Neurologic complications of cancer
Lisa M. DeAngelis, MD, FAAN
Overview
Neurologic complications of cancer are common and being seen with increased frequency as patients survive longer with better systemic disease control. Metastases can involve the brain, dura, subarachnoid space, spinal cord, roots, and nerve plexus. Chemotherapy can cause acute toxicities, such as encephalopathy from ifosfamide, which impede the delivery of a full course of effective anticancer treatment. In addition, the long-term complications of radiotherapy such as radiation myelopathy, and chemotherapy such as peripheral neuropathy, can markedly impair quality of life. Early identification, treatment, and prevention strategies can reduce these toxicities in our growing population of survivors.
Neurologic complications of cancer can be either metastatic or nonmetastatic (Table 1). Metastatic lesions affect the nervous system by direct invasion (e.g., brachial plexus metastasis), compression [e.g., epidural spinal cord compression (SCC)], or compromise of vascular supply (e.g., sagittal sinus occlusion from skull metastases). Brain metastases are discussed in 78. Although any tumor can metastasize to the nervous system, certain tumors have a predilection for causing particular central or peripheral nervous system disorders (e.g., leukemias frequently metastasize to the leptomeninges, but rarely to the brain). Prostate cancer commonly causes epidural SCC because of its tendency to metastasize to the vertebral bodies, but leptomeningeal or brain involvement is much less common.1, 2
Table 1 Neurologic complications of cancer
| Metastatic |
| Intracranial (usually to brain; see Chapter 70) |
| Spinal (usually epidural) |
| Leptomeningeal (usually base of brain and cauda equina) |
| Cranial nerves (usually from base of skull lesions) |
| Peripheral nerves (usually brachial or lumbosacral plexus) |
| Muscle (rare) |
| Nonmetastatic |
| Complications of treatment (radiation, chemotherapy) |
| Vascular disorders (hemorrhage, infarcts) |
| Metabolic, nutritional disorders |
| Paraneoplastic syndromes |
| Infections |
Nonmetastatic neurologic complications are often tumor specific. Metabolic derangements are more likely to occur with tumors that metastasize widely to vital organs, such as liver (colon cancer), or that cause changes in fluid and electrolyte balance, such as hypercalcemia (breast cancer) or inappropriate antidiuretic hormone secretion (small-cell lung cancer). Central nervous system (CNS) infections are more common in patients whose cancer is associated with immune suppression, as in Hodgkin disease. Vascular complications are more common in hematologic malignancies than in solid tumors.7 Paraneoplastic syndromes affecting the nervous system are much more frequent with certain tumors, such as small-cell lung and ovarian cancers.3 Clinically, identifying the site of neurologic dysfunction by the patient’s symptoms and signs will help to determine the diagnosis (Table 2).
Table 2 Neurologic complications in cancer patients by site
| Site | Usual causes | Typical symptoms and signs |
| Brain | Metastasis | Headache |
| Leptomeningeal metastasis | Confusion | |
| Metabolic/toxic encephalopathy | Hemiparesis | |
| Infection (meningitis, brain abscess) | Seizures | |
| Radiation encephalopathy | Ataxia | |
| Cerebral hemorrhage or infarction | ||
| Paraneoplastic (limbic encephalopathy) | ||
| Spinal cord and cauda equina | Epidural metastasis | Back pain |
| Leptomeningeal metastasis | Paraparesis | |
| Intramedullary metastasis | Sensory level | |
| Epidural abscess or hematoma | Incontinence | |
| Radiation myelopathy | ||
| Myelopathy following intrathecal chemotherapy | ||
| Paraneoplastic myelopathy | ||
| Cranial and peripheral nerves | Extrinsic compression by tumor or other mass (e.g., hematoma) | Focal pain Sensory loss |
| Direct infiltration by tumor | Motor weakness | |
| Drug toxicity | Decreased reflexes in nerve distribution (focal lesion) or distally in hands and feet (polyneuropathy) | |
| Varicella-zoster infection | ||
| Radiation plexopathy | ||
| Paraneoplastic neuropathy | ||
| Neuromuscular junction | Drugs (aminoglycoside antibiotics) | Weakness without sensory loss |
| Paraneoplastic disorders (Lambert–Eaton myasthenic syndrome, myasthenia gravis) | Respiratory insufficiency | |
| Muscle | Metastasis | Proximal weakness |
| Steroid myopathy | Weakness without sensory loss | |
| Cachectic myopathy | ||
| Paraneoplastic polymyositis or dermatomyositis |
Vertebral metastases are common in patients with metastatic cancer, but skeletal complications, including SCC, have been reduced by the use of bisphosphonates.6 SCC usually results when a vertebral body metastasis extends into the spinal canal or paraspinal tumor invades the epidural space through a neural foramen (Figure 1). Lymphomas may invade the spinal canal through neural foramina without destruction of bone. Epidural lesions may also result when tumors in the colon, kidney, prostate, or head and neck area grow directly into the spinal bony structures. When a metastasis causes the vertebral body to collapse, bone, tumor, and ligament may extend into the spinal canal to compress the spinal cord.
