Neoplasms of the prostate
Christopher J. Logothetis, MD  Jeri Kim, MD
Jeri Kim, MD  John W. Davis, MD, FACS
John W. Davis, MD, FACS  Brian F. Chapin, MD
Brian F. Chapin, MD  Deborah Kuban, MD, FACR, FASTRO
Deborah Kuban, MD, FACR, FASTRO  Eleni Efstathiou, MD, PhD
Eleni Efstathiou, MD, PhD  Ana Aparicio, MD
Ana Aparicio, MD
Overview
Cancer of the prostate is the most commonly diagnosed nonskin neoplasm and the second leading cause of cancer-related mortality in men in the United States. Considerable advances have been made in screening, diagnosis, and therapy options, particularly in advanced disease, but controversies about the diagnosis and management of prostate cancer, especially in the areas of screening and choice of therapy, continue to evolve. Controversies in advanced disease states have shifted from prognostication to prediction, and current treatment considerations are focused on optimization of sequence or combinations of therapy, determining the role of local control and bone targeting. It is anticipated that addressing these knowledge gaps will lead to an integrated and more effective treatment strategies. Further advances in therapy can be achieved by development of new agents with unique mechanisms of action and rational integration into combination therapies.
Prostate cancer awareness, clinical application of improved biopsy schemes, and advances in imaging combined with the widespread use of prostate-specific antigen (PSA) have resulted in increased detection of prostate cancer. The evolving use of the serum PSA concentration and its change over time have not been paralleled by studies that tested the relevance of those findings until the results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) and the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial were first published in 2009.1 Though many of the apparent discrepancies between these trials can be accounted for by trial design and patient cross-contamination, they brought to the forefront the dilemma of overdiagnosis and overtreatment and the urgent need to improve the accuracy of clinically significant prostate cancer. It is hoped that replacement of the current morphologic and anatomic classification of prostate cancer with one based on improved understanding of biology will lead to molecular classification and bring closer a personalized management of this complex disease.
Salient features that distinguish prostate cancer from other malignancies and that frame the dilemmas surrounding it are its striking age-dependent incidence, with progressively increasing frequency with increasing age; the variable lethality of morphologically identified cancers; the central role of androgen signaling; and the preponderance of bone-forming metastases on its lethal progression. The important advances made in each of these areas will, in the near future, modify the approaches currently used to prevent, prognosticate, and treat prostate cancer.
Biology of prostate cancer
Normal anatomic and histologic features of the prostate
The prostate gland sits in the pelvis, surrounded by the rectum posteriorly and the bladder superiorly, and it is anchored to the bladder pelvic floor; the urethra communicates between the bladder and the prostate into the penis (Figure 1). The prostate is composed of stromal, ductal, and luminal epithelial cells and is organized around branching ducts and individual glands lined with secretory epithelial cells and basal cells.2 The secretory epithelial cell is the major cell type in the gland. These androgen-regulated cells produce PSA and prostatic acid phosphatase (PAP). The central role of androgen signaling in prostate cancer biology likely accounts for the utility of PSA and PAP in determining disease status clinically. The vast majority of prostate cancers have cells that share properties with the secretory epithelial cells. Unlike the epithelial cells, the basal cell layer is not directly controlled by androgen signaling. Investigators have suggested that the basal cell population contains the prostate stem cells from which the epithelial cells develop. If correct, this view has obvious implications for the prevention and treatment of all stages of prostate cancer.
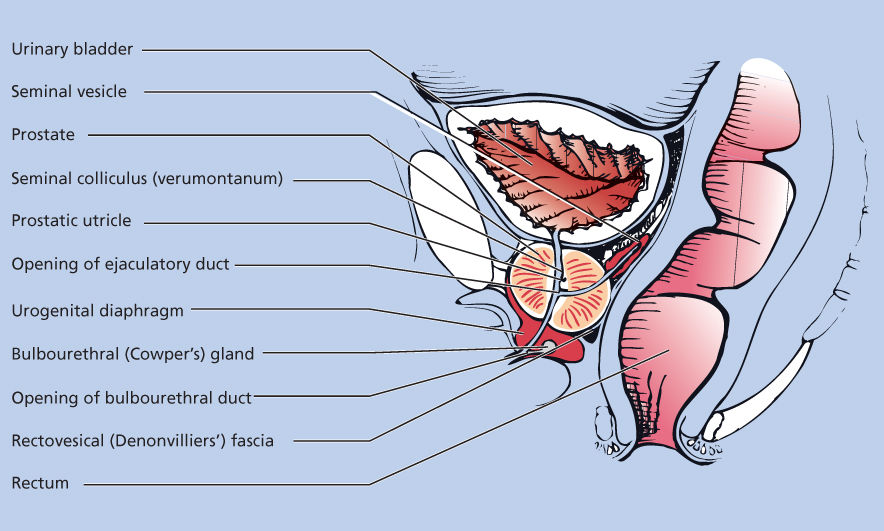
Figure 1 Normal prostate anatomy.
As in other human tissues, cells belonging to the neuroendocrine system are also present within the prostate. Neuroendocrine cells contain secretory granules and extend dendrite-like processes between adjacent epithelial cells or toward the acinar or urethral lumina.3, 4 A variety of secretory products can be found within the granules, including serotonin, calcitonin, gastrin-releasing peptide, and somatostatin.3, 4 Neuroendocrine cells are commonly identified immunohistochemically by the presence of markers such as chromogranin A or synaptophysin in the cytoplasm. They are terminally differentiated cells that are thought to regulate the growth, differentiation, and function of coexisting prostatic cells, but their exact role remains to be fully understood.
The view that the prostate has a lobar pattern has been challenged. McNeal et al.5 conducted detailed studies of the normal and pathologic anatomy of the prostate and introduced the transforming concept of anatomic zones rather than lobes to describe the gland. There are four major zones within the normal prostate: peripheral, central, transition (constituting 70%, 20%, and 5% of the glandular tissue, respectively), and anterior fibromuscular stroma (Figure 2). The peripheral zone, which extends posterolaterally around the gland from the apex to the base, is the most common site for the development of prostate carcinomas. The central zone surrounds the ejaculatory duct apparatus and makes up the majority of the prostatic base. The transition zone constitutes two small lobules that abut the prostatic urethra and is the region where benign prostatic hypertrophy (BPH) primarily originates. Some reports suggest that transition zone cancers have a lower malignant potential, but other studies report no difference in outcome compared with those originating in the peripheral zone, when controlled for grade and stage.6, 7
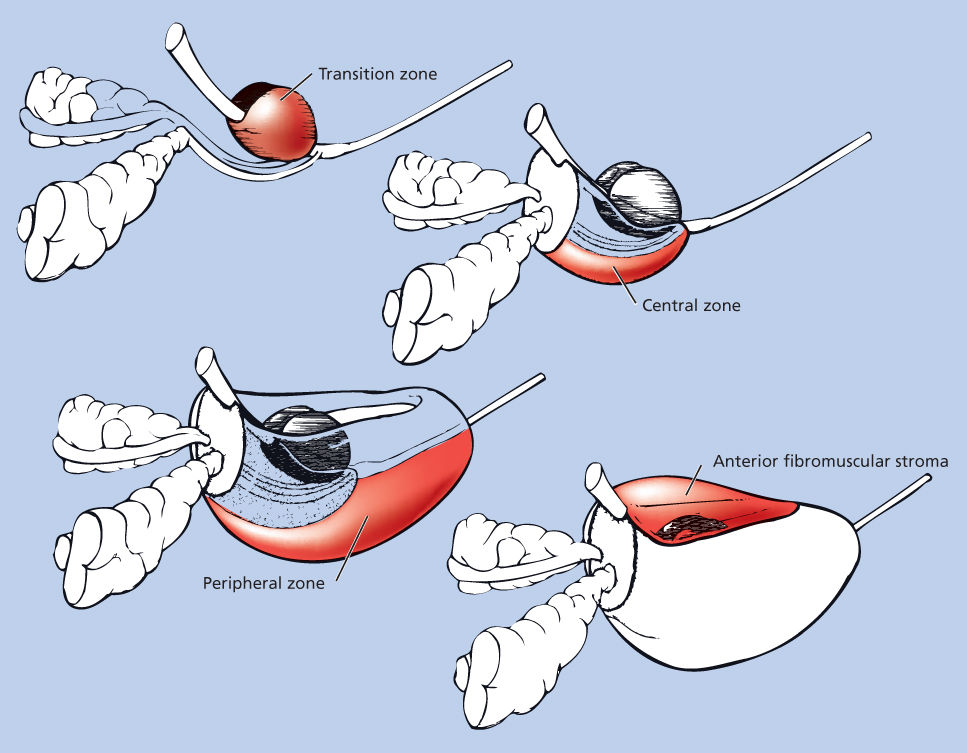
Figure 2 Zonal anatomy of the prostate: the three glandular zones of the prostate and the anterior fibromuscular stroma.
Surrounding the gland is stroma, which includes fibroblasts, smooth muscle, nerves, and lymphatic tissue. The roles of stromal–epithelial interactions in prostate physiology and cancer development are being elucidated. Recent insights suggest that these interactions are critical in normal function, and increasing evidence implicates them in prostate carcinogenesis as well. The stromal–epithelial interactions may exert both tumor-promoting and carcinogenesis—and progression—inhibitory effects. Furthermore, the stromal–epithelial interacting pathways implicated in the development of the tumor microenvironment in prostate cancer progression may be those that are shared by the prostate and bone in their normal development and function.8
There are varying distinct anatomic barriers surrounding the prostate. The smooth muscle of the prostatic stroma gradually extends into fibrous tissue that then ends in loose connective and adipose tissue. Of particular relevance is the absence of any semblance of a capsule at the gland’s apex and anteriorly. This understanding of anatomic detail allows clinicians to determine the adequacy of prostate surgery by accurately defining the surgical margin with increasing confidence. It also allows the surgical delineation of disease as “organ confined” or “specimen confined.” The organ-confined cancers do not extend beyond the confines of the prostate, whereas specimen confined indicates that the cancer does not extend beyond the cut margins. The distinction between these two terms is important because they are used to determine the adequacy of surgery and inform the use of postoperative radiation therapy in selected patients.
A final anatomic note about the prostate is that Walsh and Donker9 described the presence of two neurovascular bundles that pass adjacent to the gland posterolaterally. The neurovascular bundles are essential for normal erectile function, and defining their presence outside of the posterior–lateral prostatic fascia allowed Walsh to develop a “nerve-sparing” radical retropubic prostatectomy procedure that improves the odds of preserving potency.10
Premalignant prostatic lesions
Paradigms that are used to explain the progression of other solid tumors may not apply to prostate cancer.11 Extensive information about the genetic and epigenetic phenomena associated with prostate cancer progression has been developed recently but has yet to pass the threshold of clinical utility to be truly useful. Many clinicians accept that premalignant lesions existing in the prostate may precede the development of cancer by many years. But given the lack of knowledge about the nature or rate of their progression, the morphologic identification of premalignant lesions on biopsy specimens only serves to provide rationale for close monitoring of patients.
Morphologically heterogeneous lesions are included under the single term prostatic intraepithelial neoplasia (PIN) (Figure 3).12 PIN is defined as the presence of cytologically atypical or dysplastic epithelial cells within architecturally benign-appearing glands and acini. Although three different grades have been described, 1 (mild), 2 (moderate), and 3 (severe), grades 2 and 3 PIN are often combined as “high grade” and worthy of note. PIN is presumed to be a premalignant lesion because it is commonly present adjacent to prostate adenocarcinomas.13 The finding of PIN is associated with the existence of cancer in sites not sampled on biopsy and implies increased risk of developing a morphologic cancer although the risk of progression has not been established or quantified.
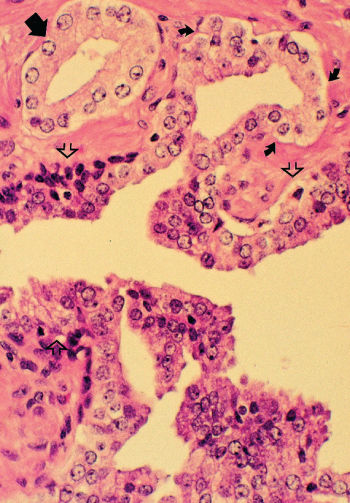
Figure 3 Photomicrograph of high-grade prostatic intraepithelial neoplasia (PIN) with basal cell layer (open arrows) with budding microacinus lacking basal cells (curved solid arrows). A microacinus of invasive Gleason pattern 3 adenocarcinoma is seen in the adjacent stroma (straight solid arrow). Hematoxylin and eosin × 160.
Source: Courtesy of Thomas M. Wheeler, MD.
Prospective studies have been small but have reinforced the hypothesis that PIN is the morphologic manifestation of a precursor lesion to morphologic prostate cancer. The data have suggested that the presence of high-grade PIN predicts the subsequent development of cancer, perhaps through a multistep carcinogenesis process. However, close clinical follow-up remains the standard of care after diagnosis of high-grade PIN alone.
Another lesion that may represent a premalignant change is atypical adenomatous hyperplasia (AAH), although existing data on this are scantier than they are for PIN. The characteristic appearance with AAH is the fulfillment of the architectural criteria for malignancy, with disruption of the basal cell layer, mainly in the transition zone, but without the cytologic changes diagnostic of cancer.14 Some authors have suggested that a prostatic lesion composed of focal areas of epithelial atrophy associated with chronic inflammation (called proliferative inflammatory atrophy, or PIA) is a precursor of PIN and eventually prostate cancer.15 Evidence for this hypothesis includes the observation that PIA often occurs adjacent to areas of PIN and prostate cancer16 and that somatic genetic abnormalities seen in PIA often resemble those seen in prostate carcinoma.17 Of particular relevance is that PIA implicates inflammation in the progression of prostate cancer.18 If this hypothesis is confirmed and causally implicated with greater confidence in prostate cancer progression, that finding may lead to more effective prevention strategies.
Histologic features of prostate cancer
Cancers that arise in the epithelium account for >95% of prostate cancers (Figure 4).19 The reported low frequency of histologic variants may reflect the fact that frequency determinations of variants are principally derived by the examination of primary cancers, among which the more common forms may be overrepresented. However, less common histologic varieties have been described, including mucinous or signet ring tumors, adenoid cystic carcinomas, carcinoid, large prostatic duct carcinomas (including the endometrial type), adenocarcinomas, and small-cell undifferentiated cancers. These unusual subtypes are reported to occur in low frequencies. Because the histologic variants often present with advanced disease clinically, they are not subjected to surgery as often as the more common forms of prostate cancer. In addition, they occasionally manifest only in metastases during progression, thus in sites not often sampled. As a consequence, we may be underestimating their frequency because of their manifestation. This fact may be particularly important when attempting to estimate the true frequency of small-cell carcinomas of the prostate, which have been reported with increasing frequency.20 Nonetheless, it is important to recognize these unusual variants of prostate cancer because standard hormonal therapies may be less effective in their treatment while they may be more responsive to chemotherapy than the more common type.17, 21
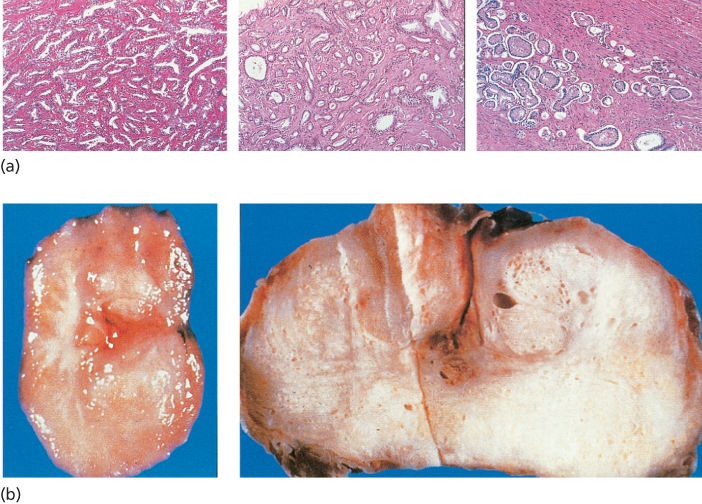
Figure 4 (a) Microscopic histologic appearance of prostate adenocarcinoma. (b) Gross histologic appearance of prostate adenocarcinoma.
Tumors with a neuroendocrine appearance (i.e., carcinoid and small-cell undifferentiated types) may arise from Kulchitsky cells, which are found in the basal regions of the prostatic epithelium.22 Small-cell carcinomas of the prostate share histologic and clinical features with other extrapulmonary small-cell carcinomas. These cancers have been described as a histologic continuum, perhaps in some instances reflecting progression of acinar adenocarcinomas.23 Thus, these “neuroendocrine” cancers of the prostate are likely to be a mechanistically and clinically heterogeneous grouping. Of importance is that they predict a specific pattern of anatomic progression: nonosseous visceral spread with lytic bone metastases and the probability of responsiveness to chemotherapy. These are recognized as a unique and aggressive variant of prostate cancer that account for a significant portion of far advanced prostate cancers.
Transitional cell carcinomas involving the substance of the prostate may also be mistaken for prostate adenocarcinoma. It may be difficult to distinguish a transitional cell carcinoma arising in the transitional epithelium of the distal prostatic ducts from a tumor arising in the bladder epithelium and spreading into the contiguous prostatic ducts.
Study findings have confirmed the primary prognostic importance of the degree of histologic differentiation of prostate adenocarcinoma. The degree of this differentiation is typically determined by patterns of gland formation and, less importantly, by cytologic detail. The most widely accepted grading scheme for adenocarcinoma of the prostate is that developed by Gleason (Figure 5).25 Gleason created a system for classifying prostate tumors based on two levels of scoring that recognize the heterogeneous nature of prostate carcinomas. The primary pattern of differentiation is assigned a Gleason grade of 1 to 5 according to the dominant morphologic features of the specimen and its departure from normal appearance; the next most common pattern is also assigned a grade. This results in a two-digit score; for example, 3 + 4 = 7. The Gleason system has been criticized for inadequately recognizing the proportion of the tumor that is composed of the secondary pattern as well as for lacking adequate distinction between good and poor prognoses in patients whose cancers have Gleason scores of 5–7 (most patients). However, the reproducibility and reliability of Gleason grading between pathologists have consistently been shown to be excellent. Gleason’s original work demonstrated a clear association between a higher score and a higher mortality rate, which others have since confirmed.26 Many other predictors of the clinical behavior of prostate cancer have been explored, but the Gleason score still remains the most broadly applicable and prognostically useful histologic grading system.
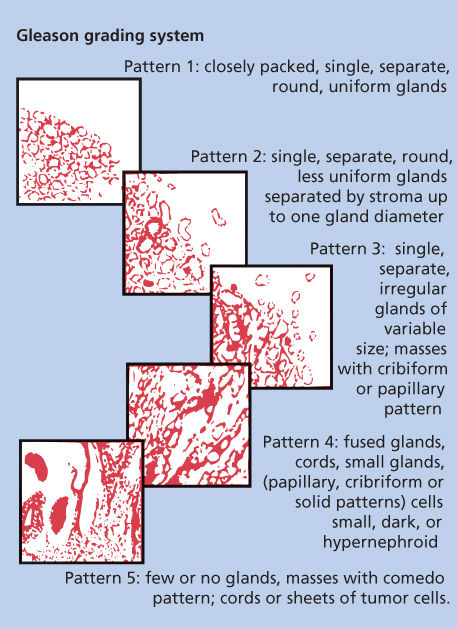
Figure 5 Histologic grading scheme for adenocarcinomas of the prostate.
Source: Kattan 2007.24 Reproduced with permission of Nature Publishing Group.
Molecular pathogenesis
Unlike the case of breast cancers, in which clinically relevant subsets of cancer have been identified on the basis of molecular profiles, the morphologic characterization of prostate cancer remains the standard.27 Prostate cancer cells harbor a number of somatic mutations, and in advanced disease, additional alterations accumulate. Alterations that affect the development and progression of prostate cancer include those in the hormonal and growth factor milieu, in hormonal and growth factor receptors, in intracellular signaling pathways, and in cell cycle regulation and apoptosis. The identification of chromosome 8q24 as a susceptibility locus supports the hypothesis that a significant portion of prostate cancers have genetic origins.28
Technological advances in genome sequencing made possible to identify germline mutations that have been linked to increased risk of prostate cancer. The HOXB13 G84E variant is the first bona fide prostate cancer susceptibility gene to be identified. In a study of 94 unrelated patients, the HOXB13 G84E variant was associated with a significant risk of early-onset hereditary prostate cancer.29 Additionally, germline BRCA1 and BRCA2 mutations confer high risk of prostate cancer and prognostic of more aggressive prostate cancer.30 However, these germline mutations have not yet translated into therapeutic relevance.
The hormonal and growth factor milieu to which the prostate is exposed has been consistently associated with the pathogenesis of cancer. In population-based studies, two hormones have been implicated: testosterone and insulin-like growth factor I (IGF-I). The association between testosterone and prostate cancer progression is well known. Several lines of evidence also suggest that IGF may be important in prostate cancer growth. First, several prostate cancer cell lines and prostate xenograft models express both IGFs I and II and their receptors.31, 32 Second, Chan et al.33 reported on the relationship between plasma IGF-I concentration and prostate cancer, citing a relative risk of 4.3 for men in the highest quartile, compared with men in the lowest quartile. Moreover, a higher incidence of prostate cancer has been noted in patients who had relatively high IGF-I concentrations in plasma samples that had been obtained 5 years prior to the cancer diagnosis,34 supporting the concept that IGF-I may be important early in the development of prostate cancer. Although this observation has yet to be confirmed, it does implicate IGF-I signaling in prostate cancer progression. It is likely that growth factor and other stromal–epithelial interacting pathways cooperate in prostate cancer progression. Thus, a simple model centered on a single pathway is unlikely to lead to the understanding of human prostate cancer. Other growth factors—including epidermal growth factor, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor beta (TGF-β)—may also be dysregulated in the development of prostate cancer (Table 1).35
Table 1 Growth factors implicated in prostate cancer
| Transforming growth factor beta |
| Fibroblast growth factor |
| Epidermal growth factor |
| Insulin-like growth factor |
| Platelet-derived growth factor |
| Vascular endothelial growth factor |
| Neurotensin |
| Endothelins |
| Colony-stimulating factors |
The central role of androgen signaling in an endocrine fashion has dominated the understanding of the pathway in prostate cancer. It is clear that androgens are a major mediator of progression, though their role in prostate cancer susceptibility remains poorly understood. In fact, several hormonal receptors are altered in prostate cancer cells. Perhaps the best example is alterations in the androgen receptor (AR). In early cancer, AR mutations are relatively uncommon,36 but germline variation (CAG repeats) in the AR gene has been shown to be a predictor of cancer aggressiveness37 and may play a role in the frequency and aggressiveness of prostate cancer in African-Americans.38 It is interesting that AR mutations are more commonly seen in androgen-independent (castrate-resistant) prostate cancer,36, 39 arguing that the AR gene remains central in the growth and survival of prostate cancer even after the need for the ligand (androgens) has been mitigated. Several theoretical frameworks have been proposed for the development of androgen independence, most of which still postulate a cancer cell that depends on a functional AR but one that is amplified, oversensitive, promiscuous, or activated by upregulated coactivators or downregulated by corepressors.40 For example, in LNCaP prostate cancer cells, an AR mutation, T877A, which is a substitution of alanine for threonine at position 877, results in an AR that is activated by other steroid hormones and by the androgen antagonist flutamide.41 This AR mutation could help explain the “antiandrogen withdrawal syndrome.” However, AR mutations occur too infrequently to account for the eventual evolution of most metastatic prostate cancers to a castrate-resistant state.
AR splice variants have been identified in prostate cancer. AR splice variants lacking the ligand-binding domain (ARVs), originally isolated from prostate cancer cell lines derived from a single patient, are detected in normal and malignant human prostate tissue, with the highest levels observed in the late stage, castrate-resistant prostate cancer (CRPC). Approximately 20 AR splice variants have been identified to date, and they are not exclusively found on prostate tissue. The first AR splice variant was identified in the placenta. “The most studied variant (called AR-V7 or AR3) activates AR reporter genes in the absence of ligand and therefore, could play a role in castration resistance.” Correlative studies have associated the presence of ARV7 in prostate cancer-infiltrated bone marrow biopsies and circulating tumor cells from patients with metastatic CRPC who have primary resistance to novel androgen signaling inhibitors abiraterone acetate (CYP17 inhibitor) and enzalutamide (second-generation antiandrogen).42, 43
Recent observations support the view that “intracrine” production of androgens acting in both autocrine and paracrine fashion are implicated in the progression of prostate cancer. Several lines of evidence support the concept that CYP17 lyase is implicated in remodeling the prostate cancer tumor microenvironment: androgens in the microenvironment are at a higher concentration than they are in the serum, CYP17 expression occurs in stage-dependent cancer progression, and tumor regression occurs clinically in castrate-resistant cancers. These data support the hypothesis that androgen signaling can be considered a stromal–epithelial interacting pathway. Difficulty in measuring the concentration of androgens in the local microenvironment continues to plague this concept, and although it is appealing, it remains unproven.
True androgen independence is likely to arise from alternative stromal–epithelial interacting or other signaling pathways. Several bone and prostate development pathways have been noted to be involved in prostate cancer progression and to be associated with higher-grade cancers.44 This attractive hypothesis could account for the bone-homing and bone-forming phenotype of prostate cancer and for its resistance to therapy in an organ-specific manner.
Molecules that alter intracellular signaling pathways also may be important in the pathogenesis and progression of prostate cancer to a castrate-resistant state. The clearest example to date is the tumor suppressor gene PTEN. This gene encodes for a phosphatase that is important in modulating the signal generated by activated growth factor receptors. Somatic mutations in PTEN occur at high frequency in prostate carcinoma cells, suggesting this as a frequent target for inactivation. One study documented a 60% rate of PTEN mutations; most of these were found in cell lines from metastatic disease, although mutations were also seen in primary cancers.45 In fact, another group demonstrated higher rates of PTEN loss or mutation in tumors of advanced stage and grade.46 Another pathway that may be inappropriately activated in prostate cancer is the hedgehog pathway. Normal hedgehog signaling is important in early development and patterning of the prostate epithelium. Recent work by Karhadkar et al.47 demonstrated that activation of the hedgehog pathway distinguishes prostate cancer from normal prostate cells and, further, metastatic prostate cancer from localized cancer. Moreover, they demonstrated that hedgehog pathway inhibition results in PC3 xenograft regression. Both the PTEN and hedgehog pathways are therefore attractive targets for drug development.
Small-cell prostate carcinomas, which often emerge during the progression of CRPC, have garnered much attention in recent times as a model of primary and possibly secondary resistance to AR-directed therapies. Most small-cell prostate carcinomas lack markers of prostatic luminal differentiation such as AR, PSA, PSAP, PSMA, and p501s.48–56 Instead of these, they often express markers that are characteristic of neural progenitor cells, such as ASCL1, POU4F2, and MYCN.50, 57, 58, 59 A decrease in the expression of RE-1-silencing transcription factor (REST), a master repressor of neuronal differentiation, has been proposed as a mechanism involved in this transdifferentiation.60, 61 In addition, small-cell prostate carcinomas are characterized by high Ki67 staining and high levels of expression of genes involved in cell cycle and mitosis, including AURKA, AURKB, PLK1, and UBE2C.58, 60, 62 It is noteworthy that concordant AURKA and MYCN amplification has been found in approximately 40% of small-cell prostate carcinomas58 and that REST knockdown resulted in a derepression of cell cycle genes in prostate cancer models61 supporting a connection between the aberrant neural development and mitotic programs. Finally, small-cell prostate carcinomas have also been shown to bear frequent Tp53 mutations49, 50, 51, 63, 64, 65 as well as RB1 and PTEN losses and a high rate of copy number alterations.50, 58, 62, 63, 65, 66
Finally, alterations in molecules that regulate the cell cycle and apoptosis offer promising avenues for further investigation. TP53, p27, p21, and Rb have been studied, and the results have provided variable levels of evidence that they participate in the pathogenesis of prostate cancer.67 Of particular relevance is that each of these molecules has been reported by some investigators to serve as prognosticators. The utility of these molecules as independent prognosticators or as part of a signature has yet to be prospectively validated. Signatures that predict disease recurrence after surgery have yet to find a role in the clinic.68
Early detection of prostate cancer
Early detection of prostate cancer, when it remains confined to the prostate, brings patients and their physicians face to face with the controversial question of how localized disease may best be managed, that is, how patients can avoid overtreatment and preserve quality of life while simultaneously escaping from life-threatening disease.
Prostate cancer screening and early detection themselves are controversial because for every 18 cases detected, only 3 will result in death. The cost of radical prostatectomy, which removes the threat of disease if there is no metastasis, may be the risk of impotence and urinary incontinence, which might affect these disease-specific quality-of-life domains.69 To determine whether screening for prostate cancer and three other cancers reduces mortality, the National Cancer Institute’s (NCI’s) Division of Cancer Prevention undertook the randomized, controlled PLCO Screening Trial at 10 sites in 1993. The prostate screening arms have been published with commentary.1, 70 Participants underwent annual PSA screening examinations for 6 years and follow-up of 13 years for the latter publication.71, 388
With a sample size of 76,685 men allocated to intervention (38,340) or control (38,345), the extended follow-up diagnosed 4250 cancers in the intervention arm and 3815 in the control. These events corresponded to 158 deaths in the intervention arm and 145 in the controls. The key conclusion was that PSA screening increased detection but did not affect mortality. A common critique of this study as echoed by Smith70 was significant contamination in the arms in the form of noncompliant screening in the intervention arm and opportunistic screening in the control arm.
Also launched in the 1990s was the ERSPC, which is also testing whether screening saves lives. It was published along with the PLCO trial in 2009. Updated results truncated at 11 years and at 13 years.71, 72 In this trial, there was significantly less contamination of the arms. The ERSPC’s results showed a significant relative reduction in prostate cancer mortality by 21% and 29% after adjustment for noncompliance.382 To prevent one death, the number needed to be screened was 1055 and to detect was 37. The additional follow-up time improved the screening metrics as expected. The authors caution that all-cause mortality was not affected and that overdetection/overtreatment remains a problem with PSA screening.398 The trial is often criticized in its design as not a unified multicenter study but rather a merger of several screening studies with differences in methodology and outcomes. As a result, the US Preventive Services Task Force (USPSTF) has issued a “D” rating for PSA screening (discourage the use of this service), yet this remains highly controversial.73, 74
Identifying early disease using PSA and PSAV
Strategies to manage the diagnosis of localized prostate cancer include watchful waiting, radical prostatectomy, and radiotherapy and will result in superior recurrence-free outcomes at earlier stages of intervention. The early detection of prostate cancer debate has centered around using absolute PSA cutoffs versus prostate-specific antigen velocity (PSAV) or PSA isomers. The PSAV, one measure used in monitoring patients with localized disease, has been scrutinized as a tool for use in the diagnosis and prediction of outcomes. It is calculated using the log slope of at least three PSA values calculated over at least 2 years with no less than 6 months between measures.75 Conceived as a way to capture the variability of prostate cancer or its progression, PSAV measures are used preoperatively and postoperatively. Researchers sometimes rely on measures taken closer together, consider fewer than three measures, and reduce the longitudinal period to less than 2 years.
Prostate cancer screening can be oversimplified into an algorithm in which all patients with a certain threshold of PSA (e.g., 2.5 or 4.0 ng/mL) or abnormal digital rectal exam (DRE) findings are referred to a urologist for evaluation and possible biopsy. However, patients’ overall interests are better served if a more comprehensive evaluation takes place that considers whether they are at increased risk of having prostate cancer because of ethnicity (e.g., African-Americans are at increased risk), age, and/or family history and whether a prostate cancer diagnosis would be likely to affect their overall survival because of a younger age and fewer competing comorbid conditions. A comprehensive PSA history may be beneficial for calculating PSAV, and the complexed PSA test may be useful as a frontline screening tool because it has slightly better specificity than total PSA in the total PSA range of 2.5–4.0 ng/mL.76
The PSA blood test has been described as a test that “neither excludes benign disease nor wholly predicts meaningful malignancies.”75 Ian Thompson, principal investigator of the Prostate Cancer Prevention Trial (PCPT), and his colleagues studied 8575 men from the study’s placebo group to estimate the receiver operating characteristic (ROC) curve for PSA and concluded that no absolute cutoff value had the high degree of sensitivity and specificity simultaneously required for identification of a risk-free value.77 Instead, they endorsed viewing all PSA values as a continuum of risk for prostate cancer.77
Single measures of PSA performed on blood samples taken decades before a patient’s diagnosis have been statistically associated with levels of risk and have garnered great attention because of their apparent simplicity and efficiency and their ability to stratify patients for screening, but their reliability awaits verification.78, 79 These studies relied on blood samples drawn from 21,277 men in Sweden from 1974 to 1986 and on Sweden’s cancer registry. From among these samples, prostate cancer diagnoses and blood samples were ascertained for 462, who were matched to controls. The median PSA level was about 0.6 ng/mL in the low-risk group. These investigators’ most recent work bases prediction of cancer risk on a single PSA test before age 40.79 Although the USPSTF recommended against PSA-based screening for prostate cancer in 2012, under the Affordable Care Act, for men over age 50 with Medicare PSA, the test is covered every 12 months.
More widely investigated have been measures of PSAV, a calculation of rising PSA level that was introduced in the early 1990s as a marker of prostate cancer development, a means to reduce unnecessary biopsies, and a way to improve the specificity of PSA testing. However, current standards that shorten the minimum longitudinal monitoring period for calculating PSAV and push ever lower the levels of PSAV considered worrisome (now 3.5 ng/mL/year in men with a PSA <4 ng/mL in one algorithm) actually increase the likelihood of biopsy.80–83 Cautious investigators82 argue that to use PSAV to monitor men with a PSA <4 ng/mL, it is necessary to have evidence that such measures ensure that enough cases will be detected within the “window of curability” to make them worthwhile and that the financial and emotional costs of overdiagnosis will not undermine other advances. They also point out that relying on findings about PSAV in undiagnosed cases, which have been largely lacking, would be very different from relying on posttreatment PSAV findings and applying them to the detection setting. They and others have said that prospective studies are needed.82, 84
An early study on the PSAV was one of men enrolled in a geriatric trial. Carter et al.80 concluded that in men with PSA values <4 ng/mL, a PSAV <0.75 µg/mL/year indicated absence of prostate cancer and a PSAV ≥0.75 µg/mL/year indicated its presence. In a work published 15 years later, Krejcarek et al.85 reported that in the undiagnosed patients they studied who had PSAV values <1.0 ng/mL/year, only 6% of those younger than 70 years with cT1c disease had high-grade cancer; however, they found that a median PSAV value of 2.71 ng/mL/year, age, and clinical T stage were significantly related to high-risk disease (Gleason score 4 + 3). Because these subjects had undergone radiotherapy, the findings are not generalizable to patients treated with other therapeutic modalities. The study by Krejcarek et al. was a retrospective evaluation in 358 men to identify those at higher risk, so they could improve outcomes by adding androgen-suppression therapy to radiotherapy and by improving the selection of radiotherapy fields.
A prospective trial conducted at the Royal Marsden Hospital and reported in 2008 studied 237 patients enrolled in an active surveillance trial who had a median PSA level of 6.5 ng/mL at the outset and a median pretreatment PSAV of 0.44 ng/mL/year.86 The investigators determined that PSA density was a statistically significant independent determinant of PSAV in untreated patients: those with a PSA density measure >0.185 ng/mL had a median PSAV of 0.92 ng/mL/year, and those with a PSA density measure <0.185 ng/mL had a median PSAV of 0.35 ng/mL/year. Because PSA density is a measure available at the outset of diagnosis and does not require longitudinal data collection, it will be a more efficient marker than PSAV is if others confirm this finding.
As a recent commentary by Vickers et al.87 points out, the use of PSAV in prostate cancer early detection and management of clinically localized disease is questioned. The main arguments against PSAV are lack of clinical utility that it does not add to established predictors of prostate cancer diagnosis, methodologic variability, and it being a poor prognosticator for mortality after conservative management and after prostatectomy.
Staging of prostate cancer
Staging of cancer, which is integral to the treatment decisions that follow, comprises initial clinical staging based on findings from physical examination of the patient and diagnostic tests and pathologic staging based on findings at surgery and on subsequent pathologic study of the removed prostate gland and other tissues. Less definitive than pathologic staging, clinical staging relies on palpation of the prostate; imaging studies, which for patients at low and intermediate risk are sometimes omitted; and needle biopsy results. Physicians can combine the clinical staging with two other significant prognostic factors, the Gleason score and the preoperative PSA value, to classify the case according to the D’Amico system, as low, intermediate, or high risk.88 This system was first described in 1998 in the report of a retrospective study in which D’Amico et al. evaluated 1872 men with prostate cancer who had been treated with radical prostatectomy, external beam radiotherapy, or radioactive implant with or without neoadjuvant androgen-deprivation therapy. In that study, clinical staging was based on DRE findings alone (American Joint Committee on Cancer tumor stage89). The researchers found that with that system, men who had been classified as being at low or intermediate risk had outcomes that were not statistically significantly different from others within their class. Most of this reliability is probably attributable to the Gleason score and the PSA level.
In the staging of prostate cancer, physicians rely on the tumor, node, and metastasis (TNM) system of the American Joint Committee on Cancer to classify cases89 (Table 2). It reports the extent of the tumor (T), the presence or absence of disease in the regional lymph nodes (N), and the extent of metastasis (M). In a second step of the staging process, the Gleason score is combined with the TNM classification, and cases are identified as stage I, II, III, or IV, progressively representing advances in the extent of disease89 (Table 3).
Table 2 TNM clinical and pathologic staging of prostate cancer
| Clinical stage | Pathologic stage | ||
| Primary tumor | |||
| TX | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| T1 | Clinically inapparent tumor neither palpable nor visible by imaging | ||
| T1a | Tumor incidental histologic finding in 5% or less of tissue resected | ||
| T1b | Tumor incidental histologic finding in more than 5% of tissue resected | ||
| T1c | Tumor identified by needle biopsy (e.g., because of elevated PSA) | ||
| T2 | Tumor confined within prostatea | pT2b | Organ confined |
| T2a | Tumor involves one half of one lobe or less | pT2a | Unilateral, involving one-half of one lobe or less |
| T2b | Tumor involves more than one-half of one lobe but not both lobes | pT2b | Unilateral, involving more than one-half of one lobe but not both lobes |
| T2c | Tumor involves both lobes | pT2c | Bilateral disease |
| T3 | Tumor extends through the prostate capsulec | pT3 | Extraprostatic extension |
| T3a | Extracapsular extension (unilateral or bilateral) | pT3a | Extraprostatic extensiond |
| T3b | Tumor invades seminal vesicle(s) | pT3b | Seminal vesicle invasion |
| T4 | Tumor is fixed or invades adjacent structures other than seminal vesicles: bladder neck, external sphincter, rectum, levator muscles, and/or pelvic wall | pT4 | Invasion of bladder, rectum |
| Regional lymph nodes | |||
| NX | Regional lymph nodes were not assessed | pNX | Regional nodes not sampled |
| N0 | No regional lymph node metastasis | pN0 | No positive regional nodes |
| N1 | Metastasis in regional lymph node(s) | pN1 | Metastasis in regional nodes |
| Distant metastasise | |||
| MX | Distant metastasis cannot be assessed (not evaluated by any modality) | ||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| M1a | Nonregional lymph nodes | ||
| M1b | Bone(s) | ||
| M1c | Other site(s) with or without bone disease | ||
a Tumor found in one or both lobes by needle biopsy, but not palpable or reliably visible by imaging, is classified as T1c.
b There is no pathologic T1 classification.
c Invasion into the prostatic apex or into (but not beyond) the prostatic capsule is classified not as T3 but as T2.
d Positive surgical margin should be indicated by an R1 descriptor (residual microscopic disease).
e When more than one site of metastasis is present, the most advanced category (pM1c) is used.
Source: Edge 2010.90 Reproduced with permission of Springer.
Table 3 Prostate cancer stages
| Stage | TNM classification and Gleason score |
| I | T1a, N0, M0, Gleason score 1 |
| II | T1a, N0, M0, Gleason score 2, 3–4 |
| T1, T1b–T2, N0, M0, any Gleason score | |
| III | T3, N0, M0, any Gleason score |
| IV | T4, N0, M0, any Gleason score |
| Any T, N1, M0, any Gleason score | |
| Any T, any N, M1, any Gleason score |
Source: Edge 2010.90 Reproduced with permission of Springer.
Prostate cancer is the most commonly diagnosed cancer in US men, with the exception of skin cancers and in situ cancers.91 About 3/4 of US men report having been screened at least once, and early prostate cancer, because it has no symptoms, is often diagnosed in outpatient settings. Distinguishing between high- and low-risk localized prostate cancer, maximizing disease control and survival, and avoiding overtreatment, especially in men likely to die of comorbidities, are challenges physicians who treat these men face daily.92
The American Urological Association has characterized localized disease into three risk categories93 (Table 4). Low-risk disease is generally characterized by a PSA value ≤ 10 ng/mL, a Gleason score ≤ 6, a lack of symptoms, and absence of both diseases in the lymph nodes and metastases (i.e., clinical stage T1c or T2). Disease is nonpalpable on DRE, but evidence of tumor may be detected by a transurethral resection of the prostate (TURP) performed because of what was thought to be BPH or by needle biopsy prompted by a high PSA level. PSA values >10 ng/mL but ≤20 ng/mL and/or a Gleason score of 7 (3 + 4 or 4 + 3) are associated with intermediate risk. PSA values >20 ng/mL and/or Gleason scores of 8–10 indicate high-risk cases.
Table 4 Risk stratification for localized prostate cancer
| Risk level | PSA level (ng/mL) | Gleason score | Clinical stage | ||
| Low | ≤10 | and | ≤6 | and | T1c or T2a |
| Intermediate | >10–20 | or | 7 | or | T2b but not qualifying for high risk |
| High | >20 | or | 8–10 | or | T2c |
Source: Thompson 2007.93 Reproduced with permission of Elsevier.
It is noteworthy that there is intense interest in further staging prostate cancer with endorectal coil magnetic resonance imaging (MRI) with multiparametric (mp) techniques.94 Studies have shown that a suspicious lesion on MRI is an independent risk factor for adverse pathology after radical prostatectomy.95 On the other hand, a normal lesion or one of low suspicion on mpMRI has a high negative predictive value for clinically insignificant disease.96 Neither MRI nor any other imaging modality is incorporated into the clinical risk groups for localized disease, nor staging of the primary tumor.
Validated pretreatment nomograms that combine PSA, Gleason score, and clinical stage have been developed to give estimates of pathologic stage, which may be valuable to clinicians planning treatment.97–100 In a 2007 update of the Partin tables, Makarov et al.100 analyzed 5730 men who had undergone prostatectomy between 2000 and 2005 at Johns Hopkins Hospital and confirmed that, as these researchers had previously shown, clinical stage contributes significantly to the prediction, as do PSA level and Gleason score, and that cumulatively they are better predictors than any one alone. No patient’s disease was clinically staged higher than T2c, and at prostatectomy, almost 75% had disease confined to the prostate. None of the 123 of 164 patients with a clinical Gleason score ≥ 8 who had a workup was found to have metastatic disease. In their series, as in others, the proportion of men presenting with organ-confined disease has been increasing: 54% in 1993,97 48% in 1997,98 64% in 2001,99 and 73% in 2007 (year of publication).100 New in this series was the absence of Gleason scores of 2 to 4, reflecting pathologists’ belief that such scores represent sampling error.101 Among patients with higher clinical stage, the authors reported a trend toward more accurate staging in their 2007 report over that in 2001 and perhaps indicating a broader need for surgery in those patients with higher clinical stage and Gleason scores. A 2013 update to the tables confirms a similar distribution of pathologic stage, updated Gleason scoring, and a more contemporary prognosis.67, 68
Following up on previous work to improve the accuracy of identifying cases of low-volume, low-grade disease,102, 103 researchers at The University of Texas M.D. Anderson Cancer Center have refined a nomogram specifically for identifying men for active surveillance.104 This nomogram includes age, PSA density, and tumor length in a biopsy core. The low number of factors, the ease in ascertaining their values with only laboratory tests and extended biopsy, and their nonsubjective nature combined with an area under the curve (AUC) measure indicating good discriminatory power (i.e., 0.727) make this nomogram attractive. The authors admitted that they cannot explain why their analysis indicates that older age would reduce the probability of low-volume, low-grade cancer and that younger men with values appropriately low in the other categories would be good candidates for surveillance. Nonetheless, their work offers for validation a new, practical tool for identifying these low-risk men.
Including a molecular marker as a predictor is another way investigators have attempted to improve a nomogram’s accuracy. PCA3, a prostate-specific noncoding mRNA, is readily detected in urine when prostate cancer is present because it is overexpressed 60- to 100-fold 90% of the time.105 Deras et al.105 undertook a prospective, multisite study of 570 men immediately before they underwent prostate biopsy and found PCA3 to be reliably sensitive and specific across PSA values <4 and >10 ng/mL and across various values of prostate volume and number of prior biopsies. Overall, PCA3 sensitivity was 54% (95% CI 0.49–0.59) and specificity was 74% (95% CI 0.71–0.77).
Staging is meant to refine the risk of oncologic end points and can be augmented with commercialized genomic prognostic biomarkers taken from biopsy specimens. Cuzick et al.106 reported a panel of cell cycle progression (CCP) genes known from breast cancer studies and validated them in a cohort of patients managed conservatively. The CCP score, a numerical representation of average CCP gene expression compared to a housekeeping gene panel, was statistically superior in predicting 10-year mortality rates compared to clinical features. Another panel of genes was validated by Klein et al.107 that mixed several pathways (stromal response, androgen signaling, proliferation, and organization) and linked elements in a small sample of a prostate biopsy with long-term radical prostatectomy outcomes. The development and validation efforts have created a genomic score that estimates adverse pathology (Gleason ≥ 4 + 3 and/or pT3 stage) at radical prostatectomy, from patients with favorable biopsy findings (Gleason 3 + 3 to 3 + 4). Both biomarkers have strong statistical validation but need additional studies on clinical utility impact such as changing recommendations between active surveillance and immediate treatment and correlating such decisions with superior oncologic and quality-of-life outcomes. The theme of disease prognosis from genomics can continue into the postradical prostatectomy space, CCP score in this setting will predict for biochemical recurrence rates along with clinical features, and another genomic classifier (commercialized as Decipher, GenomeDx, San Diego, CA) specifically estimates early metastatic progression from patients with known high-risk pathology. Table 5 compares key clinical end points, clinical utility, and cost.
Table 5 Comparison of key features of three commercialized genomic tests for prostate cancer.108
| Decipher | Oncotype DX | Prolaris | |
| Tissues tested | RP for high risk—pT3, positive margin, PSA rise | Biopsy—for NCCN very low to intermediate risk | Biopsy or RP |
| Clinical end points | Early regional nodes or bone metastasis | Risk of unfavorable pathology—pT3 and/or ≥ Gleason 4 + 3 | Biopsy—10-year mortality with conservative management RP—biochemical recurrence risk |
| Clinical utility | Adjuvant/salvage therapy | Active surveillance or immediate therapy | Biopsy—active surveillance or immediate therapy RP—adjuvant/salvage therapy |
| Cost (USD) | 4250 | 3825 | 3400 |
RP, radical prostatectomy; pT3, pathologic stage with extraprostatic extension and/or seminal vesicle invasion; PSA, prostate-specific antigen; NCCN, National Comprehensive Cancer Network; USD, United States Dollar equivalent.
Imaging of prostate cancer
Although bone and CT scan are standard imaging tools to establish metastatic disease, the staging and detection tasks have been only modestly assisted with T2 weighted MRI. Recently, the addition of novel imaging sequences such as diffusion weighted imaging and dynamic contrast-enhanced imaging together with the T2 weighted images can combine to form an “mp” sequence. There is renewed interest in the MRI for the purposes of either advanced screening (i.e., prior negative biopsy with rising PSA) or enhanced staging (i.e., estimating if a patient with low-volume/low-grade disease is likely to be harboring undiagnosed higher-grade/volumes of disease).109
Novel software/hardware packages are commercially available that allow the biopsying physician to “fuse” MRI suspicious lesions with the otherwise normal ultrasound images.110 These additional “targeted” biopsies lead to novel metrics in prostate detection such as increase in tumor upgrading and/or volume measurement per core of tissue. MRI/fusion biopsies, however, do not appear sensitive enough to omit standard extended core biopsies, consisting of random sampling by zone: right and left apex, mid, base, and lateral horns for at least 10–12 cores. Although these systematic biopsies appear necessary to detect some MRI invisible lesions, the targeted cores are more likely to detect higher-grade tumor and omit low-grade detection. In a recent prospective cohort study of 1003 men undergoing both MR/fusion biopsy and standard (sextant) biopsy, targeted biopsy detected 30% more high-risk (Gleason score ≥4 + 3) and 17% fewer low-risk prostate cancer.111
Although these conclusions are exciting in the field, the technique and standardization of the imaging have a way to go before being standard. As Emberton112 commented more than a million biopsies are performed annually in Europe, and if each one would be MRI driven, there would be significant logistical needs to build out proper equipment, technique, and training. In addition, the standardization of reporting is newly reported and ongoing in clinical adoption.113, 114 The current state-of-the art programs are selecting patients for active surveillance or prior negative biopsy/rising PSA for MRI/fusion biopsy and combining teams of dedicated uroradiologist and urologists to advance this exciting new field. The significance will be fewer patients with false negative biopsies and few patients incorrectly selected for active surveillance. Additionally, further prospective studies are needed to evaluate predictive value of this technology for clinical outcomes (e.g., disease reclassification in active surveillance, prostate cancer specific mortality).
Therapy options and applications
Active surveillance
In the pre-PSA era, “watchful waiting” implied an alternative to active treatment and described a period when patients were monitored but not treated until the disease progressed and/or symptoms developed. With the advent of PSA testing, a paradigm shift occurred, in which we now diagnose considerably more early prostate cancers, including those destined to remain clinically insignificant. New strategies are needed for managing select cases of low-risk prostate cancer without imposing immediate therapy. Such an approach has been called different terms, including “watchful observation with selective delayed intervention”115 and “active surveillance.”116 This new strategy is to forego immediate treatment but closely follow patients with low-risk prostate cancer, pursuing early detection of tumor progression when the disease is still curable and initiating definitive therapy appropriately. For this strategy to fulfill its promise, two clinical tools are mandatory: a method of identifying a priori patients harboring small low-grade, indolent tumors and a surveillance strategy that reliably detects tumor progression when the disease is still curable. Data supporting conservative management of cases with clinically localized prostate cancer can be gleaned from population-based studies117, 118 and a meta-analysis.119 These pre-PSA era studies had a preponderance of older patients and patients with clinically evident cancers; therefore, their results cannot be directly extrapolated to the PSA-screened population. Other problems included the way patients had been diagnosed—many had not undergone a full workup for metastasis, and for many, diagnosis was based on fine-needle biopsy results118—and the fact that the researchers did not centralize pathology review.117 Despite their limitations, these observational studies showed that men with low-grade prostate cancer have a protracted course of indolent disease and a very small risk of disease-specific death, even after 20 years of follow-up.120
In contrast, the risk of death from disease progression is higher for men with Gleason scores of 7–10. Watchful waiting and prostatectomy were compared prospectively in an important study by Swedish investigators who followed up their initial report with further analyses 3 years later and an estimated 15-year results.78, 121, 122 The researchers studied 695 men with T0d, T1b, T1c, or T2 disease who were randomly assigned to undergo radical prostatectomy (n = 347) or watchful waiting (n = 348). Two-thirds had palpable tumors, but fewer than half in each group—43.8% of those undergoing prostatectomy and 39.7% of those assigned to watchful waiting—had symptoms. In the recently reported extended 23.2 years of follow-up analysis,79 the investigators observed statistically significant differences at 18 years of follow-up between those who underwent prostatectomy and those who were not treated until androgen-deprivation therapy was used [42.5% vs 67.4%, RR 0.49 (95% CI 0.39 to 0.60; P <0.001)], distant metastasis developed [26% vs 38.3%, RR 0.57 (95% CI 0.44 to 0.75; P < 0.001)], and disease-specific mortality occurred [17.7% vs 28.7%, RR 0.56 (95% CI 0.41 to 0.77; P = 0.001)]. The benefit from prostatectomy was confined to men younger than 65 years of age. Also, it is important to note that a large proportion of men in the watchful waiting arm did not require any palliative treatment. Whether these findings would be replicable in a US study population is unknown because prostate cancer is typically diagnosed earlier here than it is in Sweden.123 The Prostate Cancer Intervention versus Observation Trial124 conducted in the United States compared prostatectomy with watchful waiting in 731 of mostly screened men with localized prostate cancer and life expectancy of at least 10 years. At median follow-up of 10 years, there was no significant statistical difference in all-cause mortality and prostate cancer-specific mortality between the two groups. There was trend toward lower prostate cancer-specific mortality with surgery among men with PSA levels >10 ng/mL and subgroups with higher-risk cancers. In fact, for men with low-risk prostate cancer, there was nonstatistically significant increase in prostate cancer-specific mortality by 15%. Two of the longest-running prospective cohort studies have examined the feasibility of active surveillance, or expectant management. Additionally, there are other large cohort prospective studies underway.125 Carter et al.126 studied 81 men believed to have T1c low-volume prostate cancer for a median of 23 months (range 12–58 months). Their median age was 65 years (range 52–73 years). At baseline, all men had a PSA density ≤ 0.15 ng/mL/cm3 and a Gleason score of <7. Free PSA in the men was a median of 17% (range 4.3–37%). Every 6 months, subjects underwent PSA measurement (both free and total) and DRE. Every 12 months, patients underwent transrectal ultrasound-directed biopsy, including evaluation of at least 12 cores. After at least 1 year in the study, 56 (69%) of the men were free of progression and still on surveillance. The other 25 men (31%) met the criteria of progression, which were adverse findings on prostate needle biopsy, including a Gleason score ≥ 7, any Gleason pattern of 4 or 5, more than two cores with cancer involvement, or 50% cancer involvement in any core. Their median time to disease progression was 14 months (range 12–52 months). The researchers found that in men who experienced progression by their definition, the PSA density was statistically significantly higher and the free PSA value statistically significantly lower than those values in men who did not experience progression.
In a larger phase II study, Klotz127 reported findings on 299 men who at baseline had prostate cancer of grade T2b or lower, a PSA of <15 ng/mL, and a Gleason score ≤ 7. All subjects were older than 70 years. Surveillance included PSA measures, serial bone scans, transrectal sonography (every 6 months for first 2 years and then annually thereafter), and biopsy within 1.5 or 2 years of entering the trial. Criteria for progression were that patients demonstrate PSA, clinical, and histologic disease progression. PSA progression was defined as having a PSA doubling time of <2 years (measured at least three times during a minimum of 6 months), a final PSA of >8 ng/mL, and a regression analysis of ln (PSA) on time P < 0.05. Clinical progression was defined as one of the following: doubling of the product of the maximum perpendicular diameters of the primary lesion (measured digitally), TURP necessitated by local progression, ureteral obstruction, or clinical or radiologic evidence of distant metastasis. Histologic progression was defined as a Gleason score ≥ 8 at subsequent biopsy. At 55 months, 60% remained on surveillance; at 96 months, disease-specific survival was 99%, and overall survival was 85%. Thirty-five percent had a PSA doubling time of >10 years (median doubling time 7.0 years). Reasons for abandoning surveillance included patient preference (16%), rapid biochemical progression (12%), clinical progression (8%), and histologic progression (4%). In a recently published update of the study with the median follow-up time of 6.4 years from the first biopsy (range 0.2–19.8 years), Klotz et al.128 reported the prostate cancer-specific mortality in AS of 1.5%. The risk of dying of another cause was 9.2 times greater than the likelihood of dying from prostate cancer. As AS methods move toward integration of novel imaging and biomarkers, investigators are challenged with including more men with early prostate cancer, minimizing risk of cancer progression, and maximizing quality of life.
Prostate Testing for Cancer and Treatment (ProtecT) will compare active surveillance with active therapies.129 Investigators of ProtecT, begun in 2001, expect to enroll more than 1500 men in the United Kingdom and to randomize them to treatment with conformal radiotherapy, prostatectomy, or active surveillance. Results are expected in 2016. This trial should offer investigators more information about localized disease detected through PSA screening, helping physicians and patients understand the risks and benefits better and collaborate better in decision-making.
Curative therapy: an anatomic discussion of the challenges of disease control and minimizing side effects
The patient with early disease has the option to pursue one of a number of definitive therapeutic options, each with its own variations in technique. Fundamentally, the options are a radical prostatectomy or dose-escalated radiation therapy. Both treatment categories aim to treat the entire gland by surgical removal or radiation-based destruction. Alternative treatments have also emerged, such as cryotherapy and high-intensity focused ultrasound, that treat all or a portion of the gland. All treatments are associated with a risk of treatment recurrence and varying degrees of quality-of-life side effects specific to prostate cancer treatments: erectile dysfunction, urinary incontinence, urinary irritation and/or obstruction, and bowel dysfunction. The desire to diminish side effects and treatment recurrences has left the field with numerous updates in technique, entirely new technologies, and numerous comparisons. For each question involving treatment efficacy and side effects, the patient and practitioner want to know both the average results expected and any contributing features that help predict whether an individual patient will experience the favorable or unfavorable end of the range of results. In addition, studies have shed light on whether a particular procedure is reproducible across the range of treatment centers.130–132 Most patients diagnosed today are very much aware of the potential for side effects and the concept that a practitioner’s experience may affect outcomes.
The selection of patients for treatment is often derived by considering the slow natural history of prostate cancer, the life expectancy of the aging man, and the personal wishes of the patient. The most commonly accepted recommendation is that a patient may benefit from treatment if he has 10 or more years of life expectancy. However, this estimation may be a moving target because death from cardiac disease is declining with better treatments. Men should not be denied treatment on the basis of age alone,133 but the study by Albertsen et al.118 demonstrated significantly reduced prostate cancer-related death when the disease was diagnosed at age 70 and higher, especially for men with Gleason scores <7. The recent update of the Bill-Axelson study that randomized radical prostatectomy and watchful waiting has been highly beneficial in decision-making.79 The majority of survival benefit in the radical prostatectomy cohort was observed in men < age 65; however men ages 65–75 had secondary benefits in reduced hormonal therapy, palliative therapy, and metastatic progression.
Radical prostatectomy: a model for the treatment dilemmas concerning therapy for early prostate cancer
The challenges of treating early prostate cancer can be illustrated by an anatomic tour of a radical prostatectomy operation and by using the steps of the operation to highlight what the surgeon and radiotherapist must consider in achieving cancer control with minimal side effects. Refer to Figure 6 as we narrate our way through the intricate anatomy surrounding the prostate gland.
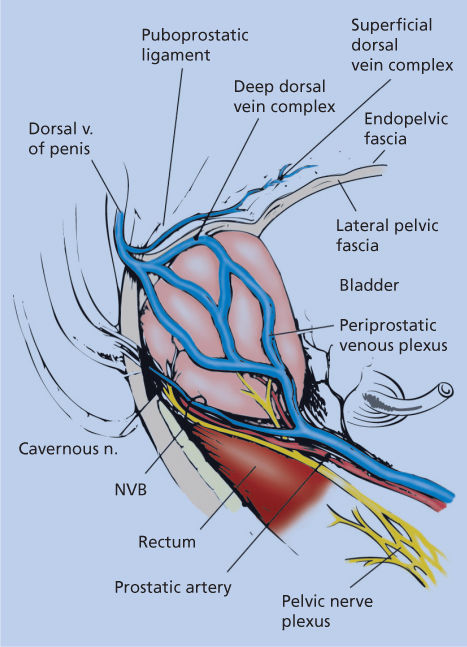
Figure 6 Surgical anatomy of the prostate in relationship to the deep dorsal vein complex, neurovascular bundle (NVB), and other surrounding periprostatic structures (lateral view).
The radical prostatectomy operation involves complete removal of the prostate gland, seminal vesicles, and distal vas deferens. Conceptually, the prostate gland can be thought of as a conical structure with open ends—the bladder neck and the urethra. The sides of the cone have a capsular structure (although not a true histologic capsule) and are surrounded by endopelvic fascia laterally and by Denonvilliers fascia posteriorly. At its apex, the prostate is surrounded by the rhabdosphincter muscle and the dorsal vein complex, which is narrow over the urethra and then spreads into an apron-like structure as it traverses over the midprostate, base of the prostate, and then over the bladder. Regardless of approach and technique, the removal of the prostate requires an intimate understanding of the intricate anatomic structures to be encountered, and a set of allowed surgical motions can be defined.
Access to the prostate
The prostate gland is among the more difficult structures to access for surgery. It is covered anteriorly by the pubic arch, distally by the dorsal vein complex and rhabdosphincter, inferiorly by the rectum, inferolaterally by the nerve bundles, and superiorly by the bladder. The prostate can be exposed with a lower midline abdominal incision from the pubic bone to the umbilicus, and the exposure progresses through extraperitoneal spaces. Alternative approaches include minilaparotomy, laparoscopic access via 5 or 6 ports in the lower abdomen (extraperitoneal or transperitoneal), and perineal access. The minilaparotomy incision is generally 8–10 cm rather than the 15–20 cm long needed for the standard laparotomy. Visualization of the prostate is similar in the two open abdominal approaches, but in the minilaparotomy, the surgeon will rely more on instrument dissection than on manual dissection. The laparoscopic approach has become increasingly popular with the availability of robotic surgical systems to increase the laparoscopic surgeon’s dexterity with instruments, with seven degrees of motion and three-dimensional camera view.
The choice of surgical approach depends on both the surgeon’s training and the patient’s characteristics. The retropubic approach has been taught in most residency programs worldwide; it provides access to the prostate and lymph nodes and entails a familiar transabdominal orientation. The perineal approach may be associated with less pain, and the scar is certainly less visible. There may be an advantage to this procedure in the circumstances of morbid obesity. However, the lymph nodes are not accessible, and this approach may be difficult for larger prostates, for example, those >60 g. The laparoscopic approach requires a steep learning curve of more than 100 cases, whereas the robot-assisted laparoscopic approach requires fewer.134–136 Differences in postoperative pain and hospital discharge are not reliably seen between open retropubic and laparoscopic approaches137, 138 but may be decreased with the perineal approach.139 Both perineal and laparoscopic approaches are associated with less bleeding, but in expert hands the transfusion rates are probably not significantly different.140 Results from nonrandomized comparisons show increased transfusion rates in retropubic prostatectomy if the rates for this group are more than 10–15%.141
Moving forward with this discussion, we will discuss only the open retropubic and laparoscopic (both manual and robot-assisted types) operations. However, it is worth noting that although historic discussions on the perineal operation suggest that the outcomes may increase positive margin rates, decrease potency rates, and cause de novo rectal incontinence,142 several high-volume centers have published very competitive outcomes,139, 143 and there is arguably a cost savings relative to the use of robotic approaches.144, 145
Alternative treatments must also consider access to the prostate in their application. Brachytherapy is a form of whole-gland radiation treatment in which radioactively labeled seeds are inserted into the prostate by transperineal access using transrectal ultrasound for guidance. In cases of BPH, the anterior portion of the prostate may extend around the pubic bone’s arch, creating a form of interference to needle placement. Thus, in the application of brachytherapy and cryotherapy (another transperineal access ablative therapy), the prostate must be of a certain size (generally <60 g) and shape to allow for access. In contrast, modern external beam radiation treatments can handle a broader range of prostate sizes and shapes. The intensity-modulated radiation therapy (IMRT) technique, for example, uses multiple beams from different angles to boost the dose to the prostate while limiting the dose to surrounding organs such as the bladder and the rectum. Proton beam radiation has recently become popular as another way to limit the dose to normal tissue.
Exposure and dissection of the apex
The anterior and lateral surfaces of the prostate are covered by endopelvic fascia. This fascia can be cut sharply or by using cautery, with care to avoid or ligate varying networks of veins that course along the prostate and often penetrate the apex at 11 and 1 o’clock. The pubovesical ligaments are cut by most surgeons to allow distal ligation of the dorsal vein complex. Mistakes in this region can cause significant blood loss in the open operation, although less so in the laparoscopic and/or robotic approaches because of the positive pressure of the CO2 pneumoperitoneum.
The rhabdosphincter surrounds the urethra distally, and the apex of the prostate has no capsule-like structure. Therefore, there is tremendous potential for mistakes in this region, and this may be the step of the operation that improves the most with experience. In essence, the surgeon must control the dorsal vein complex with proximal and distal sutures and then make a tangential cut that is as close to the apex as possible to avoid damaging the rhabdosphincter complex yet avoid a positive apical margin. Numerous technique descriptions are available and cannot be fully catalogued, but the objectives of cancer control (i.e., negative surgical margins) and urinary control are strongly influenced by this step.
Alternatives to surgery must also completely treat the apical region while avoiding side effects. Dose-escalated external beam and brachytherapy will inevitably reach both the apex and surrounding rhabdosphincter. However, because those structures are not specifically disrupted, stress incontinence results significantly less often than with surgery. Cryotherapy techniques include temperature monitors at the sphincter to avoid freezing outside of the apex.
Exposure and dissection of the bladder neck
Dissection of the bladder neck is by comparison much easier than that of the apex in the open operation. The Foley catheter can be used as a guide, and electrocautery can be used safely. Care must be taken to preserve the posterior plate of the bladder neck and divide it away from the ureteral orifices. The bladder neck-sparing technique has been reported as possibly beneficial in avoiding urinary continence but is possibly associated with an increased incidence of positive margins.146 A nonbladder neck-sparing plane can be reconstructed with sutures to match the urethral size for the anastomosis.
Alternatives to surgery must completely treat the base of the prostate while avoiding damage to the bladder. In conventional-dose radiation to the pelvis, the surrounding dose to the bladder and rectum was always a dose-limiting factor. Dose-escalation techniques, however, whether IMRT, proton therapy, or brachytherapy, effectively increase the dose to the prostate while holding down the dose to the bladder. Nevertheless, some of the dose does affect the bladder, accounting for the differing distribution of urinary side effects, including irritation, frequency, and hematuria.
Exposure and dissection of the seminal vesicles
The seminal vesicles present their own surgical challenges. These structures lie immediately posterior to the bladder, with their tips coursing laterally. The vesicles are surrounded by several small arterial branches that must be controlled with clips or sutures. If uncontrolled, these branches may cause significant postoperative bleeding, which may require a second surgery. However, electrocautery must be avoided if possible because the tips of the vesicles lie immediately medial to the neurovascular bundles. Some researchers have reported the concept of leaving the tips intact to avoid nerve damage.147 Laparoscopic surgeons may address this challenge by dissecting the seminal vesicles posterior to the bladder through the pouch of Douglas. For the radiotherapist, the seminal vesicles cannot be adequately treated by implant therapy but can be targeted by external technique. MRI with an endorectal coil can be used to stage the seminal vesicles for deciding whether to include them in the treatment plan—the trade-off being increased bladder toxicity.
Neurovascular bundle dissection
The technique for neurovascular bundle dissections is usually retrograde (apex to base) for open surgery and anterograde (base to apex) for laparoscopic surgery. For the retrograde approach, the dorsal vein and urethral division steps are completed, and the plane posterior to the Denonvilliers fascia is developed with blunt finger dissection. The bundles on each side can then be palpated. Visually, the neurovascular bundles blend well into the sides of the prostate through a series of lateral fascial layers. A triangle of fascia exists, with its borders being the prostatic fascia medially, the endopelvic fascia laterally, and the Denonvilliers fascia posteriorly. Regardless of the technique, the nerve bundle must be released at two junctions: the anterolateral junction of the prostatic fascia and levator fascia and the medial posterior junction of the Denonvilliers fascia.
During the course of neurovascular bundle dissection, the use of electrocautery must be avoided or the thermal transmission may produce irreparable nerve damage. The portion of the bundle from middle to apex has mostly parallel vessels and a few perforating veins that can be controlled with clips or just transected and left to clot. In contrast, the portion of the bundles near the base gives off perforating arteries to the prostate that must be controlled with clips to avoid hemorrhage. Alternative coagulation devices have been described that produce less thermal discharge, but the nerve bundles are very sensitive to heat, and an athermal technique is preferable. Two different planes of nerve-sparing dissection have been described: intrafascial and interfascial. Surgeons must use judgment in this area because although the closer margin obtained from the intrafascial approach may improve postoperative potency, it moves the inked margin of the resection closer to the prostate gland.148
Surgeons may choose to sacrifice the nerve bundles depending on the estimated risk of extraprostatic extension, as determined from pretreatment parameters such as PSA, clinical stage, biopsy Gleason score, number of biopsies with cancer, and volume of cancer on biopsies, and possibly by imaging with sonography or endorectal coil MRI. Nomograms may assist with arriving at this estimate,100, 149, 150 but the surgeon’s intuition and experience always play a role that is difficult to measure. In general, most patients prefer to have a nerve-sparing operation as long as cancer control can be maintained.
The proximity of the nerve bundle and the prostate capsule also relates to radiotherapy planning. With brachytherapy, the dose delivered can be quite high within the peripheral zone of the prostate but will steeply drop off outside the gland. As a result, intermediate- to high-risk disease may not be adequately treated when there is higher risk of microscopic extraprostatic extension. Many centers will recommend either radiotherapy, as the dose planning can be driven outside of the capsule, or a combination of brachytherapy and radiotherapy. Cryotherapists can also customize treatment in this region by either driving the ice ball extraprostatically if there is a concern or warming the neurovascular bundle region and thus protect it from the ice ball if no cancer is present on a given side.
Urethral division
The urethra must be divided close to the prostate apex, essentially right near the verumontanum. The surrounding rhabdosphincter should be preserved, and excessive trauma from urethral dilators and catheters should be avoided.
Anastomosis
Both running and interrupted suture lines have been described, the latter more popular and feasible with the laparoscopic approaches. The objective is to approximate the bladder to the urethra so that the anastomosis is watertight and the mucosal surfaces are in contact. Excessively large urethral bites that may shorten the functional urethral length should be avoided. Anastomoses that leak or separate may lead to a higher rate of scarring and contracture.151
Technical modifications for high-risk disease
A shift has been observed toward selecting more surgical patients with higher-risk disease.152 This requires additional surgical skill to obtain negative margins while maximizing feasible neurovascular bundle preservation and adding additional staging information with an extended pelvic lymph node dissection. As reviewed by Yuh et al.,153 the incidence of nerve sparing in high risk varies, and positive lymph nodes may be observed in one-third of patients.
Outcomes of treatment for early disease
Cancer control
Most modern studies use PSA recurrence-free survival as an end point because the data can be collected in a 5- to 10-year time frame rather than the 15- to 20-year time frame needed for longer end points such as disease-specific and overall survival rates. However, as the AUA guidelines93 stress, PSA recurrence is inconsistently defined and does not directly correlate with longer survival. The most commonly used definition of PSA failure for surgery is a PSA level >0.2 ng/mL, and for radiation, the updated American Society for Therapeutic Radiology and Oncology (ASTRO) recommendation is PSA nadir plus 2 ng/mL.154 Definitions of risk stratification also vary in different studies. The AUA guidelines recommend the D’Amico criteria and the options for each88:
- Low risk: PSA ≤ 10 ng/mL, a Gleason score ≤ 6, and clinical stage cT1c–cT2a.
- Intermediate risk: PSA > 10–20 ng/mL or a Gleason score ≤ 7 or clinical stage T2b.
- High risk: PSA > 20 ng/mL or a Gleason score ≤ 8–10 or clinical stage T2c.
According to these risk groupings, the expected cancer control outcomes of brachytherapy, external beam radiotherapy, and radical prostatectomy in terms of PSA recurrence-free survival are seen in Figure 7.93 For each modality, the 5-year range of outcomes are low risk, 75–95%; intermediate risk, 70–90%; and high risk, 30–80%. At 10 years, the ranges are low risk, 60–90%; intermediate risk, 40–80%; and high risk, 20–60%. On the basis of the limitations of lack of standardized reporting, different definitions of failure, and lack of head-to-head randomized controlled trials, the AUA panel stated that there are insufficient data to conclude that one treatment is superior to another. For patients choosing radiation therapy, the panel cited two randomized controlled clinical trials showing that higher-dose radiation may decrease the risk of a PSA recurrence.155, 156
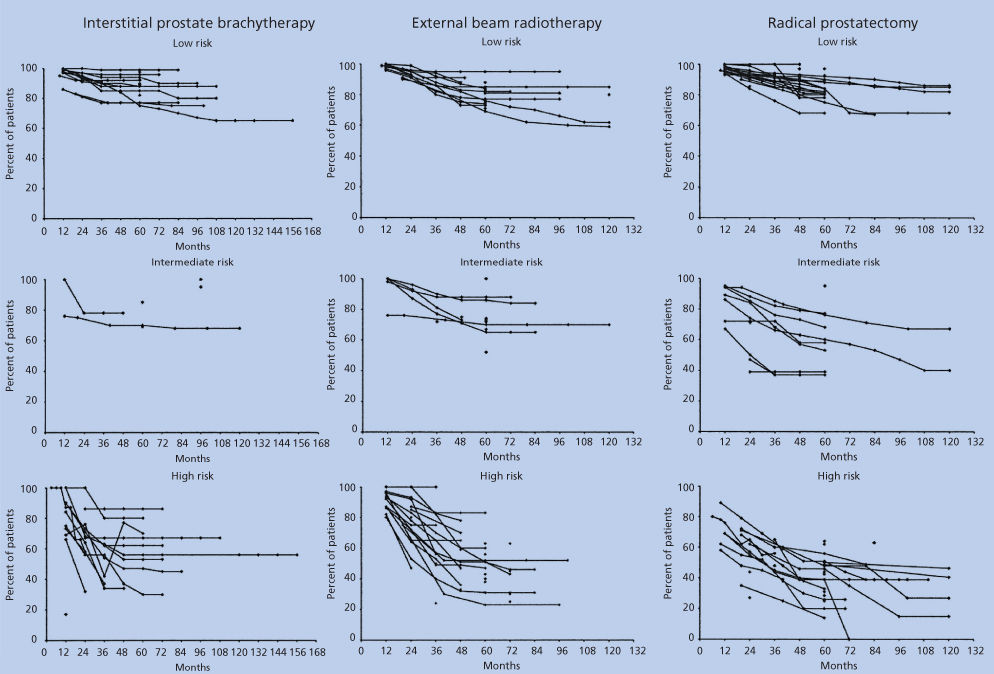
Figure 7 Prostate-specific antigen (PSA) recurrence-free survival in patients with low-, intermediate-, and high-risk prostate cancer treated with interstitial prostate brachytherapy, external beam radiotherapy, or radical prostatectomy.
The topic of neoadjuvant and/or adjuvant androgen deprivation was also addressed by the AUA panel. Randomized clinical trials of neoadjuvant androgen deprivation plus radical prostatectomy showed no benefit in terms of PSA recurrence-free survival.157, 158 However, for intermediate-risk patients treated with radiotherapy, one trial showed that neoadjuvant and concurrent androgen deprivation for 6 months may prolong survival after radiotherapy.159 For high-risk patients treated with radiotherapy, trials demonstrated a survival benefit for a longer duration of adjuvant androgen deprivation,160 in the 2- to 3-year range. However, it is noteworthy that the radiotherapy used in these trials was conventional and not dose escalated.
In summary, the AUA guidelines list active surveillance, brachytherapy, radiotherapy, and radical prostatectomy as treatment options for low-, intermediate-, and high-risk disease. For radiotherapy, randomized controlled trials are cited regarding dosages and androgen deprivation use. For the high-risk patient, it is noted that recurrence rates are high and that patients should consider “clinical trials examining new forms of therapy, including combination therapies, with the goal of improved outcomes.” It is also worth noting that the AUA panel concluded that first-line hormonal therapy is “seldom indicated in the patients with localized prostate cancer.”161
The European Association of Urology has also issued a guideline statement on prostate cancer, in which it cites many of the same randomized clinical trials regarding watchful waiting, surgery, and radiotherapy.162 Additional recommendations based on lower levels of evidence are also cited. Some selected recommendations regarding early disease are as follows:
- Brachytherapy “may be proposed to patients cT1-T2a, Gleason score <7 (or 3 + 4), PSA ≤ 10 ng/mL, prostate volume ≤ 50 mL, without a previous TURP and with a good IPSS (International Prostate Symptom Score) (level of evidence: 2b).”
- Cryotherapy “has evolved from an investigational therapy to a possible alternative treatment method for CaP (prostate cancer) in patients unfit for surgery or in those with a life expectancy <10 years (grade C recommendation).”
- “All other minimally invasive treatment options, such as HIFU (high-intensity focused ultrasound), RITA (radiofrequency interstitial tumor ablation), microwaves, and electrosurgery, are still experimental or investigational. For all of these procedures, a longer follow-up is mandatory to assess their true role in the management of CaP (grade C recommendation).”
Another large-scale effort to summarize the state of the literature was prepared for the Agency for Healthcare Research and Quality and published in the Annals of Internal Medicine.163 Again, the researchers met with difficulties because of variations in reporting and definitions, and only three randomized controlled trials compared the effectiveness between primary treatments (none with patients “primarily detected with PSA testing”), so, essentially stated, no conclusions could be drawn.
Complications of treatment
General
As a general statement, radical prostatectomy results in more urinary incontinence than radiotherapy or active surveillance do.163 Brachytherapy and radiotherapy are associated with more bladder irritation and/or hematuria side effects and less incontinence. Radiotherapy has the higher risk of bowel urgency. All treatments have sexual side effects: although the pattern with radical prostatectomy is one of early loss with gradual improvement, the pattern with brachytherapy or radiotherapy is one of more gradual and delayed loss of function. Younger age and better preexisting function will predict better outcomes from all treatments.
However, there are numerous sources of bias and variability in comparisons, and there is no evidence that any one therapy “has a more significant cumulative overall risk of complications.”161 Although patients often request a single statistic, such as an incontinence rate or potency rate, it is accepted that more accurate quality-of-life research will result from the use of (1) validated instruments that ask multiple questions and offer a range of potential answers; (2) an instrument that is administered by someone other than the treating practitioner; (3) a prospective longitudinal design, including a pretreatment baseline measurement; and (4) an instrument that maintains response rates at >70% throughout the study period. The recent multicenter study by Ferrer et al.164 evaluated the three standard treatments (radical prostatectomy, brachytherapy, and radiotherapy) with such an ideal method (except for treatment randomization). In the absence of comparisons of side effects in randomized controlled trials, various categories of studies can assist with our understanding of side effects: (1) results from multicenter community or academic series (i.e., voluntary reporting of what goes on in the community), (2) single-surgeon high-volume series (i.e., idealistic results), and (3) Medicare- or claims-based studies (i.e., involuntary outcome reporting).
Multicenter studies
Penson et al.165 reported the results from the Prostate Cancer Outcomes Study, which is a community-based cohort study conducted at six centers where men underwent radical prostatectomy in 1994 and 1995. In this study, the only relevant predictive information was that the men underwent a radical prostatectomy in the community, that is, no description of technique or quality analysis of the surgeon and/or surgery was given. Among the 1288 patients studied, frequent urinary leakage occurred in 3% at baseline, in 19% at 6 months, in 13% at 1 year, in 9% at 2 years, and in 11% at 5 years. At 5 years, the urinary control level was described as having total control in 35%, occasional leakage in 51%, frequent leakage in 11%, and no control in 3%.
Additional data were presented regarding pad use, irritative symptoms, and bother. Urinary bother started as no problem in 87% at baseline; at 5 years, 45% reported no problem, 42% reported a slight problem, and 13% reported a moderate to great problem. The percentages of patients who reported experiencing erections sufficient for intercourse were 81% at baseline, 9% at 6 months, 17% at 1 year, 22% at 2 years, and 28% at 5 years. Although urinary function and bother scores improved between 2 and 5 years, the percentages of men with no sexual activity were 15% at baseline, 44% at 1 year, and 46% at 5 years. Bilateral nerve sparing predicted a better return of erections at 5 years: 40% for bilateral compared with 23% for unilateral and 23% for nonnerve sparing. Age was also a predictor: in the most favorable group, 61% of men 39–54 years old reported erections.
Sanda et al.69 reported on a multi-institutional cohort from nine university-affiliated centers, with surgery completed with open, laparoscopic, and robotic-assisted techniques. In theory, this group of surgeons has a high volume, but again, specific technique was not reported. Using the 100-point Expanded Prostate Cancer Index Composite (EPIC) scale, patients who underwent radical prostatectomy had a baseline score for urinary continence of just 90; the score dropped to 50 at 2 months, improved to 70 at 6 months, and plateaued at 80 at 12–24 months.
However, as the results from these two large studies demonstrate, the literature contains varying definitions of incontinence. Sexual function was adversely affected by both radical prostatectomy and radiotherapy. Among patients who underwent radical prostatectomy, potency was better preserved with nerve-sparing techniques. Among patients who underwent radiotherapy, however, potency was better preserved in patients treated with monotherapy than in those given a combination with hormonal therapy (even after a short duration of 6 months). Bowel function was most affected by radiotherapy and brachytherapy even after 1 year—9% with distress related to bowel function.
The AUA guideline panel review93, 161 reported a range of 3–74% for urinary incontinence and suggested that there are insufficient data to provide an overall assessment of urinary outcomes. Those AUA guidelines also provide a large-scale review of published results of erectile dysfunction after radical prostatectomy without details of surgical technique and experience. Rates of erectile dysfunction after 1 year are as high as 90%, and nerve-sparing techniques are helpful.
Expert series
The nerve-sparing operation was initially described by Walsh et al. in the early 1980s,166 and it became increasingly popular and oncologically safe after the introduction of PSA screening. As one can imagine, patient demand for Walsh’s services and the services of other surgeons dedicated full time to this operation became quite high. Walsh et al. published a validated quality-of-life survey study that demonstrated the return of urinary control at 1 year in 93% of patients and a potency rate of 86% at 18 months.167 A high-volume robot-assisted prostatectomy series also demonstrated excellent results: 1032 of 1110 patients (93%) wore one or no pads per day at 1 year, and potency was reported in 79.2%.168 Although other studies have looked closely at factors affecting urinary control or potency rates, a recent trend has been to estimate the odds of achieving the “trifecta” of desired outcomes: cancer control, urinary control, and potency. The group from Memorial Sloan Kettering Cancer Center has published the concept as a nomogram.169
Expert comparisons have extended across technique choices. Touijer et al.170 performed a single-institution study involving two high-volume surgeons, one of whom performed laparoscopic and the other, open surgeries. There was an unexpected finding of better return of continence (defined as patient reports of no leakage or not requiring a pad) in the open-surgery group: at 12 months, 75% versus 48%. Descriptions of the surgical techniques were cited, but the true difference that affected outcomes was not described. Those authors suspected the result was due to the apical dissection in the laparoscopic approach and stated that further prospective analysis is needed.
Thus, a clear need in outcomes research in early disease is to better link technique to outcome. An example is seen in the study by Masterson et al.171 which demonstrated that a specific technical improvement in nerve sparing can be described and the results measured. In the standard technique, the apex is dissected starting with the dorsal vein complex, cutting the urethra and then bluntly mobilizing the posterior plane before releasing the posterolateral neurovascular bundles. In the modified technique, the sequence starts with dissection of the entire neurovascular bundle off the lateral aspect of the prostate from apex to seminal vesicle before the urethra is cut and posterior dissection performed. This avoids excessive traction applied to the neurovascular bundles. The 6-month recovery of erections improved from 40% with the standard technique to 67% with the modification. Such analogies can be seen in the literature on brachytherapy, in which the D90 analysis of the implant quality is a significant predictor of long-term biochemical disease-free interval.172
Variations in outcomes and Medicare databases
In multicenter studies and expert series, researchers voluntarily submit their own results and therefore have the ability to decide whether to participate. In the case of other studies, accessible data are used without such a decision to participate from each physician. Even among expert surgeons, complications vary.132 The Medicare database and the Surveillance, Epidemiology, and End Results (SEER) registries are common sources for data from studies such as these. The advantages of these studies include their large numbers of patients and the opportunity they offer for studying a more average community cohort of patients. Their limitations include sampling only patients over age 65 and that their data end points are designed for billing purposes more than for research and can be incomplete in their assessment of the outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree