at an intravenous dose of 10–20 mg/kg (loading dose) to stop prolonged seizures.38 If the patient has intermittent seizures but has stopped convulsing before any medications are given, 5 mg/kg can be given instead of 10 mg/kg to prevent further seizures.39
For stopping a prolonged seizure (defined here as longer than 15 or 20 minutes), intravenous lorazepam, phenobarbital, or phenytoin is usually recommended.38,39,40,41,42,43 These drugs all have potential dangers. All primary care physicians and emergency rooms should have a written plan or protocol readily available for the control of prolonged seizures.
Possible Etiologies
CNS Infection
Between 15–25% of all patients with meningitis will have seizure with fever either at the time of presentation or sometime during the course of the disease. Patients with meningitis almost always have symptoms other than seizure that suggest the diagnosis. Meningitis or encephalitis should always be excluded by examination of the spinal fluid if there is any question of the state of consciousness or meningeal irrigation.
Non-CNS Infection
Infection not involving the CNS with seizure precipitated by fever or toxins, such as shigellosis, pneumococcal bacteremia, or infection with human herpesvirus type 6 (HHV-6) is another category of causes. There was a lot of enthusiasm for HHV-6 as a cause of febrile seizures after it was discovered that one third of patients up to the age of two years presenting to the emergency department with the clinical syndrome of simple febrile seizure had HHV-6 infection.44
However, a subsequent case-control study found evidence of acute HHV-6 infection in 15 (43%) of 35 patients with febrile seizures and in 15 (45%) of 33 controls.45 The conclusion of these authors was that HHV-6 infection is not a major factor in the pathogenesis of febrile seizures. A more plausible interpretation might be that HHV-6 infection is a frequent cause of high fever in the age group at risk for febrile seizures, and that some children are predisposed to the development of seizures with fever.
Toxic and Metabolic Causes
Convulsion secondary to a specific cause such as lead encephalopathy or hypoglycemia can accompany fever caused by an infection and need to be excluded if suggestive clinical findings are present.
Seizure Disorder
Idiopathic convulsive disorder (“epilepsy”) with seizure precipitated by fever is the proper diagnosis if there is an abnormal electroencephalogram (EEG) obtained at least a week after the seizure or if convulsions also occur without fever.
Simple Febrile Convulsion
This is by far the most common cause of seizure with fever, occurring in approximately 4% of children between the ages of 6 months and 5 years. Simple febrile seizures are a benign condition whose main complication is recurrence, which happens in about one-third of patients. Recurrence risk is difficult to predict, but seems to be higher in children who present at a younger age.46 The diagnosis of simple febrile seizure is fairly straightforward in older toddlers who have a classic history and physical examination, but it is more difficult in younger patients.
Diagnostic Approach
Lumbar Puncture
There has been much discussion about the need for lumbar puncture in the evaluation of seizure and
fever; clearly, the yield is low in cases where the diagnosis of simple febrile seizure is clinically suggested. However, meningitis can present with seizure and fever, and in young children, other signs of meningitis may be subtle. Because of this, the American Academy of Pediatrics (AAP) practice parameter recommends that excluding CNS infection by lumbar puncture be strongly considered in children younger than 12 months, and considered for children between 12 and 18 months of age.47
fever; clearly, the yield is low in cases where the diagnosis of simple febrile seizure is clinically suggested. However, meningitis can present with seizure and fever, and in young children, other signs of meningitis may be subtle. Because of this, the American Academy of Pediatrics (AAP) practice parameter recommends that excluding CNS infection by lumbar puncture be strongly considered in children younger than 12 months, and considered for children between 12 and 18 months of age.47
A recent review of 503 cases of meningitis (97% of which were proven or suspected to be bacterial) showed that 115 presented with seizures (23%). Of these patients, 105 (91%) were obtunded or comatose, and thus obvious candidates for lumbar puncture. Of the other 10, 6 had nuchal rigidity, 1 had prolonged focal seizures, and 1 had multiple seizures and a petechial rash, all independent reasons to obtain spinal fluid by lumbar puncture. Two were suspected, on clinical grounds, of having viral meningitis.48
In an older review of 152 children with purulent meningitis, 27 (18%) had fever and seizures.49 Of these 27 children, 11 (41%) had no recorded meningeal irritation, no change of consciousness, and no bulging fontanelle; all of these children were less than 18 months of age. It is often stated that clinicians with experience can exclude meningitis on clinical grounds; however, science supporting this statement is absent. In fact, one of the authors of this last study argued that after years of experience it is still difficult to exclude meningitis in young children on clinical grounds alone, and he would do a lumbar puncture on all children under 16 months of age who present with fever and a convulsion.50
In a retrospective review of 709 outpatients undergoing lumbar puncture, 225 (32%) had fever and a convulsion as the reason for the puncture.51 Only five had abnormal CSF, and most of these also had signs of meningeal irritation. A prospective emergency department study that allowed physicians to decide which patients required lumbar puncture (and thus biased the results toward finding a higher percentage of children with meningitis) found a total of 7 cases of meningitis (3 bacterial) in 102 patients who underwent lumbar puncture. There were 98 patients who were thought not to need the test. Most of the children with meningitis had lethargy, irritability, or vomiting; all had features of complex febrile seizure.52 The “catch-22” is this: those who clearly have simple febrile seizure need not undergo lumbar puncture; however, absence of meningitis is one of the criteria for establishing the diagnosis of simple febrile seizure. The author of Clinical Pediatric Neurology says that “a brief, generalized seizure from which the child recovers rapidly and completely is not caused by meningitis, especially if the fever subsides spontaneously…”53 Certainly, lumbar puncture in a child with fever and a convulsion can be selectively obtained, and other findings must be considered.
Criteria suggested for electing lumbar puncture are:
Any clinical suspicion of meningitis
Under 18 months of age
Unusually slow recovery of normal function after a febrile seizure54
Complex febrile seizure, especially focal seizure.
Electroencephalogram
An EEG is usually not indicated immediately after the convulsion.54 It will often be abnormal even after a simple febrile convulsion, although an expert can often distinguish between a simple postictal abnormality and abnormalities suggesting epilepsy. If the EEG reveals epileptogenic activity several weeks after the seizure, the correct diagnosis is more likely to be “convulsive disorder precipitated by fever.” The pattern of activity seen on acute EEG is not predictive of recurrence risk.55
Other Tests
Skull roentgenogram and blood glucose, calcium, sodium, or blood urea nitrogen (BUN) measurements are unlikely to reveal an abnormality and are not recommended unless there is some clinical basis for suspecting an abnormality.54,56,57,58
Hospitalization is not recommended except for severe or multiple seizures or when the parents are too frightened or otherwise unable to observe the child.54
Prevention
Antipyretic Medication
Various antipyretic medications, given at the onset of fever and at a fixed interval throughout the course of a febrile illness, have been tested in prophylaxis against febrile seizures. Prospective randomized trials of acetaminophen59 and ibuprofen60 have failed to show a reduction in the number of
recurrences. Although antipyresis may have other beneficial effects, there appears to be no scientific basis for the use of antipyretics in the prevention of febrile seizures.
recurrences. Although antipyresis may have other beneficial effects, there appears to be no scientific basis for the use of antipyretics in the prevention of febrile seizures.
Anticonvulsant Medication
About one-third of patients will develop recurrence of febrile seizure, and half of those patients go on to a third episode. These recurrences can be decreased by continuous anticonvulsant therapy with either phenobarbital or valproic acid, but these medications are not without risk. Prophylaxis against recurrent febrile seizure with phenobarbital has been shown to lower achievement scores even 5 years out.61 Valproic acid is more effective at prevention of seizures,62 but carries the risk of idiopathic and irreversible severe hepatotoxicity.63
In one study, diazepam, taken at the onset of fever, was also shown to decrease the incidence of febrile seizures; 39% of recipients, however, developed side effects of the medication.64 Other studies failed to duplicate the beneficial effect.62 Some authorities believe that prophylaxis is even less likely to be needed if the convulsion is associated with roseola, shigellosis, or viral meningitis, because these illnesses have a tendency to be associated with convulsions. A lower recurrence rate among children whose first febrile seizure was associated with HHV-6 (the causative agent of roseola) has been confirmed by a prospective clinical trial.65 A major factor to consider is that in the grand majority of cases, febrile seizure, even if it recurs, is a benign disorder with an excellent prognosis. The risk of most prophylactic regimens outweighs the potential benefits.
Exceptions could include children with neurologic disease, those with focal seizures, a family history of epilepsy, or a febrile seizure lasting more than 15 minutes or followed by a neurologic abnormality.66
Postictal Pleocytosis
Occasionally the question arises as to whether seizure activity alone may be responsible for the finding of leukocytes in the CSF. In approximately 5% of cases, WBCs may be found in the CSF within 72 hours of a seizure, and most commonly within 12 hours of a seizure.67,68,69 The maximum number of WBCs in the spinal fluid is usually less than 15 per mcL, but may rarely be as high as 80 per mcL.70 Mildly increased protein may also be observed after seizures in about 10% of cases.67 Postictal pleocytosis can occur after simple, complex partial, or generalized tonic-clonic seizures.68 Clearly, this is a diagnosis of exclusion, and infectious causes should be pursued vigorously.
Purulent Meningitis
Definitions
Purulent meningitis is a medical emergency. It is usually manifested by clinical signs of acute neurologic infection and cloudy spinal fluid. Typically, the CSF has more than 1000 leukocytes/mcL with a predominance of neutrophils, low glucose (often 0–10 mg/dL), and elevated protein (usually more than 100 mg/dL). Some patients with early bacterial meningitis have cell counts, glucose, and protein in the same range found in nonpurulent meningitis; that is, aseptic meningitis syndrome, which is discussed in the following section. Prior oral antibiotic treatment decreases yield of CSF culture, but does not significantly alter the CSF parameters; total number of white blood cells, glucose levels, and protein levels are not affected. In order to avoid jumping to etiologic conclusions, it is useful to use the terms “purulent” and “nonpurulent” meningitis until a bacterial etiology is confirmed or excluded by culture. In the patient with apparent purulent meningitis but a negative Gram stain, the possibility of a parameningeal infection (such as a brain abscess or subdural empyema) should be considered.
Ventriculitis may occur without meningitis, particularly if the CSF flow is obstructed. This is most likely as a complication of neurosurgical shunting operations for hydrocephalus and is discussed later in this chapter.
Age
In the past, purulent meningitis occurred predominately in children. The advent of the protein conjugate H. influenzae type b vaccine has had a dramatic effect on the epidemiology of bacterial meningitis. In 1986, 62% of bacterial meningitis in the United States occurred in children younger than 2 years of age and 79% occurred in children younger than 18. By 1995, children younger than 2 years old accounted for 25% of all cases of bacterial meningitis and those younger than 18 accounted for 48%.71
It is reasonable to assume that widespread use of the protein conjugate pneumococcal vaccine will decrease the incidence of bacterial meningitis in young children even further.