Metastases
Spinal metastases
Metastatic lesions compressing the spinal cord or cauda equina are, after brain metastases, the most common symptomatic neurologic complication of metastatic cancer.1, 2, 4 The spinal cord ends at the L-1 or L-2 vertebral body but compression of the cauda equina below that level is usually also considered SCC because the diagnosis and treatment are identical.1 SCC causes pain and, if untreated, paralysis and incontinence. Patients who become paraplegic as a result of cancer usually die within a matter of months; however, studies indicate that early diagnosis and treatment maintain a patient’s independent ambulation and usually result in longer survival.
Approximately 5% of patients dying of cancer have evidence of SCC at autopsy, suggesting 18,000–20,000 new cases of SCC annually in the United States.1, 5 Breast, lung, prostate, and lymphoma are the most common primary cancers causing SCC (Table 3). SCC usually occurs in the late stages of metastatic cancer, but in up to 20% of patients, cancer was unsuspected before the neurologic symptoms.
Table 3 Primary cancer causing symptomatic spinal cord compression in 583 patients at Memorial Sloan Kettering Cancer Center (MSKCC)
| Primary tumor | No. of patients (%) |
| Breast | 127 (22) |
| Lung | 90 (15) |
| Prostate | 58 (10) |
| Lymphoreticular | 56 (10) |
| Sarcoma | 52 (9) |
| Kidney | 39 (7) |
| Gastrointestinal | 29 (5) |
| Melanoma | 23 (4) |
| Unknown primary | 21 (4) |
| Head and neck | 19 (3) |
| Miscellaneous | 69 (12) |
| Total | 583 |
The thoracic spine is the most common location of SCC, followed by the lumbosacral and cervical spine in a ratio of about 4 : 2 : 1. Two or more contiguous vertebral bodies are involved by metastatic disease in approximately 25% of patients with SCC, but, unlike infection, the intervertebral disc is preserved. As many as 32% of patients have other sites of SCC in addition to the clinically suspected location, emphasizing the importance of imaging the entire length of the spinal canal when evaluating a patient for suspected epidural disease.
Pathophysiology of spinal cord compression
Epidural tumor is found both anterior and posterior to the spinal cord in almost 50% of patients; in approximately 20%, the tumor is circumferential. Histologic studies show that the most common abnormalities are demyelination with infiltration by lipid laden macrophages, interstitial edema, and focal axonal swelling, but infarction is rare, even in patients who develop the sudden onset of paraplegia. Compression of the epidural venous plexus (possibly contributing to spinal cord edema) occurs early in SCC, whereas decreased spinal cord blood flow takes place much later; therefore, venous infarction may contribute to the acute paraplegia, which can happen unpredictably, making SCC a neurologic emergency. The release of potentially neurotoxic substances, including prostaglandins and serotonin, by compressed neural tissue may also play a role in neurologic disability.
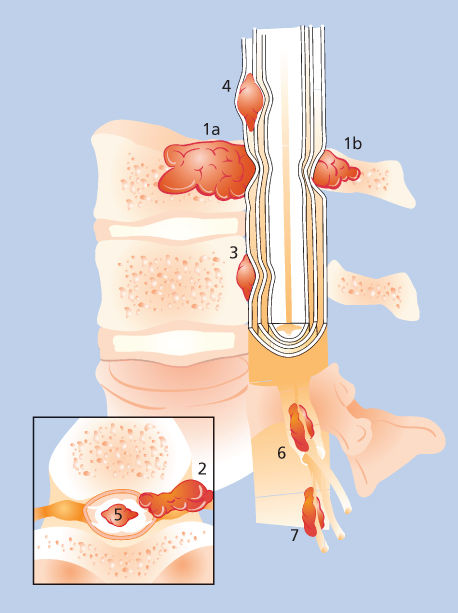
Figure 1 Neoplastic epidural spinal cord compression results from direct extension of a bony metastasis to the vertebral body (1a) or posterior elements (1b), by paraspinal neoplasm infiltrating through neural foramina (2), or a direct metastasis to the epidural space (3). Unusual causes of spinal metastases include subdural metastasis (4), intramedullary metastasis (5), and paraspinal metastasis to the radicular vessels (6) or root (7).
Clinical findings and diagnosis
The symptoms and signs depend on the level of compression (e.g., cervical vs thoracic). Back pain is the first symptom of SCC in virtually all patients (Table 4).5 The pain may be local (at the involved area of the spine), radicular (radiating into arm, trunk, or leg), or both. Local pain is dull, aching, and progressive and usually localizes to the involved area of the spine. Local pain aggravated by movement implies spinal instability. In the cervical and lumbosacral regions, radicular pain is often unilateral, but in the trunk, it is usually bilateral (band-like), a finding highly suggestive of epidural disease. Pain from SCC is typically worse when the patient is supine, which helps to distinguish SCC from a herniated disc where the pain improves with recumbancy. The pain of SCC is exacerbated by coughing, straining, or valsalva. Pain may be absent in patients whose SCC is identified incidentally on chest or abdominal CT or MRI done to evaluate the patient’s primary cancer.
Table 4 Symptoms and signs of spinal cord compression in 213 patients at Memorial Sloan Kettering Cancer Center (MSKCC)
| First symptom | Present at diagnosis | |||
| No. | % | No. | % | |
| Pain | 201 | 94 | 207 | 97 |
| Weakness | 7 | 3 | 157 | 74 |
| Autonomic dysfunction | 0 | 0 | 111 | 52 |
| Sensory loss | 1 | 0.5 | 112 | 53 |
| Ataxia | 2 | 0.9 | 8 | 4 |
Weakness is the second most common feature of epidural SCC; it usually follows the onset of pain by weeks to months and is most obvious in proximal muscles of the legs. By the time weakness occurs, tone in the lower extremities is usually increased, reflexes are hyperactive, and the plantar responses are extensor. If the SCC occurs below the cord involving the cauda equina, hyporeflexia or areflexia is found. If pain limits strength testing, analgesics should be administered to permit adequate evaluation.
Bowel and bladder dysfunction usually occur late in SCC. However, when the conus medullaris is the site of compression (vertebral lesions from T-10 to L-1), bladder dysfunction may be the first and only sign. The patient may be unaware of urinary retention because bladder sensation is lost; examination reveals a distended bladder, and a postvoid ultrasound reveals urinary retention. The anal sphincter is usually flaccid. Sensory symptoms include numbness and paresthesias that begin in the toes and spread proximally. Except in conus compression, the sacral segments may be spared, even when a sensory level is found on the trunk. In a few patients, gait or truncal ataxia mimicking cerebellar disease may be the only neurologic finding although back pain usually precedes the ataxia. Varicella-zoster eruption may occur at the dermatomal level of epidural metastasis. If it precedes the diagnosis of the spinal metastasis, it can delay recognition of SCC.
None of the clinical symptoms or signs of epidural SCC are specific (Table 5). Some disorders that mimic SCC such as herniated disc or spinal stenosis are common and may confuse the initial evaluation of an individual cancer patient. Others, such as epidural hematomas and abscesses, may be directly related to the cancer or its treatment.
Table 5 Differential diagnosis of epidural spinal cord compression
| Diagnosis | Example(s) | Diagnostic test |
| Intramedullary tumor | Glioma | MRI with gadolinium |
| Metastasis | ||
| Extramedullary-intradural tumor | Meningioma | MRI with gadolinium |
| Neurofibroma | ||
| Leptomeningeal tumor | Metastasis | MRI with gadolinium, CSF cytology |
| Primary lymphoma | ||
| Radiation myelopathy | Previous RT to spine | MRI with gadolinium |
| Arteriovenous malformation | Post-RT cavernous angioma | MRI with gadolinium, myelogram, arteriogram |
| Transverse myelopathy | Postinfectious myelopathy, multiple sclerosis | MRI with gadolinium |
| Epidural hematoma | Thrombocytopenia (history of lumbar puncture) | MRI or CT |
| Epidural abscess | Sepsis, epidural catheter | MRI with gadolinium/culture |
| Degenerative spinal disorder | Herniated disc, spinal stenosis | MRI |
| Osteoporosis | Vertebral collapse | MRI/biopsy |
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; MRI, magnetic resonance imaging; RT, radiation therapy.
Source: Adapted from DeAngelis and Posner, 2008.1 Reproduced with permission from Oxford University Press.
Patients with cancer who develop back pain have SCC until proved otherwise. Evaluation should proceed urgently because high-grade compression may cause abrupt compromise of the spinal cord and severe neurologic dysfunction. The only necessary diagnostic test for spinal lesions caused by cancer is an MRI (Figure 2). The entire spine should be imaged, but contrast is not necessary to detect spine metastasis or epidural tumor. However, contrast is essential to identify intramedullary or leptomeningeal metastasis (LM). Patients unable to have an MRI (e.g., pacemaker) should be imaged by CT-myelography where full reconstructed images can provide excellent views of the spine in all dimensions.
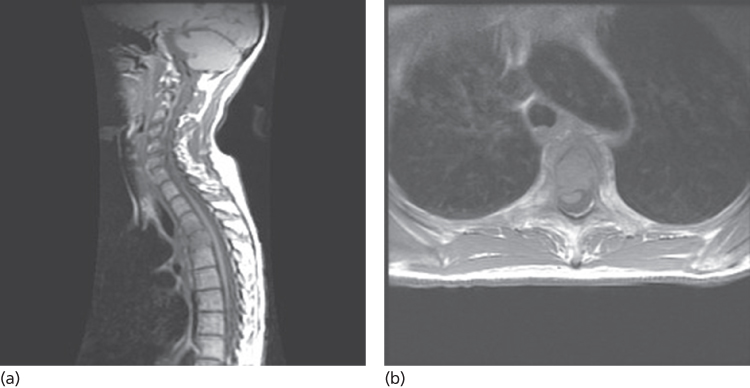
Figure 2 MRI demonstrating spinal cord compression from metastatic breast cancer. (a) Tumor in the vertebral body compressing the spinal cord arteriorly. (b) Axial image at the same level demonstrating anterior and lateral compression and distortion of the cord.
Treatment
SCC requires urgent therapy directed at reduction of tumor mass and prevention of regrowth.1, 4 Radiation therapy (RT) is the primary treatment for most patients, but surgery and chemotherapy are important modalities in individual patients. The initial treatment for all patients is corticosteroids, which can have an oncolytic effect relieving SCC by shrinking tumors, especially lymphoma, but in most instances, their salutary effects result from the reduction of spinal cord edema. Other treatment is usually started concurrently so the effect of corticosteroids is difficult to evaluate, and the optimal dose has not been determined.
For patients with pain only, dexamethasone at 16 mg every 24 h can be started increasing the dose if pain persists or new symptoms develop. For patients with severe pain, or evidence of myelopathy, an intravenous bolus of 100 mg of dexamethasone should be administered, followed by 100 mg every 24 h in divided doses. The drug should be tapered as the patient is treated with more definitive modalities. Corticosteroids cause side effects, and some may be more prominent in patients with epidural SCC. Perforation of the gastrointestinal (GI) tract may occur in as many as 1% of patients; constipation, a frequent complication of SCC, appears to increase the risk of GI rupture. Thus, a rapid taper, particularly of high initial doses, is essential.
RT is the most common treatment for patients with SCC, many of whom are poor surgical candidates because of advanced cancer or multiple vertebral body metastases.1, 5 RT to a total dose of 3000 cGy (300 Gy × 10 fractions) is administered to the site of compression and one or two vertebral bodies above and below that level. Multiple courses of RT for patients who respond initially but then relapse may be helpful and carry only modest risk.7 Intensity-modulated stereotactic radiotherapy (IMRT) is effective and safe and can be administered with good results to a previously irradiated site or in place of standard external beam RT. 8–10
Surgery plays an increasingly important role in SCC treatment. Traditionally, surgery was restricted (1) to patients who developed SCC at sites already irradiated, (2) to patients in whom a diagnosis of cancer had not been established, (3) when epidural defects result from displaced bone or disc fragments, (4) when spinal instability results from bone destruction, and (5) to patients with radio resistant tumors (e.g., renal cancer). However, a prospective phase III trial demonstrated the superiority of surgery plus RT compared to RT alone for SCC.11, 12 Tumor resection that usually required resection of the vertebral body had a superior outcome with significantly better neurologic function for longer, including a greater proportion of patients who regained ambulation and continence. Length of survival was not significantly different between the two groups, but there was a trend toward longer survival with surgery (129 days vs 100 days, p = 0.08). These data suggest that surgery should be a consideration in all patients with SCC. However, the patients enrolled in the study were highly selected and it is not clear that the excellent outcome reported can be expected for all patients with SCC. More recently, vertebroplasty and kyphoplasty have proven effective for selected patients with SCC, especially those with vertebral compression fractures and severe pain.13
Chemotherapy, with or without RT, is useful in some patients with tumors that are sensitive, especially lymphoma and occasionally other solid tumors.14 Excellent responses of SCC from chemosensitive tumors to appropriate chemotherapy are seen, but this approach is limited to those who have minimal to no neurologic compromise. If myelopathy is present, RT or surgery must be the primary therapy. Regardless of treatment type, the majority of patients who are ambulatory at the beginning of therapy remain ambulatory. Some paraparetic patients will recover sufficient function to walk again, but only a few who are paraplegic recover useful function.
Leptomeningeal metastasis
LM is less frequent than brain metastasis or SCC but is becoming increasingly common, particularly in patients with small-cell lung, breast, and ovarian cancers. Quality of life and duration of survival are both severely compromised by LM, and fewer than one-half of those treated by currently available therapy receive benefit.
Reliable estimates of the incidence of LM are difficult to obtain, but LM is found in approximately 5% of patients with metastatic cancer. Few studies of LM give incidence figures and the diagnosis is heavily dependent on the diligence with which it is pursued. Recognizing these limitations, the frequency of LM in different cancers varies widely (Table 6).
Table 6 Frequency of leptomeningeat metastases (LM) in various cancers
| Cancer | Percent developing LM | Features |
| Carcinoma | ||
| Breast | 5 | Incidence may be increasing, more common with infiltrating lobular carcinoma |
| SCLC | 9–25 | Incidence is increasing, risk increases with duration of survival |
| NSCLC | ? | Less common than breast |
| Melanoma | 23 | 50% at autopsy |
| Leukemia | ||
| AML | <5 | 10% without prophylaxis; associated with high WBC count, elevated LDH, extramedullary disease at diagnosis, and monocytic morphology |
| ALL | 11 | 30% without prophylaxis; associated with T-cell phenotype, Burkitt morphology, and high WBC count |
| CLL | Rare | May occur during blast crisis |
| Lymphoma | ||
| NHL | 4–10 | Associated with diffuse large B-cell and lymphocytic histology, bone marrow involvement |
| HD | Rare | |
| Overall | 8.6 | LM present at autopsy in 56 of 649 brains examined at Memorial Sloan Kettering Cancer Center |
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; HD, Hodgkin disease; LDH, lactic acid dehydrogenase; NHL, non-Hodgkin lymphoma; NSCLC, nonsmall-cell lung cancer; SCLC, small-cell lung cancer; WBC, white blood cell.
Pathophysiology
Malignant cells may enter the subarachnoid space by several routes (Figure 3). Subarachnoid invasion can occur via infiltration of the wall of veins and of the marrow trabeculae; malignant cells can attach to leptomeningeal capillaries and move directly into the subarachnoid space. Brain parenchymal lesions can erode into the ventricle or subarachnoid space or be spilled at surgery, causing LM. LM develops in 40% of patients following resection of a cerebellar metastasis, but in only 2–3% of those who had resection of a supratentorial lesion. The choroid plexus is a rare route of entry. Malignant cells may invade the subarachnoid space by direct infiltration along nerve roots, and possibly via epineural lymphatics.
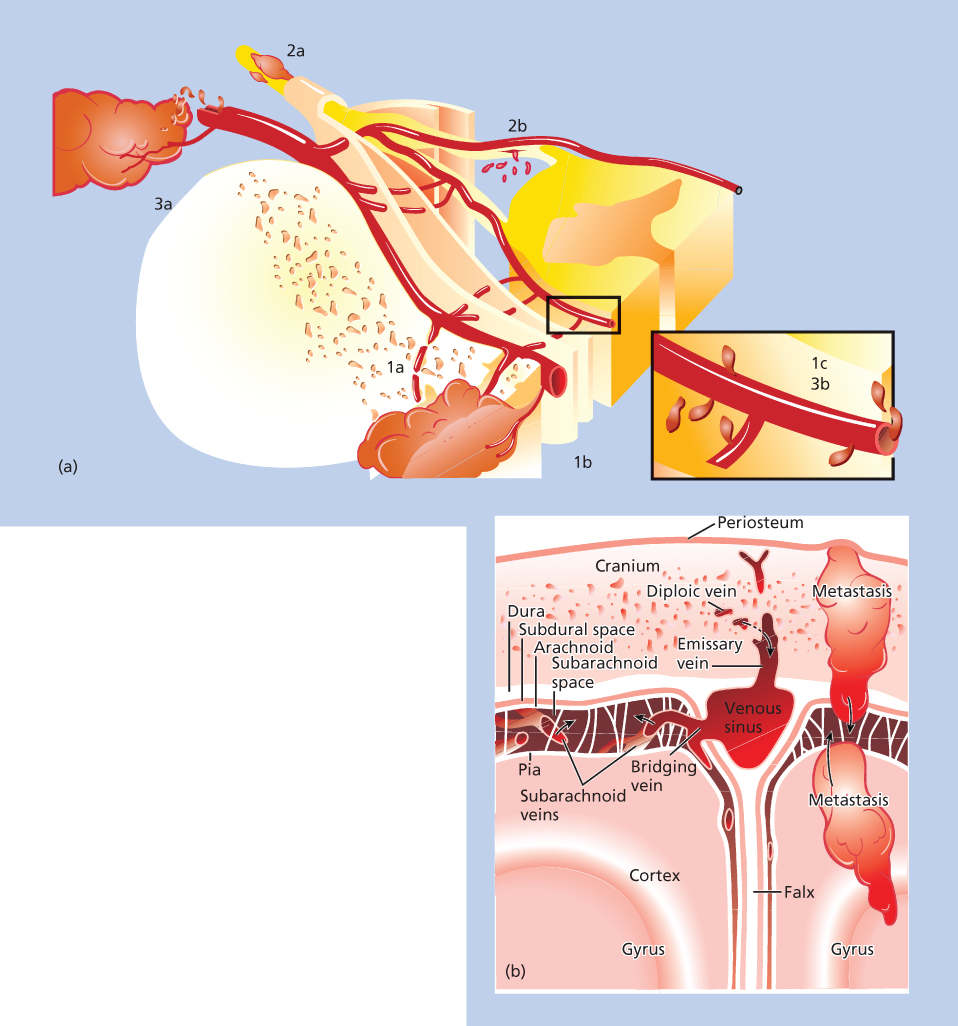
Figure 3 Pathophysiology of leptomeningeal metastases. (a) Mechanisms of tumor cell entry into the spinal subarachnoid space. Tumor may invade the vertebral body (1a) and grow along vertebral veins (1b) into the subarachnoid space (1c). Tumor may invade peripheral nerves or nerve roots outside the vertebral canal (2a) and grow along the nerve sheath into the spinal canal to seed the leptomeninges (2b). The tumor can invade blood vessels outside the central nervous system (3a) and transverse subarachnoid veins into the subarachnoid space (3b). Source: From Ref. 1. (b) Possible mechanisms of tumor entry into the cerebral subarachnoid space. Tumor may enter the cranial subarachnoid space via metastases either to the skull or brain, to the diploic veins of the skull, or directly from subarachnoid veins. The choroid plexus (not shown) is also an occasional site for the formation of leptomeningeal tumor.
LM causes nervous system dysfunction by several mechanisms:1 (1) direct infiltration of malignant cells into the brain, spinal cord, cranial nerves, or spinal roots that interferes with neural function, (2) interruption of CSF flow leading to increased intracranial pressure (ICP) with or without hydrocephalus, and (3) infiltration of tumor along the Virchow–Robin spaces may reduce blood supply to the brain causing cerebral infarction.
Symptoms and signs
LM is strongly suggested by the patient having symptoms or signs at multiple sites of the neuraxis.1, 2 These include (1) headache, particularly early in the morning or posturally induced headache in the absence of brain metastases; (2) cranial nerve palsies, particularly, diplopia or facial weakness; (3) neck pain in the absence of cervical spine metastases; (4) radicular pain in the arms or legs, particularly when accompanied by weakness but without local spine pain; (5) unexplained constipation, impotence, or urinary incontinence or retention; (6) asymmetric leg weakness and diminished reflexes in the absence of pain or sensory changes; and (7) confusion, memory loss, or other cognitive abnormalities.
Diagnosis
The diagnostic gold standard of LM is the demonstration of malignant cells in the CSF. However, imaging is a much more common means of diagnosis currently.15 A cranial MRI may reveal enhancing cranial nerves or enhancing tumor in cortical sulci; communicating hydrocephalus may suggest LM. A gadolinium-enhanced spine MRI may demonstrate tumor nodules on spinal roots, particularly in the cauda equina, even when symptoms of nerve root dysfunction are absent (Figure 4). The whole neuraxis should be imaged to identify sites of bulky disease. When characteristic findings are identified, this suffices to establish the diagnosis. MRI is 76% sensitive and 77% specific. Occasionally, FDG-PET imaging may identify LM.16 However, negative imaging does not exclude the diagnosis in a patient with typical features. A lumbar puncture should be performed and the opening pressure, cell count, protein, glucose, and bacterial and fungal studies should be obtained; the cytology specimen should contain at least 10 mL of CSF optimally and should be processed quickly according to the laboratory’s protocol.
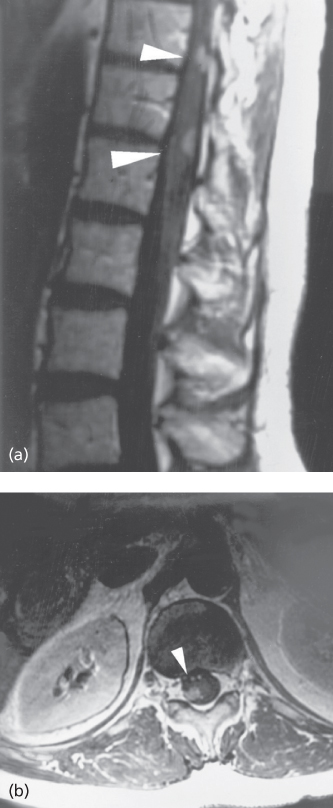
Figure 4 Gadolinium-enhanced MRI demonstrating leptomeningeal metastases from lung cancer. (a) Sagittal and (b) axial images demonstrating enhancing nodules within the thecal sac. This patient had a positive cerebrospinal fluid cytology for malignant cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