Risk Factors
Males are slightly more likely to acquire meningitis than are females. There is a suggestion that meningitis is more common in poor populations. The incidence of pneumococcal meningitis is 8- to 24-fold higher in blacks, irrespective of socioeconomic status or crowding.72 Patients with asplenia/polysplenia, sickle cell disease, or other hemoglobinopathies that lead to splenic dysfunction are at higher risk than the general population. Patients with malignancies or immunodeficiencies have a higher rate of meningitis and are more likely to be infected with uncommon bacteria. Malnourishment causes immune dysregulation that is the probable cause of increased risk in these children.73 Patients with occult or known dermal sinuses or dural defects are at increased risk. Children with cochlear implants have a 30-fold increased risk for pneumococcal meningitis.74 Finally, systemic diseases, especially diabetes mellitus or chronic renal disease, may confer a higher risk of meningitis.
Clinical Presentation
Patients suffering from purulent meningitis are generally ill-appearing. They usually have some combination of fever, headache, nausea, vomiting, photophobia, and neck stiffness. They may be irritable or lethargic. Obtundation and coma are late signs. On physical examination, nuchal rigidity may be found; this finding is less common in infants. Classically, Kernig and Brudzinski signs are sought. Kernig sign is elicited as follows: with the patient in the supine position with the hip flexed 90 degrees (knee pointing straight up), the knee joint is extended by slowly raising the foot upwards. Kernig sign is positive if this motion causes extreme discomfort. With the patient remaining supine, Brudzinski sign is positive if the hips are involuntarily flexed when the examiner bends the head down toward the chest. As with simple testing for nuchal rigidity, Kernig and Brudzinski signs are meant to help the physician detect inflammation of the meninges. Although these signs are often discussed, rigorous examination of their clinical utility is largely lacking. One prospective study of 295 adults with suspected meningitis found that neither the Kernig sign nor the Brudzinski sign was of clinical utility in differentiating patients with meningitis from those without meningitis.75
Possible Infectious Causes
Most purulent meningitis is caused by Neisseria meningitidis, Streptococcus pneumoniae, or Haemophilus influenzae type b (Hib). The epidemiology of meningitis has changed drastically since the introduction of the Hib vaccine. Prior to the vaccine, H. influenzae was by far the most common cause, especially in children between the ages of 1 month and 5 years. The incidence of Hib meningitis dropped from 19.4 cases per 100,000 in 1980 to 3.7 cases per 100,000 in 1991.76 The vaccine was introduced in October of 1990. There has been a continued decline in the number of cases of Hib meningitis to even lower levels. The vaccine induces protection against nasopharyngeal carriage, which allows even the unvaccinated some measure of protection. From 1–23 months of age, pneumococcal meningitis is slightly more common than meningococcal meningitis. In children between the ages of 2 and 18 years, N. meningitidis accounts for 59% of cases; in adults S. pneumoniae predominates.71
In the first 30 days of life, the most common causes are Group B streptococci and enteric bacteria (particularly E. coli). Other bacteria that rarely cause meningitis except in the newborn period include other enteric gram-negative rods, Listeria monocytogenes, and Staphylococcus aureus. These agents also are an occasional cause of meningitis in the first few months of life, especially in prematurely born or debilitated infants.
Unusual infectious causes are discussed in the section on therapy of unusual infections.
Early Diagnosis
In young infants, it is important to do a lumbar puncture and examine the spinal fluid whenever the neck is questionably stiff or the anterior fontanel is questionably bulging and the patient appears ill. Disturbed consciousness (lethargy, irritability) and crying when handled are especially important symptoms suggesting early meningitis, as nuchal rigidity may be absent or appear late in young infants.
Treatment Before Lumbar Puncture
In rare cases, the illness may be so severe that supportive therapy should be started before the diagnostic studies. Any of the three major pathogens of meningitis can cause septic shock or cerebral edema. For example, in patients in whom meningococcemia is suspected because of hypotension and
purpura, good intravenous access should be established and treatment of shock begun before doing a lumbar puncture. Meningococcemia can occur without meningitis, and the early treatment of septic shock is more important than determining if meningitis is present. An intravenous bolus of ceftriaxone can be given as soon as access is established. Some patients with evidence of life-threatening cerebral edema may be treated with mannitol before a lumbar puncture is done. Priorities in the emergency management of purulent meningitis are listed in Box 9-1.
purpura, good intravenous access should be established and treatment of shock begun before doing a lumbar puncture. Meningococcemia can occur without meningitis, and the early treatment of septic shock is more important than determining if meningitis is present. An intravenous bolus of ceftriaxone can be given as soon as access is established. Some patients with evidence of life-threatening cerebral edema may be treated with mannitol before a lumbar puncture is done. Priorities in the emergency management of purulent meningitis are listed in Box 9-1.
BOX 9-1 Emergency Treatment of Meningitis
|
In patients who are going to be transported to a hospital, if lumbar puncture cannot be done locally, presumptive antibiotic therapy should be begun without obtaining CSF if meningitis is suspected and transport will delay treatment.77
Children with purulent meningitis should generally be admitted to the critical care unit for close neurological monitoring, at least for the first 24 hours of illness, when complications such as shock, herniation, cerebral infarction, and seizures are most common.
Spinal Fluid Examination
Indications and technique of lumbar puncture and examination of the spinal fluid are described in an earlier section. Complete examination of the CSF should be done in order to detect any abnormality that may be helpful in the diagnosis. A Gram-stained smear should be examined even when few or no white blood cells are found. A few organisms can sometimes be found that originate on the slide or in the stain, but in rare instances, many organisms are found in spinal fluids that have no pleocytosis, especially in pneumococcal meningitis. The meningococcus is the organism most frequently missed on smear but found on culture, and H. influenzae is often misinterpreted as another organism.
Glucose and protein should be determined and are discussed in the following section.
Antibiotic therapy should not be delayed until the spinal fluid studies are available, especially if the fluid is grossly cloudy. As soon as the CSF is obtained, a third-generation cephalosporin such as ceftriaxone, 80 mg/kg as a loading dose,78 should be given as an intravenous bolus. If there is likely to be a delay in starting the infusion into a small vein, the antibiotic should be given into a large vein, such as an antecubital or external jugular vein, or even intramuscularly if an intravenous route is not readily obtainable. As soon as an intravenous line is available, vancomycin should also be administered.
Prior Antibiotic Therapy
Often, patients are receiving oral antibiotic therapy (as for otitis media) when the clinical and CSF findings of purulent (or nonpurulent) meningitis occur. This should not be called “partially treated meningitis.” “Meningitis during antibiotic therapy” is more accurate and does not imply a missed bacterial meningitis.
Meningitis during antibiotic therapy represents one of the most frequent and difficult situations in pediatrics. Prior antibiotic therapy is associated with a longer duration of symptoms,78,79,80 especially in children with H. influenzae type b infection, which led to the theory that H. influenzae meningitis has two forms, one rapid and one slower in onset.80 It has been suggested that the slower-onset form has a lower mortality rate,80 but bacteriologically confirmed H. influenzae meningitis with prior oral antibiotics has a higher incidence of neurologic sequelae.81
The definitive study of the effects of prior oral or intramuscular antibiotic therapy on modifying the CSF findings in bacterial meningitis has not been done, nor is it likely to be done. The design needed was described by Wheeler in a 1970 editorial.82 In 1975, in a chart review, the authors concluded “little more can be learned by a chart review of cases with positive cultures” and indicated that a prospective study with better diagnostic methods was needed.80
The prospective (and unlikely) study that would approach the issues directly would involve following, without antibiotic therapy, children who had developed clinical signs of meningitis while receiving oral antibiotics, who have CSF findings of 10–1000 white blood cells/mcL (with or without a predominance of “polys”), normal or abnormal glucose, and normal or abnormal protein.
In the absence of such a study to evaluate variables of age, dose and duration of antibiotics, and the predictive value of low glucose, high protein, or CSF leukocyte count, clinicians decide whether to treat “as if” the patient had a bacterial meningitis by making an individualized clinical judgment based on these variables.
An infant less than 1 year of age is more likely to be treated for bacterial meningitis because the risk of brain damage is greater in the developing brain. Similarly, either a low glucose or an elevated protein in the CSF is much more likely to reflect a bacterial than a viral meningitis. As described later in the section on atypical presentations, however, the actual number and type of cells is sometimes the opposite of the expectation, even when no prior antibiotics have been given. Finally, patients with typical purulent meningitis (low glucose, high protein, high number and percent of neutrophils) have negative cultures in about 5–10% of cases with no preceding antibiotics and have just as bad a prognosis as those with positive cultures.79
In the absence of the definitive prospective study, several types of imperfect alternate studies are sometimes cited, although they do not provide conclusive guidance:
Proved bacterial meningitis. The CSF findings in proved H. influenzae meningitis do not differ significantly between patients with and without prior antibiotics when the culture is positive.80 One study concluded an oral antibiotic preceding admission would not alter the CSF findings in most patients to an extent that would preclude establishing a diagnosis of H. influenzae meningitis.81 In a study in Denmark that included 569 patients with pneumococcal, meningococcal, or Haemophilus meningitis, prior antibiotic therapy did not statistically change the frequency of CSF WBC counts below 1000/mcL, nor was there any increase in mortality rate or late sequelae.83
Examining CSF after IV therapy. Studies regarding the effect of antibiotics on CSF parameters have reached conflicting results. In one study, full appropriate IV antibiotic therapy of established bacterial meningitis for 44–68 hours did not alter the findings characteristic of bacterial meningitis (i.e., low CSF glucose, predominance of polymorphonuclear leukocytes) on the second
lumbar puncture.84 In that study, three children with meningococcal meningitis had a normal CSF glucose and negative culture after 48 hours of therapy, but the remaining 65 children showed no statistically significant alterations in CSF cytology or biochemistry after about 48 hours’ treatment. However, in another study of 42 patients, which was done to compare ampicillin and chloramphenicol against H. influenzae meningitis, many test values in both groups fell into a range of normal for glucose, protein, and total WBC count after 1–4 days of therapy.85
Comparing bacterial with nonbacterial meningitis. In some studies, the two groups compared were defined by positive or negative CSF cultures, and the CSF findings are statistically significantly different in terms of mean values, although overlap of values for each measure is present. In one study that included 38 children given antibiotic therapy in the 48-hour period before lumbar puncture, two patients with a positive CSF culture had cell and differential counts characteristic of “aseptic meningitis,” with a slightly decreased glucose and definitely elevated protein.86 No patient with a positive bacterial culture had all CSF findings compatible with aseptic disease, but the range of cell count, percentage of polymorphonuclear cells, glucose, and protein clearly overlapped those of the prior-antibiotic group whether or not the culture was positive. These authors interpreted their data to support Wheeler’s 1970 editorial that “there is a small but important group” in whom prior antibiotic therapy may significantly alter the CSF laboratory values. However, others have speculated that it would be rare for prior antibiotics to alter all measurements simultaneously.87 There is no evidence that bacterial antigen tests are more sensitive than Gram stain for the detection of meningitis in patients pretreated with antibiotics.88
Investigators continue to search for a biologic marker that would clearly differentiate viral meningitis from bacterial meningitis with antibiotic treatment. One study showed that CSF ferritin levels were greater than 18 ng/mL in 46 (98%) of 47 cases of bacterial meningitis, and that these levels did not correlate with CSF neutrophil count, CSF protein concentration, serum ferritin levels, or the age of the patient.89 Furthermore, in 16 (84%) of 19 patients who had additional lumbar punctures performed, the ferritin levels remained elevated for an average of 15 days, despite appropriate intravenous antibiotic therapy. However, 15 (3%) of the 441 control patients also had ferritin levels greater than 18 ng/mL; 12 had bacteremia or pneumonia, 2 had relapsed CNS leukemia, and 1 had hemorrhagic herpes encephalitis.
Another group of investigators measured the N-acetyl neuraminic acid (NANA) levels in the CSF of 68 patients with bacterial meningitis, 37 of whom had pyogenic organisms and 31 of whom had tuberculous meningitis. They found that free NANA levels were elevated only in patients with pyogenic meningitis, and that the increase was not related to cell count or CSF glucose levels.90 Unfortunately, however, this paper did not address the fate of the NANA levels after antibiotic treatment, so it does not directly address the question.
In spite of the various interpretations of the available studies, reasonable guidelines for continuing antibiotic therapy in a child developing meningitis while receiving antibiotics can be proposed, recognizing that experienced clinicians disagree. We suggest continuing IV antibiotic therapy if any of the following is present:
Significant neurologic signs such as lethargy, vomiting, paresis, convulsions
Age less than 1 year (some would include older infants)
CSF glucose or protein clearly abnormal
CSF WBC count exceeding 300/mcL or exceeding 60% polymorphonuclear cells
Any early complication of bacterial meningitis.
If the patient is neurologically normal at 72 hours when the CSF culture is negative and maximum temperature is less than 101°F (38.4°C), antibiotics can be discontinued. If bacteria are seen on initial Gram stain and found on review, or if neurologic abnormalities are present, therapy should be continued for the usual duration.
A second lumbar puncture may be indicated if significant fever or neurologic signs persist, with consideration of appropriate studies for the numerous causes of nonpurulent meningitis. A CT scan may be indicated for persistent fever or neurologic abnormalities.
Fortunately, most patients with viral meningitis will be clinically very much improved after 72 hours. A patient with bacterial meningitis sufficiently modified by prior antibiotics who has none of the above criteria for continuing antibiotics is
likely to be fully cured by 72 hours of further IV antibiotics.
likely to be fully cured by 72 hours of further IV antibiotics.
In a school-age child with a good state of consciousness and no significant neurologic signs, with fewer than 300 cells/mcL (predominately mononuclear), with normal CSF glucose and protein, the clinician can elect to stop antibiotics and observe carefully. If the patient is not definitely improved in 8–12 hours, the CSF can be reexamined, with the various causes of nonpurulent meningitis kept in mind for further study.
Atypical Presentations
Bacterial meningitis may not develop in the usual clinical pattern (Table 9-3). Probably the most common atypical presentation is mistaken for pneumonia; fever and rapid breathing, presumably caused by central hyperventilation, dominate the clinical picture. Weakness or ataxia also is presumably of CNS origin.
Even after the newborn period, signs of meningeal irritation were absent in 16 (1.5%) of 1064 of patients in one series.91 Acute hearing loss has been reported as the presenting sign in a 6-year-old with meningitis after a posttraumatic basilar skull fracture.92
It is important to note that fever is not uniformLy present in children with bacterial meningitis. Neonates with meningitis are at least as likely to have a normal or low temperature as they are to have an elevated temperature at the time of presentation. In children outside the neonatal period, more than 85% will have fever at the time of presentation.93 In one review of children older than 6 years old with bacterial meningitis, 11 (44%) of 25 were afebrile on presentation, suggesting that fever may be less common in older children.94 Overall, the classic triad of fever, stiff neck, and mental status changes occurs in only one-half to two-thirds of patients with bacterial meningitis.93
TABLE 9-3. ATYPICAL PRESENTATIONS OF MENINGITIS | |
|---|---|
|
Bacterrhachia Without Pleocytosis
Atypical CSF findings include spinal fluid with cell count, glucose, and protein within normal limits, a situation where bacteremia “seeds” the meninges and the bacteria are culturable before the inflammatory reaction has occurred. A review found that 7 (3%) of 261 children with bacterial meningitis had CSF findings within normal limits when first seen.95 All appeared sick enough to be hospitalized, and all but one child were immediately treated for sepsis, indicating that children with bacteremia producing positive CSF cultures and normal CSF findings usually appear sick enough to be hospitalized and treated for suspected septicemia. Another report described the atypical finding of cloudy spinal fluid with innumerable pneumococci and few leukocytes.96
Low CSF cell counts (nonpurulent meningitis) may also occur with bacteremia. The prognosis in this situation depends on the prognosis for the bacteremic disease more than on the prognosis for meningitis if appropriate antibiotic therapy is given. Early meningococcemia or infective endocarditis may produce a “seeding” of the CSF with fewer than 100 leukocytes/mcL and normal CSF glucose and protein.
Other Atypical Patterns
Prior antibiotic therapy is probably the most frequent factor causing delay in diagnosis of bacterial meningitis.97 Previous immunization with the polysaccharide H. influenzae type b vaccine occasionally resulted in a more gradual onset in H. influenzae meningitis, presumably related to partial protection by antibodies stimulated by the vaccine. This clinical pattern has not been seen following receipt of the protein-conjugate Hib vaccine.
A predominance of mononuclear cells with low glucose and high protein can occur with Listeria or tularemia.98 On the other hand, several virus infections (such as enteroviruses or La Crosse virus) may present with a leukocyte count above 1000/mcL, although the CSF glucose and protein are typically
normal or near normal (see section on purulent meningitis with negative culture).
normal or near normal (see section on purulent meningitis with negative culture).
Eosinophilic meningitis is discussed in the section on non-purulent meningitis.
Initial Antibiotic Therapy
Purulent meningitis is an extremely serious disease, and the outcome can range from complete recovery to brain damage or death. For this reason, no area in pediatric infectious diseases has had so many changing recommendations for antibiotic therapy. The epidemiology of the disease has also changed, due mostly to the efficacy of the conjugated Hib vaccine, but also to the overuse of antibiotics and subsequent spread of penicillin-resistant S. pneumoniae isolates. The two most likely pathogens in children outside the neonatal period are the pneumococcus and N. meningitidis; empiric therapy should be directed against these two pathogens. Third-generation cephalosporins such as ceftriaxone or cefotaxime penetrate CSF well and have good activity against all isolates of N. meningitidis; they are also active against all penicillin-susceptible and most penicillin-resistant isolates of S. pneumoniae. Unfortunately, a recent rise in the percentage of pneumococcal isolates that are only intermediately sensitive to these cephalosporins has mandated the inclusion of vancomycin in the empiric treatment of suspected bacterial meningitis in children outside the neonatal period. Vancomycin is a large molecule that crosses the blood-brain barrier poorly; therefore, children should be given large doses (20 mg/kg/dose) to enhance penetration into CSF. Once an organism has been identified and susceptibilities are available, therapy should be tailored appropriately.
Empiric therapy for neonates should be directed against Group B streptococci and the enteric gram-negative rods. The combination of ampicillin and gentamicin, or ampicillin and cefotaxime, are reasonable choices. Some experts would use all 3 drugs initially if purulent spinal fluid is obtained. Ceftriaxone should probably be avoided in the first 6 weeks of life because of its propensity to displace bilirubin from albumin binding sites and to cause “sludging” of bile in the gall bladder; both of these effects raise serum bilirubin levels.
Specific Antibiotic Treatment
Pneumococcal Meningitis
As soon as S. pneumoniae is identified as the cause of meningitis and penicillin- and cephalosporin-resistance have been excluded by an oxacillin disk diffusion, minimal inhibitory concentration (MIC) test, and/or the e-test, penicillin alone is sufficient therapy and is less expensive than ampicillin. The dose is 250,000 units/kg per day divided into four to six doses. Penicillin-allergic patients can be treated with ceftriaxone, unless there is a history of anaphylaxis, in which case beta-lactam agents should probably be avoided. Vancomycin plus rifampin may be used in this unusual circumstance.
Patients infected with penicillin-resistant strains should be treated with a third-generation cephalosporin. In the event the isolate is intermediately or completely resistant to third-generation cephalosporins as well, vancomycin should be continued for the entire course of therapy. The cephalosporin should not be discontinued, as levels achieved in the spinal fluid may exceed the minimum inhibitory concentration of even “resistant” strains. For cases of meningitis caused by highly resistant strains, some experts advocate a repeat lumbar puncture 48–72 hours into therapy to document sterilization of the CSF.
Meningococcal Meningitis
Penicillin, ampicillin, or third-generation cephalosporins are effective. Penicillin is the drug of choice for susceptible strains. Usually, patients allergic to penicillin can be treated with a third-generation cephalosporin. Prophylaxis of household and other intimate contacts (such as daycare contacts) should be carried out as detailed in Table 21-9. There are several drugs that have been shown to eradicate carriage of meningococci; these drugs are effective prophylactic agents. Rifampin 600 mg twice a day for four total doses is effective in adults; children can be given 10 mg/kg/dose for four doses. Children less than one year should get 5 mg/kg/dose instead. A single dose of 500 mg of ciprofloxacin has been shown to be effective in adults and is considerably less cumbersome. One intramuscular dose of ceftriaxone is also effective,99 and may be considered in cases where compliance to the other regimens is likely to be poor, or in situations where the other agents are contraindicated, as, for example, for prophylaxis of a pregnant woman.
A deficiency of the terminal components of complement is sufficiently common in systemic meningococcal infection that the patient should be screened for this disorder with a CH50.100 It is more common in patients of African-American descent.
A second case of invasive meningococcal infection is especially suspicious. The family should be screened if the patient has a complement deficiency.
A second case of invasive meningococcal infection is especially suspicious. The family should be screened if the patient has a complement deficiency.
H. influenzae Meningitis
Unusual Bacterial Causes
Other bacterial causes are usually related to trauma, the newborn period, or to some host defect. Recommended initial chemotherapy is shown in Table 9-5.
TABLE 9-4. INITIAL EMPIRIC THERAPY OF BACTERIAL MENINGITIS | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Duration of Therapy
For the patient with uncomplicated bacterial meningitis, antibiotic therapy should be given for at least 10 days except for meningococcal meningitis, where 5–7 days is sufficient.78 Patients should generally be afebrile for 48–72 hours before therapy is stopped.
If therapy is begun late, or if the prognosis is otherwise poor, 14 days of IV therapy should be given. Duration of therapy for gram-negative meningitis should probably be longer; 21 days is considered standard.
If therapy is begun late, or if the prognosis is otherwise poor, 14 days of IV therapy should be given. Duration of therapy for gram-negative meningitis should probably be longer; 21 days is considered standard.
TABLE 9-5. ANTIBIOTICS FOR UNUSUAL BACTERIA CAUSING MENINGITIS | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
In our opinion, treatment of bacterial meningitis should be completed in the hospital by the intravenous route. When there is unusually severe illness, when the patient is a young infant, or when there is a delay in diagnosis and treatment, the clinician should choose the longer duration and the higher doses of antibiotics, using the surest route. CSF protein and WBC do not usually return to normal until well after therapy has been completed; normalization of CSF parameters should not be used as a criterion for duration of therapy.
Relative Importance of Antibiotic Therapy
The management of meningitis requires more than the choice of the best antibiotic, the best dose, and the best route. Indeed, antibiotic therapy of meningitis is relatively standardized; the anticipation, early recognition, and effective treatment of complications, particularly cerebral edema, must be emphasized.
Neonatal Meningitis
Meningitis occurring in the first month of life differs from meningitis in older individuals in a number of important respects:
Poor prognosis. The diagnosis is often late because of minimal symptoms. Also, brain complications are more likely and more severe, because the central nervous system is still developing.
Misleading clinical response. Newborn infants often have a prompt return of the temperature to normal, suck well, and appear to be fairly normal yet may ultimately develop hydrocephalus or signs of brain damage. Repeat lumbar punctures should be done to follow the response to therapy early in the course.
Infecting organisms. Group B streptococci and E. coli are the usual pathogens, but Listeria, other streptococci, staphylococci, Hemophilus, and many gram-negative bacillary species are also possible.103 E. coli is almost never a cause of meningitis after the first 6 weeks of life, although rare exceptions might occur in a very premature infant or an infant with an underlying disease, such as severe congenital heart disease.
Chemotherapy. A collaborative study showed no evidence of difference between ampicillin plus amikacin (representing aminoglycosides) and ampicillin plus moxalactam (representing a third-generation cephalosporin).104 Each regimen has its benefits and its drawbacks: the combination of ampicillin and an aminoglycoside (gentamicin, tobramycin) is synergistic for Group B streptococcal infection and effective against gram-negative meningitis as well, but in
some hospitals monitoring of serum levels is cumbersome, and aminoglycosides have well-known renal and ototoxicity. Ampicillin plus a third-generation cephalosporin (usually cefotaxime) is more convenient and less toxic, but is probably less reliable against Group B streptococci, especially those that are ampicillin tolerant (discussed later). Many experts would use all three days initially (ampicillin plus gentamicin plus cefotoxime).
A 72- to 96-hour follow-up lumbar puncture should be done in neonatal meningitis to ensure sterility as a guide to efficacy and prognosis.
In the newborn or very young infant with meningitis caused by Group B streptococci, ampicillin and gentamicin appear to be synergistic and should be used for 14 days or longer.78 Although penicillin-resistant Group B streptococci have not been identified, some isolates are penicillin tolerant (the minimum bactericidal concentration is more than 4 times greater than the minimum inhibitory concentration). Therefore, high doses of penicillin (450,000 U/kg/day) or ampicillin (300 mg/kg/day) should be used. The incidence of side effects with high-dose penicillin or ampicillin is no higher than that seen with lower doses. For gram-negative enteric bacilli, adding gentamicin to cefotaxime may produce a synergistic effect. Some gram-negative bacilli (especially Enterobacter and Citrobacter) possess a chromosomal, inducible beta-lactamase and can develop resistance to beta-lactams during therapy, even if initial susceptibility testing is favorable.105 For these pathogens, a carbapenem (meropenem) or fourth-generation cephalosporin (cefepime) plus an aminoglycoside (gentamicin or tobramycin) is appropriate therapy. Other gram-negatives (especially Klebsiella and Serratia) may also harbor extended-spectrum beta-lactamases that can render semi-synthetic penicillins and cephalosporins useless. When caring for newborns with gram-negative meningitis of any kind, consultation with an infectious diseases specialist is advised. Patients with gram-negative meningitis should be treated for 21 days or longer.
Other therapy. The poor outcomes seen with gram-negative meningitis have led some to consider trials of intraventricular or intrathecal antibiotic therapy. A multicenter, randomized, controlled trial of intravenous ampicillin and gentamicin with or without intrathecal gentamicin was conducted in 117 infants with gram-negative enteric meningitis. There were no significant differences in the mortality, morbidity, or time to CSF sterilization between the two groups.106
Seizures in neonates with gram-negative meningitis have multiple potential causes, including poor cerebral perfusion, infarcts, edema, and hypoglycemia. The development of cerebral abscesses is common and should be screened for with computed tomography. Drainage by a neurosurgeon may be necessary.
Early Complications
The complications of meningitis can be divided into early complications (those that occur during the first 24 hours and may be the immediate cause of death) and late complications (those usually recognized after several days or later) (Table 9-6). The early complications are cerebral edema, septic shock, disseminated intravascular coagulation, myocarditis, hyponatremia with water intoxication (which aggravates cerebral edema), and convulsions.107 Sensorineural deafness is also an early complication but may not be detected until later. Cerebral edema and endotoxic shock are the principal causes of death after patients have reached the hospital and are receiving antibiotics. In order to detect early signs of these severe complications, the physician should be sure the indicators of shock and cerebral edema (described below) are charted on a flow sheet every 15 minutes in the early hours of treatment, just as one would chart such vital data in a hospitalized diabetic in severe acidosis.
Diagnosis of Cerebral Edema
The recognition of cerebral edema is based on progressive changes in several physical findings108 (Table 9-7). A flow sheet is useful to follow the course (Fig. 9-1). In cerebral edema, the state of consciousness changes from alert but irritable, to lethargic but arousable, to stuporous, and finally to deep coma. Pupillary reflexes change from mid-position, equal, and reactive to light to dilated and sluggish and, finally, to dilated and fixed. The optic discs are usually not a useful guide, because rapid changes in pressure are often not reflected by anything more than minimal blurring of the discs. Such a minimal blurring in acute purulent meningitis is not a contraindication to a careful lumbar puncture.
Marked papilledema or choked discs implies a more dangerous situation, and a chronic illness such as lead encephalopathy should be considered, as discussed earlier under Indications and Risks. Eye movements change from fixation on distant objects when the neck is rotated, to rotation in concert with the head, as if staring (doll’s eye movement) late in the course of the disease. Response to pain changes from purposeful withdrawal of the extremity to which the painful stimulus is applied, to non-purposeful withdrawal and stiffening with decerebrate rigidity, to complete flaccidity.
Marked papilledema or choked discs implies a more dangerous situation, and a chronic illness such as lead encephalopathy should be considered, as discussed earlier under Indications and Risks. Eye movements change from fixation on distant objects when the neck is rotated, to rotation in concert with the head, as if staring (doll’s eye movement) late in the course of the disease. Response to pain changes from purposeful withdrawal of the extremity to which the painful stimulus is applied, to non-purposeful withdrawal and stiffening with decerebrate rigidity, to complete flaccidity.
TABLE 9.6. ACUTE COMPLICATIONS OF BACTERIAL MENINGITIS | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
TABLE 9-7. PROGRESSION OF SIGNS OF BRAIN SWELLING | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The pattern of breathing is an important guide to increased intracranial pressure. In cerebral edema, the breathing pattern changes from regular to irregular to the Cheyne-Stokes pattern. Cheyne-Stokes respirations are characterized by periods of deep and rapid respirations alternating with periods of slow, shallow respirations or apnea. The late changes in breathing and eye movements are related to compression of the brainstem and are often not seen if the child receives early chemotherapy.
The fontanel may change from flat to bulging. Convulsions may occur. Patients with meningitis who have had convulsions should not be kept deeply sedated, because this obscures changes of consciousness that are a guide to the severity of cerebral edema. Lateral rectus palsy may be seen in severe or chronic cerebral edema and is not of localizing value.
There is one report of 5 children who developed cutaneous flushing, an obvious but transient reddening
of the skin, at the same time they experienced neurologic deterioration secondary to increased intracranial pressure. The epidermal flushing involved the upper chest, face, or arms, and lasted from 5–15 minutes. The exact origin of this response is unknown, but it is postulated that it may be a centrally mediated response to sudden elevations in ICP.109
of the skin, at the same time they experienced neurologic deterioration secondary to increased intracranial pressure. The epidermal flushing involved the upper chest, face, or arms, and lasted from 5–15 minutes. The exact origin of this response is unknown, but it is postulated that it may be a centrally mediated response to sudden elevations in ICP.109
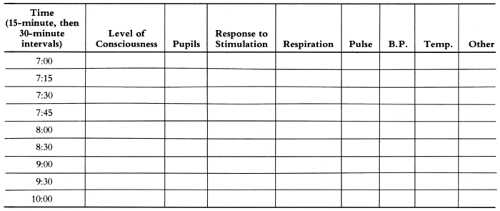 FIGURE 9-1 Flow sheet for observation for brain swelling. Use Table 9-7 for signs to be recorded. |
These changes associated with cerebral edema resemble the progression of stages during induction of general anesthesia and have also been described in patients with brain tumors when herniation is imminent. This progression can sometimes be reversed by drugs such as mannitol that create an osmotic gradient between the brain and the plasma. Osmolar agents rapidly remove water from all of the extravascular space, but it is the removal of water from the swollen brain cells that may be lifesaving. Increased cerebral blood flow also occurs during mannitol infusion and may be a factor in its cerebral effects.110
Corticosteroids such as dexamethasone also reduce cerebral edema, according to studies of patients with brain tumors.
Other agents have been shown to decrease intracerebral pressure in experimental meningitis in animal models, including the calcium-channel blocking agent nimodipine.111
The use of mannitol or dexamethasone in patients with meningitis and progressive worsening of brain signs has not yet been proved effective in any prospective controlled comparative study. The evidence for its effectiveness is derived primarily from repeated observation of reversal of the signs of brain deterioration in individual patients when this therapy is given.
Cerebral Herniation
The fatal consequence of cerebral edema is cerebral herniation and typically occurs within 8 hours of admission.112 This is the usual cause of death in the first 24 hours of H. influenzae meningitis.113 However, it is not always fatal when treated (e.g., with mannitol), as described later in this section. Prevention by limiting intravenous fluids, as described later, is better than having to treat the cerebral edema, but fluid restriction has no immediate effect in an urgent cerebral edema situation. In addition, cerebral perfusion pressure is dependent on adequate systemic blood pressure; fluid restriction is thus inappropriate in the patient with hypotension and shock.
Relation of Cerebral Edema to CSF Manometric Pressure
The presence of cerebral edema should be recognized by clinical observations. The actual measurement of opening pressure at the time of lumbar puncture is much more easily accomplished in older patients who are lucent enough to be cooperative with the examination (i.e., those who likely have normal pressures). In smaller children and in babies, attempting to measure the CNS pressure by manometry increases the risk of needle manipulation and may produce intrathecal bleeding. This
blood in the spinal fluid may make the CSF cell count and protein results, which are of critical importance in a patient with suspected meningitis, difficult to interpret. Free back-flow is often not obtained if the patient is straining. Localized cerebral edema may be present in the absence of increased spinal fluid pressure as measured in the lumbar area.114 Finally, the finding of a normal opening pressure does not rule out the presence of increased pressure a few hours later, after intravenous fluids have begun to correct dehydration.
blood in the spinal fluid may make the CSF cell count and protein results, which are of critical importance in a patient with suspected meningitis, difficult to interpret. Free back-flow is often not obtained if the patient is straining. Localized cerebral edema may be present in the absence of increased spinal fluid pressure as measured in the lumbar area.114 Finally, the finding of a normal opening pressure does not rule out the presence of increased pressure a few hours later, after intravenous fluids have begun to correct dehydration.
Treatment of Cerebral Edema
Mannitol, urea, and dexamethasone appear to be effective in reducing cerebral edema from causes other than meningitis. The use of these agents has not been adequately studied in meningitis.
Mannitol has some theoretical advantages: there is no commitment to continuous therapy, and there is no risk of adrenal suppression or gastric ulceration or of masking fever, as is the case with dexamethasone. Moreover, there is no preparation time because of addition of diluents required, and no confusion about possible renal insufficiency, as in the case of urea. Urea is seldom used.
Mannitol is usually given intravenously as a 25% solution, 0.25–0.5 g/kg/dose, over a 20- to 30-minute period, for trauma.115 A review of supportive therapy recommended mannitol at 0.5–2.0 g/kg/dose over a 30-minute period, repeated as necessary.107
 FIGURE 9-2 Effect of mannitol on intracranial pressure. (From Wise BL, Chafer N. J Neurosurg 1962;19:1038–43.) |
A decrease in CSF pressure has been noted in brain tumors within 1/2 hour of starting the infusion, with the lowest pressure reached after 2–4 hours (Fig. 9-2). Pressure returned to previous levels by 6–10 hours. Mannitol can be repeated as soon as 4–6 hours after the last infusion if signs of cerebral edema are again noted. However, after about 48 hours, or six to eight doses, the patient’s long-term prognosis is likely to be unchangeable. The decrease in cerebral edema produced by mannitol or urea is only temporary, and the result depends on improvement of the disease process itself.
Prevention of Cerebral Edema
There has been some controversy over the role of fluid restriction in the prevention of cerebral edema in patients with meningitis. Prospective studies have shown that as many as 88% of patients with meningitis develop the syndrome of inappropriate antidiuretic hormone secretion (SIADH).116 Furthermore, studies done in patients who were never fluid restricted showed that the development of SIADH correlated with poor neurologic outcomes.117 However, the diagnosis of SIADH should never be made in a patient who is dehydrated (because the release of ADH in the setting of dehydration is not inappropriate). Restriction of fluids in
the setting of even mild dehydration may decrease cerebral perfusion. A prospective study of 50 children with meningitis suggested a worse outcome in patients who were fluid restricted.118
the setting of even mild dehydration may decrease cerebral perfusion. A prospective study of 50 children with meningitis suggested a worse outcome in patients who were fluid restricted.118
After restoring circulatory perfusion, restricting fluid therapy to approximately two-thirds maintenance in patients with hyponatremia would be a rational approach. Fluid restriction should be continued only until it can be demonstrated that the patient does not have SIADH, after which fluids are liberalized. This can usually be accomplished within 24–36 hours by careful monitoring of the patient’s input, output, serum sodium concentrations, and urine specific gravities and osmolalities. Serum sodium concentration should be monitored at least twice every 24 hours, especially in patients with documented sodium levels less than 130 mg/dL. Use of less than one-half the calculated maintenance dose as a preliminary guide may contribute to low blood volume, hypotension, or cerebral vascular sludging and venous thrombosis and is therefore to be avoided.
Initial Treatment of Poor Perfusion (Compensated Shock)
Often, children with meningitis have poor circulatory perfusion when first seen, as judged by poor capillary refilling in nail beds and cool extremities. This is not the same as septic shock, which is a later, more severe, situation. Treatment consists of a rapid intravenous infusion of Ringer’s lactate, 10–20 mL/kg/dose, repeated as necessary, to restore circulatory blood volume and improve brain perfusion.
Septic Shock
Shock is usually recognized by low systemic blood pressure and a fast, weak pulse. The extremities may be warm, so that septic shock is sometimes called “warm shock.” Slow filling of the capillary nail beds may be a useful guide to septic shock.
Treatment of Shock
The therapy of septic shock is controversial and is discussed in more detail in Chapter 10. The optimal therapy is plasma volume expansion, which can be done using plasma or 5% albumin, with an estimated initial dose of 25 mL/kg/dose. Patients with meningitis and shock need to receive enough fluid to keep systolic blood pressures adequate, and to keep urine output above 0.5 mL/kg/hour. Central venous pressure should be monitored. Pressor agents such as dopamine can be used to support blood pressure when needed but are not a substitute for adequate filling volume.
The total amount of plasma or 5% albumin given should be based on the blood pressure and on the central venous pressure. When the blood pressure is low or unobtainable, blood volume replacement should be given until blood pressure is adequate or central venous pressure is high.
Disseminated Intravascular Coagulation
The patient with meningococcemia is much more likely to develop disseminated intravascular coagulation (DIC) with purpura than is a patient with bacterial meningitis of another cause. This problem is discussed further in Chapter 10.
Myocarditis
Congestive heart failure, manifested by rapid pulse, enlarging tender liver, or pulmonary edema, is not always caused by over-treatment of endotoxin shock by excessive intravenous fluids. Myocarditis, presumably secondary to endotoxin, has been demonstrated in some cases by autopsy findings of petechiae, cell infiltrates, and muscle fiber necrosis. Digoxin may be of value.
Purulent pericarditis may also occur, especially as a complication of H. influenzae meningitis. Small, self-limited effusions were detected in 20% of 100 consecutive cases.119 They were usually not significant clinically.
Hyponatremia
Decreased serum sodium concentration may be noted as early as the time of admission to the hospital, although it usually appears after the patient has had some intravenous therapy. This hyponatremia is probably secondary to brain disease and is mediated by an “inappropriate” excessive secretion of ADH. Use of 0.5 normal saline as the maintenance intravenous fluid is reasonable, with continued fluid restriction. Mannitol should be avoided when the patient is severely hyponatremic, because it increases sodium losses. It is an error to focus on correction of serum sodium and the mechanism of inappropriate ADH secretion to the neglect of the basic complication of cerebral edema and its treatment.
Convulsions
In a patient with meningitis, convulsions may occur because of cerebral edema, hyponatremia, subdural effusion, fever, and, by unexplained mechanisms, the disease itself. It is important to consider all possible underlying causes that might be improved by treatment other than anticonvulsants. Barbiturates in low to moderate doses may actually improve the state of consciousness in a patient with constant seizures. However, high doses of barbiturates or other anticonvulsants can obscure the persistence of a physiologic disturbance that should be corrected, such as cerebral edema or subdural effusions.
Hemiparesis or Focal Signs
Focal or lateralized signs observed during the first few days of purulent meningitis have several possible etiologies.120 Usually, mild focal signs such as slight asymmetry of strength or reflexes improve with observation and specific antibiotics and presumably have a vascular or inflammatory basis. However, hemiparesis or total paralysis may be caused by bleeding from DIC, indicating potential residual damage. Focal signs can also be caused by subdural effusions or asymmetric cerebral edema. CT scanning is indicated in the patient with focal neurologic signs.
Subdural Effusion
In young children with bacterial meningitis subdural effusions are very common, occurring in 44 (39%) of 113 children aged 1–18 months old in one study. Only one child had a documented subdural empyema.121
Long-term follow-up (median 5.5 years) demonstrated no increased incidence of seizures, hearing loss, neurologic deficits, or developmental delay. The authors concluded that specific invasive therapy is not indicated in infants with meningitis and subdural effusion who are otherwise improving. The rare child with a subdural empyema will usually not improve clinically on antibiotics alone or will have an initial improvement followed by relapse. In the older child, whose cranial sutures are closed, subdural effusions are potentially more serious, especially if large or if associated with midline brain shift. Neurosurgical consultation for possible drainage should be obtained immediately.
Brain Abscess
Except for gram-negative meningitis in the neonatal period, brain abscess is extremely rare in the first week of purulent meningitis. Operative drainage is not usually necessary unless intracranial pressure is increasing.
Anemia
As with other severe infections, meningitis is frequently accompanied by the anemia of inflammation.122
Diagnostic Approach
EEG
One retrospective study of neonates with meningitis concluded that a markedly abnormal EEG during the acute phase of meningitis correlated with long-term poor neurologic outcome.123 The study looked at 75 EEGs in 29 neonates; babies with normal or near-normal EEGs had good outcomes. This study also suggested that in a population of neonates, EEG may be useful for detecting subtle or subclinical seizure activity.123
Brain Scans
The availability of CT and magnetic resonance imaging (MRI) scanning has revolutionized CNS diagnosis. Scanning is useful for detecting brain abscess or subdural effusion (neither of which usually needs to be drained), large lateral ventricles (implying ventricular obstruction and ventriculitis), small lateral ventricles (cerebral edema), and diminished attenuation or focal hemorrhage (neither particularly treatable nor closely correlated with prognosis).124 Imaging may be useful for the patient who is not responding as expected after several days of adequate therapy; to look for cerebral edema when there is no clinical emergency and a chronic process is suspected; and to evaluate possible hydrocephalus. It is also useful for the patient with focal neurologic signs. However, routine brain scanning in all patients with meningitis is not indicated: one prospective study of serial CT scans in meningitis concluded that clinical management was not influenced by scan results, which failed to reveal any significant abnormalities not suspected on neurologic examination.125 Another study of 58 children with meningitis showed that positive findings with obvious clinical significance were found in
only 6 (10%) of 58 CT scans, all of which were occasioned by complex or prolonged seizures or prolonged fever.126 The procedure demonstrates what clinicians and pathologists have always known: purulent meningitis exerts a profound effect on the brain parenchyma as well as on the meninges. If imaging is deemed necessary, contrast-enhanced MRI is probably the best imaging modality.127
only 6 (10%) of 58 CT scans, all of which were occasioned by complex or prolonged seizures or prolonged fever.126 The procedure demonstrates what clinicians and pathologists have always known: purulent meningitis exerts a profound effect on the brain parenchyma as well as on the meninges. If imaging is deemed necessary, contrast-enhanced MRI is probably the best imaging modality.127
Hearing Loss
Sensorineural hearing loss is the most common adverse neurologic outcome in children who recover from bacterial meningitis. This complication occurs in about 10% of patients, with about half of these cases being bilateral.128 It is most common with meningitis due to S. pneumoniae (31%), as compared with N. meningitidis (11%) or H. influenzae (6%).128 It appears to be unrelated to the number of days of illness before admission or the type of antibiotic therapy but is correlated with ataxia, severe neurologic deficits, or initial CSF glucose of less than 20 mg/dL.128 It is thought that hearing loss is secondary to inflammation that results not only from the infection itself, but also from the bacterial products released by lysis during antibiotic therapy.
Pretreatment with dexamethasone decreases the concentrations of some inflammatory mediators and leads to decreased hearing loss in animal models of experimental meningitis, especially meningitis caused by H. influenzae. Some clinical studies also documented better hearing outcomes in patients pretreated with dexamethasone versus placebo.129 Most of the patients in these trials were suffering from H. influenzae infection, as the trials were carried out when Hib was the most common pathogen. Even though some aspects of the pathogenesis of disease are similar, extrapolation of data obtained from children with H. influenzae meningitis to those with S. pneumoniae or N. meningitidis infection may not be straightforward. A small, prospective trial of dexamethasone vs. placebo in children with pneumococcal meningitis showed a trend toward better audiologic outcomes in the dexamethasone recipients.130 Limitations of the study include that the numbers were small and that ampicillin/sulbactam was the antimicrobial therapy employed. It has already been demonstrated that hearing outcomes vary with differing antimicrobial regimens.
A large, prospective, multicenter trial demonstrated that hearing loss, when it occurred, was present within the first several hours of illness.131 There is also the theoretical concern about the ability of dexamethasone to tighten the blood-brain barrier that is opened due to the inflammation of meningitis. This would normally be considered good, as it decreases the transport of plasma proteins, etc.; however, in this era of increasingly resistant S. pneumoniae isolates, the concern is that the transport of large molecules such as vancomycin would also be decreased. In a rabbit model of meningitis, CSF concentrations of vancomycin have been shown to be lower in rabbits that received concomitant dexamethasone.132
Most experts advise pretreatment with dexamethasone in cases where the suspicion of H. influenzae infection is high (e.g., contact with a known case, gram-negative rods on Gram stain, patient from a group that gets no vaccinations for religious reasons). A recent randomized, placebo-controlled trial in adults with bacterial meningitis demonstrated decreased morality in the dexamethasone-treated group.133 However, the use of steroids in the treatment of bacterial meningitis remains controversial. The preponderance of the clinical evidence suggests that benefit for patients with pathogens other than H. influenzae, if indeed a benefit exists, is small enough to be clinically difficult to demonstrate. The risk of having meningitis secondary to a multiply-resistant pneumococcus that requires vancomycin therapy, on the other hand, is tangible.
Hearing loss, if it is going to occur, is present within 48 hours of presentation. Children with bacterial meningitis should thus have their hearing tested prior to discharge from the hospital or shortly thereafter. If it is normal, no further testing is necessary. If abnormal, repeat testing should be performed, because about one-third of children will regain hearing over the ensuing 6 months. For infants under about 1 year of age, routine electrical auditory testing is advisable. Behavioral hearing testing by a pediatric audiologist in a soundproof room is the best method for a cooperative child.
Prognosis
Purulent meningitis is still one of the most important medical emergencies. It should be suspected and diagnosed early by prompt lumbar puncture and treated vigorously. Management includes anticipation
and treatment of cerebral edema and shock as early complications. Late complications and permanent brain damage remain significantly frequent.
and treatment of cerebral edema and shock as early complications. Late complications and permanent brain damage remain significantly frequent.
The clinician should usually avoid predictions to the family about prognosis and about intellectual recovery and should instead advise parents to wait and see how the child does, using follow-up examinations and, later, specialized testing if necessary as a guide.
Later Complications
These complications occur from a few days to a few weeks after onset and include subdural effusion, hydrocephalus, and brain damage with mental retardation.
Subdural Effusion
The subdural space normally contains no fluid. Subdural effusions occur in about a third of children with meningitis, according to subdural taps done routinely.121 The best explanation of the pathogenesis of subdural effusion is that protein enters the subdural space from dural blood vessels, which are abnormally permeable in meningitis. The protein then brings in fluid from the vessels by osmotic action. In early papers, it was suggested that subdural effusions are more likely to occur when relatively large amounts of spinal fluid are removed at lumbar puncture. Although this has not been clearly documented, the theoretical risk has resulted in the consensus that the volume of fluid removed at lumbar puncture should be kept to the minimum necessary for study (a total of 2–3 mL).
Subdural effusions are least frequent after meningococcal meningitis135 and, after correction for age, are about equally frequent in H. influenzae and S. pneumoniae meningitis.
A subdural effusion is not likely to occur until several days after the onset of purulent meningitis but rarely is found on the first day that meningitis is recognized, particularly if there has been preceding antibiotic therapy.
Because small asymptomatic effusions are common, and because asymptomatic moderate effusions typically resolve without drainage, they need not be looked for and drained routinely, as was done in the past. Effusions are often blamed for persistent fever, vomiting, or other complications of meningitis; subdural effusions are common enough that you might expect to find them in patients with these clinical features. Causation is more difficult to establish. Prospective studies suggest that the neurologic outcome is no worse for those with effusions than for those without.121 Effusions presumably also occur in older children with closed fontanels and usually resolve without drainage. Subdural punctures are rarely done when small effusions are detected on older infants, because the procedure is much more invasive if the anterior fontanel is closed. CT scans demonstrate that even large effusions may resolve completely.137
Clinical indications to obtain CT scans consist primarily of persistent or recurrent neurologic abnormalities after 48 hours of therapy. These include focal neurologic signs, continuing lethargy, seizures, bulging fontanel, or increase in head circumference.138 Usually, the fever is higher than expected for the day of the illness. CT scans can only detect effusions of about 30 mL or larger, but smaller effusions are unlikely to be clinically significant.138 Transillumination is a simple, less expensive way to detect the thin layer of fluid and can be used to supplement the CT scan (Fig. 9-3).
If an effusion is found concurrently with any of the above neurologic findings, subdural needle aspiration through an open fontanel should be considered to see if improvement occurs. A second aspiration is usually not indicated unless the first aspirate is positive on culture or unless neurologic findings are relieved and then recur later to an equally severe degree. Large volumes of fluid (25
mL or more) should not be removed rapidly, because this may result in rapid expansion of the compressed brain, which may be manifested by pallor, tachycardia, or even apnea. Persistent subdural effusions have been treated by neurosurgical shunting of the fluid from the subdural space into the peritoneal cavity. However, the more conservative approach of waiting for absorption of the fluid may be just as effective in most cases.
mL or more) should not be removed rapidly, because this may result in rapid expansion of the compressed brain, which may be manifested by pallor, tachycardia, or even apnea. Persistent subdural effusions have been treated by neurosurgical shunting of the fluid from the subdural space into the peritoneal cavity. However, the more conservative approach of waiting for absorption of the fluid may be just as effective in most cases.
Subdural Empyema
This term is used when the effusion is purulent, containing more than 5000 leukocytes/mcL. It is more likely to be associated with a positive culture and severe neurologic damage.138 Subdural empyema can also occur as a complication of severe sinusitis or mastoiditis without presenting as a purulent meningitis.139 In contrast to patients with simple effusions, patients with subdural empyema usually present with focal signs, especially hemiparesis. Imaging may reveal shift of the midline structures, requiring an immediate neurosurgical referral for drainage.
Slow Improvement During Days 3 to 7
During the first 2 days of treatment for purulent meningitis, the patient often improves significantly in terms of state of consciousness and fever. From the third to the seventh day, improvement is steady but less rapid, as the temperature may remain somewhat elevated, the neck somewhat stiff, and the child irritable (Fig. 9-4). The physicians need patience during this time. They should not do unnecessary procedures (such as a CT scan or a repeat lumbar puncture, which often leads to a third). Solid clinical reasons should make the clinician “do something” diagnostic, but this is typically a time of “worry, but watch carefully.”
Follow-up Lumbar Puncture
A follow-up LP should be performed 3–4 days into therapy in neonates with gram-negative meningitis and in patients infected with drug-resistant pneumococci, and should be considered in anyone who has a poor response to therapy. Neonates with bacterial meningitis of any etiology should have a repeat LP at the end of therapy. However, routine follow-up examination of the spinal fluid is unnecessary as a test of cure.77,140 Sometimes, equivocal results on the follow-up examination as the result of laboratory variation or a traumatic (bloody) tap prolong hospitalization and lead to another lumbar puncture, both of which are clinically unnecessary.
Patients with neurosyphilis generally get a follow-up LP at 6 months and those with cryptococcal meningitis are “re-tapped” two weeks into therapy. Meningitis caused by multidrug resistant Mycobacterium tuberculosis is a vexing problem; follow-up LP may be required on more than one occasion.
Persistent Pleocytosis or Low Glucose
Persistent pleocytosis alone should not be used as a reason for prolonging therapy beyond 14 days. Repeat lumbar puncture to be sure the CSF glucose has risen to normal is not essential, if the response has otherwise been adequate. The frequency distributions of glucose, protein, and leukocyte counts show wide variation after successful treatment of meningitis.140
Persistent Fever
The most frequent causes of fever beyond the expected range are drug fever, unrelated infections, phlebitis, arthritis, and unknown etiology.141 Sometimes prolonged fever is diagnosed and evaluated based on an unrealistic expectation of the rapidity of defervescence. Prolonged and even secondary fevers (fever occurring after a period of defervescence) are not uncommon in children with bacterial meningitis, even in the absence of complications. A brain CT scan is not automatically indicated. Several studies have documented that approximately 10–13% of patients with meningitis have fever beyond 8 days, and about 15–20% develop secondary fevers.142 The presence of prolonged or secondary fever does not correlate with adverse neurologic outcome, nor does it correlate with relapse or recrudescence.143 If the child is neurologically well, and progressive improvement in clinical condition (other than fever) is noted, watchful waiting is the best approach. Plotting a graph of the child’s fever curve may also reveal a decrease in the temperature index, which is encouraging. Small undetected collections of serous fluid containing antigen-antibody complexes may be present in a joint, pleural space, pericardial space, or intracranially. This theoretical explanation is based on the detection of larger fluid collections in some patients with persistent fever.
Antibiotic therapy need not be continued beyond
the previously recommended times if the clinical condition is satisfactory.
the previously recommended times if the clinical condition is satisfactory.
Hydrocephalus
Hydrocephalus may be communicating or obstructive. Obstructive hydrocephalus can occur within a few days of the onset of the illness if there is thick pus in the ventricles that blocks CSF flow out of the ventricular system, especially in newborns or very small infants. Obstructive hydrocephalus occurring early in the illness is usually manifested by acutely increased intracranial pressure, with slow pulse, rising blood pressure, and apnea. Emergency treatment consists of insertion of a needle into the ventricles to remove CSF and relieve pressure.
Communicating hydrocephalus usually is not noted until 2 weeks or more after the onset of the illness. It occurs more frequently in patients with a delay in beginning therapy. It may also occur in young infants with meningococcal meningitis, where the onset of the disease may be slow even without modification by preceding antibiotic therapy. Hydrocephalus is sometimes first suspected by noting a “setting sun” appearance of the eyes (Fig. 9-5), and an enlarging head can be confirmed by daily measurement of the head circumference. A CT scan or sonogram is useful to determine whether the ventricles are dilated throughout (communicating) or if there is obstruction of the aqueduct with a small fourth ventricle. Hydrocephalus is a more common complication of tuberculous or cryptococcal meningitis.
Brain Damage with Mental Retardation
Severe intellectual damage is probably the most dreaded complication of meningitis. Other neurologic deficits that may result include seizures, paralysis, and deafness. Many mechanisms can contribute
to this damage. Cerebral anoxia with subsequent infarction may occur because of shock or apnea or increased intracranial pressure. Direct infectious or toxic destruction of brain tissue may also occur.
to this damage. Cerebral anoxia with subsequent infarction may occur because of shock or apnea or increased intracranial pressure. Direct infectious or toxic destruction of brain tissue may also occur.
 FIGURE 9-5 Setting sun appearance of the eyes in early hydrocephalus. The pupils and iris are the “sun,” with increased visibility of the white sclera as the “sky.” |
Statistical data are usually not helpful in discussing the prognosis of an individual patient with the parents. Bad prognostic factors include coma, hypothermia, shock, age less than 12 months, anemia, and seizures that are intractable or last (or have their onset) greater than 72 hours into antibiotic therapy. In most patients with sequelae, functional improvement tends to occur to at least some extent over time.
Hemorrhage, Thrombosis, or Infarct
With CT scanning, these findings are recognized more often, but no specific therapy is available. Infarctions are more common with meningitis caused by S. pneumoniae. Hemiparesis may be present but sometimes resolves with time. Occlusion of the internal carotid artery has been reported.120
Immune or Reactive Arthritis
Sterile arthritis representing a presumed antigen-antibody reaction has been recognized as a complication of infections such as hepatitis B, yersiniosis, salmonellosis, meningococcemia, and gonorrhea. During the course of H. influenzae meningitis, a sterile high-protein arthritis with fever is occasionally observed, which typically responds promptly after removal of the fluid. Arthritis during the first few days, on the other hand, is usually septic.144,145
Other Complications
Purulent Meningitis With Negative Culture
Bacterial Meningitis
This is presumed to be the usual cause of purulent meningitis with a negative spinal fluid culture. In many cases, the culture is negative because of preceding antibiotic therapy,148 and in a few cases, cultures may be negative because of improper collection or delay in delivery to the laboratory. This is discussed further in the following section on aseptic meningitis syndrome. Listeria monocytogenes may not be positive on culture until 72 hours of incubation.
Congenital Dermal Sinus
The CSF culture may be negative, or a skin or other “contaminant” bacterium may be found, so the culture is regarded as negative when the source is a congenital dermal sinus. Any child suffering from meningitis who is noted to have a midline dimple over any part of the vertebral column should have an MRI performed to look for the presence of an associated dermal sinus. If present, the sinus tract should be resected after recovery from the meningitis to prevent a second episode.
Anaerobic Meningitis
Anaerobes are a very rare cause of bacterial meningitis and would result in a negative conventional culture.149 Anaerobic meningitis is usually associated with prior CNS or otolaryngologic surgical procedures or penetrating trauma. The diagnosis is sometimes suspected by the finding of pneumocephaly on imaging studies.150 The principal predisposing cause in a child would be chronic sinusitis or chronic otitis media. An illustrative case of an 18-year-old with infectious mononucleosis who developed Lemierre’s syndrome and associated meningitis has been reported. The blood culture grew Fusobacterium necrophorum, but the CSF culture grew Prevotella bivia. CT scan showed pan-sinusitis.151 Another report tells of a previously healthy child who developed Fusobacterium necrophorum meningitis secondary to purulent otitis media with the same organism.152 A 3-year-old who injured her eye with a toothbrush developed meningitis secondary to Veillonella parvula, a gram-negative anaerobic coccus found in the mouth flora.153
Reactive Meningitis (Parameningeal Infection)
Acute sinusitis or other bacterial infection near the meninges may produce more than 1000 WBC/mcL, predominately neutrophils, whereas the glucose and protein are usually normal or nearly normal, and the Gram stain typically reveals no organisms. In the absence of prior antibiotics, parameningeal
infection should be suspected, and head imaging should be obtained.
infection should be suspected, and head imaging should be obtained.
Amebic Meningitis
Amebic meningitis is exceedingly rare. However, the physician should be aware that purulent meningitis with a negative culture can be due to amebae such as Naegleria species.154 The patient sometimes has a history of swimming in lake water, which may be the source of the ameba. It has been suggested that the route of inoculation is through the nose to the olfactory bulbs. The CSF cell count is usually in the purulent meningitis range, with a predominance of neutrophils. Erythrocytes are often present and may provide a useful clue. The spinal fluid glucose may be normal or slightly depressed. The CSF protein is usually elevated.
This diagnosis can be made by isolating the ameba from the brain or by observing the motile organism in the spinal fluid (Fig. 9-6). Specific chemotherapy with amphotericin B, miconazole, and rifampin has cured several cases,154,155 but the prognosis is extremely poor. In animal models, passive antibody prior to infection is protective,156 and antibody given intracisternally as therapy prolongs survival but does not prevent death.157
Contaminated CSF Collection
In this situation, there are no CSF findings of purulent meningitis except a smear showing bacteria, which may be traced to commercial lumbar puncture kits.158 False positive gram staining due to contamination of the ethanol the slides were stored in has been reported. If contaminated gentian violet is placed on a hot slide, false positive Gram stains may result; this problem is solved by placing gentian violet on slides that have cooled.159
Cerebravascular Accidents
Hemorrhage or thrombosis of the brain occasionally results in a CSF leukocytosis beyond what can be explained on the basis of the red blood cells present. It should be remembered that erythrocytes become hemolyzed in the CSF, resulting in the supernatant CSF becoming xanthochromic.
Herpes Simplex Encephalitis
As discussed later in this chapter, herpes simplex encephalitis can resemble bacterial meningitis with negative bacterial cultures.
Mycoplasma Meningitis
Mycoplasma pneumoniae produces CNS involvement in approximately 1 in 1000 patients. The primary manifestation is usually encephalitis, but meningitis and meningoencephalitis may also be seen. In meningitis secondary to Mycoplasma pneumoniae the cells may be predominantly neutrophils and the protein may be elevated, but the glucose is normal.160 Typically, there is a preceding definite middle or lower respiratory infection, as discussed in Chapter 8.
Mycoplasma hominis is carried in the genital tract of 12–50% of pregnant women. Prevalence of infection and frequency of antibody titers increase with increasing parity.161 M. hominis cannot be seen on Gram stain and is difficult to isolate on routine microbiology laboratory media. Waites et al. found evidence of CNS infection with M. hominis in 5 of 100 predominantly preterm babies being evaluated for meningitis. In 4 babies, the organism was repeatedly isolated over several weeks.162 In another study of infants with good prenatal care, M. hominis was isolated from 9 (3%) of 318 infants who underwent
lumbar puncture. Most cleared the infection without specific therapy. In all these studies, the lack of a control group of healthy infants makes interpretation of the findings difficult.
lumbar puncture. Most cleared the infection without specific therapy. In all these studies, the lack of a control group of healthy infants makes interpretation of the findings difficult.
Mollaret’s Meningitis
This is an uncommon syndrome of recurrent aseptic meningitis, usually seen in adults and rarely teenagers, characterized by cloudy spinal fluid, usually with a neutrophilic pleocytosis and normal glucose and protein. The presence of “Mollaret cells,” once thought to be endothelial but now known to be of the monocyte/macrophage family, is pathognomonic. This syndrome is now thought to be secondary to HSV-2. This conclusion is based on the PCR finding of HSV-2 DNA in the CSF of three patients with the disorder and on the known propensity of herpes group viruses to cause recurrent disease.163
Intravenous Immunoglobulin
Several cases of aseptic meningitis after receipt of high-dose immune globulin have been reported. Bacterial meningitis is often suspected initially because of the neutrophilic predominance.164,165 In a review of 11 cases,165 the mean CSF leukocyte count was 1123 per mcL (median, 451) with a mean of 74% neutrophils (median 87%).
Neonatal Intraventricular Hemorrhage
After the red blood cells have been lysed, the white blood cells and protein may remain for several days after an intraventricular hemorrhage. The CSF glucose also is usually depressed early or several weeks later, and this may persist. The finding of xanthochromic fluid is the principal clue to this cause of suspected meningitis with negative cultures.
Chronic Meningitis
Persistently low CSF glucose concentrations may occur, particularly in enteric bacterial meningitis of newborns. This finding may indicate brain dysfunction rather than persistence of infection.166
Most chronic meningitis is of fungal or mycobacterial origin.167 Malignant infiltrations usually occur in patients with known malignant disease. In adults, other noninfectious causes include vasculitis, sarcoidosis, systemic lupus erythematosus, Sjögren’s syndrome, and drug reactions. Other causes are discussed under recurrent meningitis.
Relapsing or Recurrent Meningitis
Recurrent episodes of purulent meningitis with negative cultures can be categorized under the diagnosis of Mollaret’s meningitis. An extremely rare cause of chronic CSF pleocytosis in teenagers with recurrent oral, genital, or ocular lesions is Behçet’s syndrome.168 An intracranial epidermoid cyst or other tumor can cause recurrent sterile meningitis.169,170
An immunoglobulin deficiency is rarely a cause of repeated episodes of meningitis. Deficiency of a terminal component of complement or of properdin is a cause of recurrent meningococcal meningitis.171,172
A neurenteric fistula associated with presacral mass and an abnormal sacrum is seen in Currarino syndrome, which can be a cause of recurrent or polymicrobial meningitis. Most patients have a history of constipation because of associated anal stenosis. A congenital encephalocele may extend into the sinuses and result in recurrent meningitis. The diagnosis is made with a fine-cut MRI of the sinuses.
Posttraumatic or Post-Operative Meningitis
An abnormal communication with the CSF, such as a skull or spinal wound, skull fracture, or a congenital dermal sinus in the sacral or occipital areas, should be sought early in all cases of persistent, relapsing, or recurrent meningitis.173,174 Recurrent pneumococcal meningitis suggests an occult CSF leak through a dural tear held open by a skull fracture.173 Imaging of the sacral spine may reveal abnormalities in patients with recurrent meningitis caused by enteric gram-negative rods, or in babies with polymicrobial gram-negative meningitis.
Nonpurulent Meningitis
Definitions
Nonpurulent meningitis (aseptic meningitis syndrome) can be separated from the general category of presumptive CNS infections by the absence of severe cerebral manifestations, such as a severe disturbance of consciousness, and by a spinal fluid cell count of 10–500 leukocytes/mcL (see Table 9-2).
Usually, encephalitis can be distinguished clinically
from nonpurulent meningitis. In one study of both these syndromes, mild impairment of consciousness, febrile convulsions, or mental dysfunction associated with high fever alone were not accepted as definite evidence of encephalitis.178
from nonpurulent meningitis. In one study of both these syndromes, mild impairment of consciousness, febrile convulsions, or mental dysfunction associated with high fever alone were not accepted as definite evidence of encephalitis.178
Obviously, patients with 500–1000 WBC/mcL need to be fit in to one of the categories on the basis of other criteria. This classification is intended as a preliminary one, and exceptional cases occur. In mumps virus meningitis, the CSF WBC count sometimes exceeds 1000/mcL but is typically mostly lymphocytes. In enterovirus outbreaks, especially of Coxsackie-virus meningitis, the protein may be slightly elevated or the glucose slightly depressed or the cell count may be above 500/mcL with a slight predominance of neutrophils, even on an early repeat tap. In a study of 150 children in an epidemic of echovirus meningitis, only 3% had an initial CSF glucose below 40 mg/dL and none were less than 20 mg/d1.179 However, 23% of the children had an initial CSF white blood cell count exceeding 1000/mcL, 21% had a predominance of neutrophils, and 12% had a CSF protein exceeding 80 mg/dL. (These abnormalities did not necessarily occur concurrently.)179 Thus, the preliminary categories are not absolutely perfect in predicting the possible etiologies, and patients who have findings atypical of purulent or nonpurulent categories should be judged individually by clinical findings and according to whether there is enterovirus disease in the community. Many such patients, especially infants, get treated for bacterial meningitis until their diagnosis is clarified.
“Aseptic meningitis syndrome” is the term now used most frequently. “Viral meningitis” and “meningoencephalitis” are terms with significant disadvantages. All of these terms can be further defined.
Aseptic meningitis syndrome was originally defined by Wallgren as an acute illness with meningeal signs and symptoms, a small or large number of cells in the cerebrospinal fluid, and absence of bacteria on direct smear or culture of CSF with no general or local parameningeal infection, and a relatively short benign course (Table 9-8).180 Aseptic meningitis syndrome is now usually defined on the basis of CSF findings that allow the prediction that bacterial pathogens will not be found; namely, a moderate number of leukocytes that are predominately lymphocytes and a smear negative for bacteria (Fig. 9-7). The CSF glucose and protein may or may not be abnormal by this definition. The Centers for Disease Control and Prevention (CDC) include recovery without antibiotics in its recording of definitive cases of aseptic meningitis.
The diagnosis of viral meningitis should not be regarded as equivalent to aseptic meningitis syndrome, which is a syndrome that has many other possible etiologies that are extremely important to consider (Table 9-9). “Nonpurulent meningitis” is a better problem-oriented diagnosis because it makes the clinician more likely to consider such important etiologic possibilities as tuberculosis or partially treated bacterial meningitis.
The term “meningoencephalitis” has the disadvantage of failing to distinguish patients who should be said to have “encephalitis,” which has severe cerebral signs and a higher probability of brain damage. A severe and persistent (at least 8 hours) disturbance of consciousness is an early and relatively reliable indication of a poor prognosis. Nonpurulent meningitis and acute encephalitis also differ in probable etiologies.
A single virus can produce a spectrum of severity of illness, from asymptomatic to mild to severe to fatal. Coxsackieviruses, for example, can cause a spectrum of severity from headache and fever, to nonpurulent meningitis, to paralytic poliomyelitis-like syndrome, to acute and fatal encephalitis. However, from the starting point of a single patient’s illness, it is useful to make a presumptive diagnosis of either nonpurulent meningitis or acute encephalitis and then analyze the etiologic possibilities.
Importance of CSF Glucose
A decreased CSF glucose concentration (hypoglycorrhachia) is usually present in purulent meningitis. It has little more than supportive diagnostic value because the purulent spinal fluid and Gram stain already indicate the presumptive diagnosis of bacterial meningitis. The decreased CSF glucose appears to be related to decreased glucose transport across the blood-CSF barrier and conversion of the brain metabolism from oxidative to the less efficient glycogenolysis. Thus, a lowered glucose suggests more severe brain involvement.
In nonpurulent meningitis, a low CSF glucose is correlated with more chronic or more serious etiologic agents, such as Mycobacterium tuberculosis. Therefore, in patients with nonpurulent meningitis, a decreased CSF glucose has special diagnostic value. It is thus useful to divide nonpurulent meningitis into two subgroups: those with and those without decreased CSF glucose (Table 9-2). It is
useful to define CSF glucose as definitely lowered if it is less than 40% of the blood glucose, or as less than 40 mg/dL if the blood glucose is not known.
useful to define CSF glucose as definitely lowered if it is less than 40% of the blood glucose, or as less than 40 mg/dL if the blood glucose is not known.
TABLE 9-8. DEFINITIONS OF SOME ACUTE CENTRAL NERVOUS SYSTEM SYNDROMES | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
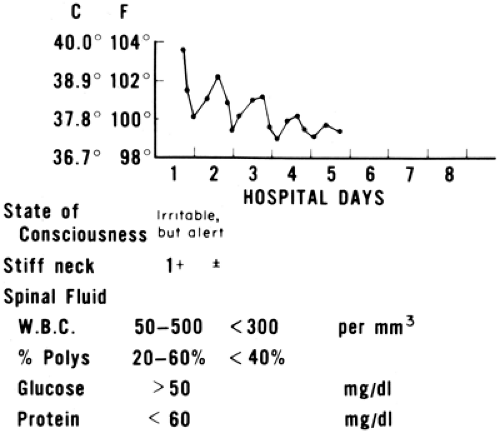 FIGURE 9-7 Typical course of coxsackievirus meningitis, showing normal glucose and protein and predominance of lymphocytes in cerebrospinal fluid after a few days of illness. |
TABLE 9-9. NONPURULENT MENINGITIS: CLASSIFICATION OF ETIOLOGIES | ||||||
|---|---|---|---|---|---|---|
|
An elevation of CSF protein concentration is often also present when there is a decreased CSF glucose. However, a slightly elevated protein may be a result of laboratory variability, of slight bleeding during the lumbar puncture, or of previous traumatic lumbar punctures or operative procedures.
Low-Glucose Subgroup
Bacterial Meningitis
When a patient develops nonpurulent meningitis while receiving an antibiotic, the most reliable clue to bacterial meningitis may be a significantly decreased CSF glucose. In this situation, the protein may also be elevated. Because prior antibiotic therapy may prevent recovery of the infecting organism on culture, the frequency of this etiology is unknown, but it is probably the most frequent cause of hypoglycorrhachia in children.181 This situation is discussed in detail in the section on purulent meningitis.
Early bacterial meningitis may also produce this CSF pattern. Listeria monocytogenes produces nonpurulent meningitis with predominately lymphocytes in a small proportion of cases. The CSF glucose is almost always low and the protein almost always high in such cases.182
Bacterial meningitis can also present as nonpurulent meningitis with a normal CSF glucose, as discussed later.
Tuberculous Meningitis
Tuberculous meningitis presents subacutely in 3 clinical stages. Stage 1 consists of nospecific signs such as fever and headache. In stage 2 the patient develops mental confusion and early focal signs, such as cranial nerve palsies. In stage 3 the patient progresses to obtundation and coma. The prognosis is directly related to the clinical stage at the time therapy is begun. Thus, it is critical to make the diagnosis as early as possible.
Tuberculous meningitis usually results in hypoglycorrhachia. In a series of 405 children with tuberculous meningitis from 1987–1998, 80% had depressed CSF glucose levels. CSF protein was greater than 100 mg/dL in 65%. Half of patients
had less than 60 WBC per mcL, 45% had 60–500 WBC/mcL, and 5% had more than 500 WBC/mcL. Just over half had evidence of pulmonary involvement, and only 16% had a positive tuberculin skin test (TST). Nearly 70% had a positive family history of tuberculosis.183
had less than 60 WBC per mcL, 45% had 60–500 WBC/mcL, and 5% had more than 500 WBC/mcL. Just over half had evidence of pulmonary involvement, and only 16% had a positive tuberculin skin test (TST). Nearly 70% had a positive family history of tuberculosis.183
Another study reported a higher percentage of pulmonary involvement: 87% of 214 children with tuberculosus (TB) meningitis in that series had an abnormal chest x-ray.184 Family or exposure history was found in 66%, and the TST was greater than 10 mm in only 30%.
A study comparing 110 children with tuberculous meningitis to 94 patients with non-TB aseptic meningitis syndrome identified 5 clinical features that suggested TB:
prodromal stage of one week or longer
optic atrophy
focal neurologic deficit
abnormal movements, and
less than 50% of CSF white blood cells of the polymorphonuclear phenotype
When tuberculous meningitis is suspected, three factors should be evaluated: TST, chest roentgenogram, and exposure history (including past travel to or residence in a high-risk area). If the TST is negative (less than 5 mm), the chest roentgentogram is normal, and there is no history of exposure to tuberculosis (or residence in high-risk areas), then tuberculous meningitis is much less likely. In contrast to past teaching, pulmonary tuberculosis that has been present long enough to calcify (more than about 6 months) may still be associated with dissemination.185 Thus, even if the three factors listed above are negative, tuberculous meningitis should be considered if the CSF glucose is low and there is a CSF lymphocytosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree