Neoplasms of the head and neck
Renata Ferrarotto, MD  Merrill S. Kies, MD
Merrill S. Kies, MD  Adam S. Garden, MD
Adam S. Garden, MD  Michael E. Kupferman, MD
Michael E. Kupferman, MD
Overview
Head and neck cancers (HNCs) comprise a diverse group of malignancies affecting the upper aerodigestive tract. The most common tumor type is squamous cell carcinoma. While the main risk factors for HNC remain tobacco and alcohol abuse, oncogenic viruses such as human papilloma virus and Epstein–Barr virus play a major carcinogenic role in tumors of the oropharynx and nasopharynx, respectively. The management of head and neck malignancies is site and histology specific, and requires a multidisciplinary team approach. In this chapter, we review the current knowledge of HNC, with focus on squamous and salivary cancers, and discuss ongoing and future research aiming to improve the management and outcomes of patients with these malignancies.
Introduction
Approximately 62,000 new cases of head and neck cancer (HNC) will be diagnosed in the United States and over 13,000 Americans will die from these malignancies in 2016, accounting for 4% of all new cancer cases and 2% of cancer deaths annually.1, 2 Tobacco and alcohol are the primary etiologic agents for squamous cell carcinomas (SCCs).3 However, a rising proportion of these cancers (particularly those found in the oropharynx) are attributable to oncogenic human papilloma virus (HPV)4 and the preventive efficacy of population-wide HPV vaccination on incidence rates has yet to be determined. HNCs have a much greater impact in certain parts of the world, especially where cigarette smoking and/or chewing of carcinogenic stimulants is more prevalent.1, 5, 6 The more widespread adoption of multidisciplinary care likely underlies improvements in survival rates for some sites (nasopharynx and oropharynx);7 however, long-term survival rates are stagnant for other sites.8
Despite marked advances in reconstructive surgery, rehabilitation, and intensity-modulated radiotherapy (IMRT), head and neck squamous cell carcinoma (HNSCC) patients continue to have significant functional deficits which affect quality of life. Combined-modality approaches involving chemotherapy and radiation are now standards of care for “nonsurgical” locally advanced disease with objectives of disease eradication and organ preservation. Understanding the biology of HNC and developing molecularly targeted therapeutic agents and chemoprevention strategies have advanced substantially. These new therapeutic and preventive approaches appear to hold great promise for improving the control of HNC and its sequelae. This chapter reviews the current status of and future investigative directions for the epidemiology, biology, diagnosis, and therapy of HNC.
Descriptive epidemiology
Incidence
In the United States, estimates for 2015 are for approximately 45,780 new cases of oral cavity and oropharynx cancer, 13,560 new cases of laryngeal cancer, 3200 nasopharyngeal carcinoma (NPC), 3100 malignant salivary gland tumors, and 2000 nasal cavity and paranasal sinus neoplasias.2 The United States has benefited from tobacco control efforts with declining smoking prevalence beginning in the 1960s and subsequent declines in incidence rates for most HNC beginning in the 1980s.9 Approximately one in two oral cavity cancers occur in women, while only one in four pharyngeal and laryngeal cancers occur in women. Death rates from oral cavity and pharyngeal cancers have declined among whites and blacks over the years from 1993 to 2007, the largest changes in black men and women with 12 years of education. Although blacks and whites have similar rates of oral cavity/pharyngeal cancer, black men have double the rate of laryngeal cancer of white men; and black women have a 40% higher rate of laryngeal cancer than that of white women. Hispanics have the lowest incidence of oral cavity/pharyngeal cancer, and Asians have the lowest rates of laryngeal cancer.10 The median age at diagnosis for HNSCC is approximately 60 years, but the incidence of these cancers in young adults (age <45 years) appears to be increasing, related to increasing numbers of oropharyngeal cancers associated with oncogenic HPV.4 HPV-positive oropharyngeal cancers are more common in white men, presumably related to the prevalence of oral sexual practices.9
Worldwide, HNC incidence was approximately 550,000 cases in 2011. Melanesia has the highest incidence of oral cavity cancer (36 : 100,000), followed by South-Central Asia, and Central and Eastern Europe.10 While mortality rates attributed to oral cavity cancer have been decreasing in most countries in Europe and Asia, rates continue to increase in Eastern European countries, particularly in females, reflecting the tobacco epidemic in that region. Although 80% of HNC cases in South-Central Asia are oral cavity and pharyngeal (excluding nasopharyngeal), in other regions of the world, laryngeal and nasopharyngeal cancers are more common. Laryngeal cancer accounts for approximately one-third of HNC in the developed world, and approximately 40% of cases in Southern and Eastern Europe. In South-Eastern Asia, nasopharynx carcinoma is the sixth most common malignancy overall in males, accounting for ∼70% of all HNC in countries like Malaysia, Indonesia and Singapore.10
Prevalence
Highlighting the impact of cancer survivorship, approximately 350,000 individuals were living in the United States with a history of HNC in November, 2007. As expected, the sex distribution of these prevalent cases reflects the sex of the incident cases. However, African Americans accounted for only 11.5% and 7.3% of the prevalent population of individuals with a history of laryngeal and oral cavity/pharyngeal cancer, respectively. These small percentages likely reflect the poorer overall survival (OS) of African Americans diagnosed with HNSCC. Over the past decade, African Americans have demonstrated survival rates approximately 20% and 15% worse than whites for oral cavity/pharyngeal and for laryngeal cancer, respectively.11
Mortality
In 2015 in the United States, 8650 deaths will be attributed to oral cavity and oropharyngeal cancer, and 3640 to laryngeal cancer.2 Broader use of and improvements in multidisciplinary care, and the declining incidence attributable to tobacco control, likely underlie the significantly improved survival rates for nasopharyngeal, oropharyngeal, and hypopharyngeal cancer patients, and trends toward improved oral cavity cancer survival rates; however, laryngeal cancer survival rates appear to be worsening.7, 8 As with other cancer sites, mortality/incidence ratios for HNC are much higher in developing countries as compared with the United States.1, 10
Risk factors
Tobacco
In the late 1950s, a landmark case-control study by Dr. Ernst Wynder established the link between tobacco use and oral cavity cancer. A year later, a cohort study of over 180,000 men demonstrated an increased risk of death from HNSCC in cigarette smokers as compared with men who never smoked.12 In 1964, the Advisory Committee to the Surgeon General on Smoking and Health published its report linking smoking to cancer.13 The strength and consistency of the association between smoking and HNSCC have been demonstrated in numerous case-control and cohort studies with significant relative risks or odds ratios in the 5- to 25-fold range.14, 15 Furthermore, a dose–response effect is consistently shown between the duration and dose of smoking with increasing risk of HNSCC and between the time since quitting and the decreasing risk of HNSCC. Other mucosal malignancies of the head and neck such as NPC and sinonasal malignancies have a weaker association with tobacco smoking.16
Although the risk of bronchogenic carcinoma appears to be less significant for cigar and pipe smokers than for cigarette smokers, these forms of tobacco use are also clearly associated with an increased risk of HNSCC.17 The pooling of saliva containing carcinogens in gravity-dependent regions may account for the site distribution of HNSCC based on consumption patterns; in the United States, floor of mouth (FOM), laryngeal, and hypopharyngeal cancers are almost exclusively found in smokers. Smokeless tobacco and related product users and pipe smokers often have a habitual position for the quid or pipe stem, and these products are also associated with cancer of the oral cavity. In South-Central Asia where the use of such products is common, the gingivobuccal region is the most common site for HNSCC.6, 18
Although smoking rates are declining in the developed world, they are rising in developing countries. In 1965, 42.4% of the U.S. adult population was actively smoking, while in 2013 only 17.8% were current smokers.19 Although the reduction in cigarette smoking has been much greater in men over the past three decades, the rate of current cigarette use remains higher in men (20.5%) than in women (15.3%), and 26.1% of Native Americans continue to smoke.20 Worldwide, striking variations in HNC sites and incidence are seen among different regions, cultures, and demographic groups and are due, in large part, to differing patterns of tobacco and other substance abuse. For instance, in South-Central Asia, “pano” (betel leaf, lime, catechu, and areca nut) is commonly chewed and is a strong risk factor independent of tobacco use for carcinoma of the oral cavity, one of the most common cancers in men and women in this region.10, 21
Alcohol
Alcohol, too, is an important promoter of carcinogenesis in at least 30% of HNSCCs.10, 22 Furthermore, alcohol appears to have an effect on HNSCC risk independent of tobacco smoking, but these effects are consistently significant only at the highest level of alcohol consumption.23 Although studies attempting to correlate the type of alcoholic beverage with specific cancer risks have been conflicting, most investigators believe that ethanol itself is the main causative factor. Nevertheless, it appears that the major clinical significance of alcohol consumption is that it potentiates the carcinogenic effect of tobacco. The synergistic relationship between smoking and alcohol results in a 30-fold increased risk of HNC.22, 24
Infectious agents
Although various infectious agents have been suggested to play a role in head and neck carcinogenesis, current scientific evidence implicates only Epstein–Barr virus (EBV) and HPV as etiologic agents. Although herpes simplex viruses have been suggested as a risk factor for oral cavity cancer,25 and Helicobacter pylori for laryngeal cancer,26 confirmation is lacking.
Human papilloma virus
In contrast to non-oropharyngeal cancers, the incidence of oropharyngeal squamous cell carcinoma (OPSCC) has increased in recent decades, specifically among younger age groups,27–31 given the rising incidence of oropharyngeal cancers associated with HPV infection.32, 33 HPV is associated with approximately 70% of OPSCC in the United States.34 HPV may also play a role in the etiology of SCCs arising in the sinonasal tract.35
HPVs are deoxyribonucleic acid (DNA) viruses with a unique affinity for human epithelia, and HPV-associated cancers arise from the tonsillar crypts without an overlying epithelial dyplasia. HPV has been well established as an etiologic agent in cervical and anal cancer,36 and over the past decade, several investigators have indicated that infection with HPV is a significant risk factor for oropharyngeal carcinoma.37–44 Over 120 different HPV types have been isolated, with low-risk types (e.g., HPV 6, 11) inducing benign hyperproliferation of the epithelium, leading to lesions such as papillomas and warts, and high-risk types (e.g., HPV 16, 18, 31, 33, 35) associated with carcinogenesis.45 The prototypical oncogenic types 16 and 18 account for over 90% of HPV-related OPSCC, and are capable of malignant transformation of primary human keratinocytes from genital or upper respiratory tract epithelia.46 This transforming potential is attributed to the two HPV oncoproteins, E6 and E7, that inactivate two human tumor-suppressor proteins, p53 and pRb, respectively.47, 48 These viral oncoproteins are not only necessary for transformation but also stimulate cellular proliferation, delay cellular differentiation, increase the frequency of spontaneous and mutagen-induced mutations, and promote focal and broad chromosomal instability in transfected cell lines.49 Furthermore, in order to maintain a malignant phenotype, a transcriptionally active viral genome appears to be necessary.50–53
The risk factors associated with HPV-associated OPSCC are distinct from HPV-negative tumors, and include oral sexual behaviors and marijuana use. Furthermore, a dose–response relationship has been identified between the risk of developing HPV-positive OPSCC with number of oral sex partners and joint years of marijuana use.54 Although oropharyngeal cancer patients presenting without the classic tobacco exposures more commonly have HPV-16-associated tumors,37 it is also possible that there may be synergistic interactions between the traditional oropharyngeal risk factors of tobacco and alcohol with HPV.41 The potential effect of HPV-16 immunizations in the prevention of cervical carcinomas may help prevent oropharyngeal cancers as well.4
EBV
The epidemiologic link between EBV and NPC is quite strong,42, 43 and is further supported by the identification of EBV DNA in premalignant nasopharyngeal lesions. Although World Health Organization (WHO) types II and III are overwhelmingly positive for EBV, the virus is also associated with well-differentiated (WHO type I) NPC.46 Both serologic and mucosal swab evidence of EBV infection has been used to enhance screening for NPC in endemic areas.42 Evidence of EBV DNA in cervical lymph node metastases of unknown primary origin has been used to identify nasopharyngeal primaries. More recently, the detection of EBV DNA in peripheral blood (both cellular and cell-free component) has demonstrated prognostic significance for predicting survival and distant metastases and may become a biomarker to follow up on these patients.51
Genetic susceptibility
Since only a fraction of smokers develop cancer, variations in genetic susceptibility may be equally important in disease etiology. A genetic component to this disease is also supported by large family studies demonstrating a three- to eightfold increased risk of HNSCC in first-degree relatives of patients with HNSCC.53, 55 According to this hypothesis, inherited differences in the efficiencies of carcinogen metabolizing systems, DNA repair systems, and/or cell-cycle control/apoptosis systems influence risk of tobacco-induced cancers.56, 57 Identifying such at-risk individuals in the general population by use of biomarker assays would have a profound impact on primary prevention, early detection, and secondary prevention strategies. Three recent high-impact publications of genome-wide association studies have identified the same lung cancer susceptibility locus in separate populations.54, 58, 59 These studies will likely be followed in the near future by similar explorations of genome-wide HNSCC association studies and suggest that tailored prevention of tobacco-associated cancers may be a realistic goal.
Environmental tobacco smoke
In a study of 173 cases of HNSCC and 176 cancer-free controls, environmental tobacco smoke was associated with a more than twofold increased risk of HNSCC, and a dose–response relationship was also observed.60 In a separate study of 44 nonsmokers with HNSCC and 132 cancer-free nonsmoker controls, environmental tobacco smoke was associated with a significantly increased risk of HNSCC, and this was particularly true for females and for those reporting exposure at work.61
Laryngopharyngeal reflux
Observational and anecdotal studies have long suggested that gastroesophageal reflux (GERD) may be associated with laryngeal cancer.62, 63 Furthermore, multiple studies have documented a high prevalence of gastric reflux into the laryngopharynx in patients with laryngeal cancer via 24-h pH probe monitoring.64 A case-control study of 10,140 hospitalized patients and 12,061 outpatients with laryngeal and pharyngeal cancer and 40,561 hospitalized and 48,244 outpatient controls has been performed using U.S. Department of Veterans Affairs databases.65 The diagnosis of GERD was associated with a significantly elevated risk of laryngeal cancer (OR, odds ratio = 2.4 and OR = 2.3 for hospitalized and outpatient groups, respectively) and of pharyngeal cancer (OR = 2.4 and OR = 1.9 for hospitalized and outpatient groups, respectively). However, a large Swedish cohort study of 66,965 patients with discharge diagnoses of heartburn, hiatal hernia, or esophagitis with a follow-up of 376,622 person-years concluded that there was no evidence of a causal association between GERD and either laryngeal or pharyngeal cancer.66
Marijuana
Marijuana smoke has a four times higher tar burden and a 50% higher concentration of benzopyrene and aromatic hydrocarbons than does tobacco smoke. Although anecdotal evidence has long suggested that marijuana is a risk factor for HNSCC, few reports have found direct evidence of marijuana as an etiologic factor for HNSCC because most users of marijuana are also exposed to tobacco and alcohol.67, 68 A recent case-control study including 240 HNSCC patients and 322 cancer-free controls demonstrated a positive association of fivefold between marijuana use and HPV-related HNSCC, with a dose–response relationship.69 However, a large retrospective cohort of 64,855 health maintenance organization (HMO) members found no association of marijuana use with tobacco-related cancers.70 Problems of underreporting of marijuana use and limited sample size of heavy users limit conclusions regarding marijuana use and HNSCC risk.71
Diet
Epidemiologic evidence from case-control studies suggests that diets high in animal fats and low in fruits and vegetables may be risk factors for HNSCC.72, 73 Case-control studies have correlated salted fish consumption with NPC risk, which may be due to the high content of nitrosamine compounds in preserved foods.74, 75 Some evidence suggests vitamin A and beta-carotene may be responsible for the protective effect of diets high in fruits and vegetables, and carotenoid deficiency has been considered to be a risk factor for HNSCC and lung cancers.76 It is not known, however, which of the more than 500 carotenoids are protective, what chemical interactions may occur, or what protective role other micronutrients in carotenoid-rich foods may play. Others have found that total intake of vitamins C and E are also protective.73 Moreover, diets are complex and difficult to assess and validate; in particular, there are often inaccuracies in translating foods into constituent nutrients. It may be impossible to determine which of the vast array of compounds is most beneficial, and controlling for other dietary variables and confounding risk factors has remained a difficult methodological problem.
Occupation/air pollution
Although occupational exposures probably play a minor role overall in the development of HNSCC, they are major risk factors for malignancies of the sinonasal region.77–79 The most important exposures occur in the metalworking, refining, woodworking, and leather/textile industries.77, 78 Indoor air pollution is a significant problem in much of the developing world where indoor stoves using biomass or fossil fuels are the primary method of cooking and heating. Although controversial, an expert committee of the National Academy of Sciences has concluded that there is sufficient evidence to consider asbestos as a significant independent risk factor for laryngeal cancer.80
Radiation
No significant association has been demonstrated between ionizing radiation and the development of HNSCC. However, SCCs of the lip, like skin cancers, are associated with ultraviolet radiation exposure. Furthermore, exposure to gamma radiation is associated with thyroid cancers, sarcomas of the head and neck, salivary gland malignancies, and paranasal sinus cancers. Although therapeutic irradiation of HNC does not appear to induce second primary SCCs of the aerodigestive tract, it is associated with an increased risk of sarcomas of the head and neck.81 This is a particular concern for children requiring radiotherapy. Furthermore, environmental, medical diagnostic, and therapeutic radiation exposure to the head and neck are all significantly associated with salivary gland malignancies, with a dose–response relationship.82, 83 Mucoepidermoid carcinomas (MECs) appear to be the most common radiation-induced salivary malignancy.82, 83 Occupational studies have suggested that indoor exposure to radon gas or volatile chemicals may also increase the risk for HNSCC.79
Ultimately, the public health goal is better prevention and early detection of these malignancies by reducing the use of tobacco and alcohol, preventive vaccination against high-risk oncogenic HPV, discovering and avoiding other causative agents, and identifying the genetically susceptible. Unfortunately, HNSCC screening has not been effective, likely due to the rarity of the disease and expertise required for examination.84 However, in parts of the world where HNSCC accounts for a major portion of the cancer burden, prevention and screening programs have had some measure of efficacy where implemented.6
Pathologic assessment and biology
Aside from stating that a particular tumor is SCC, additional information reported by the pathologist usually includes tumor grade or differentiation. Unfortunately, differentiation factors have not been consistently accurate in reflecting the biologic aggressiveness of HNSCC.85 Prognosis is influenced by many factors other than grade.86, 87 These include tumor size, nodal status, site, surface expression of epidermal growth factor receptor (EGFR), host immune response, age, and performance status. P16 expression/HPV status is the strongest independent prognostic factor for survival among patients with OPSCC.88
Features reflecting aggressive disease include lymphatic invasion, perineural invasion, lymph node metastases, and penetration of the tumor through the capsule of involved lymph nodes (extracapsular spread [ECS]). A complete discussion of the molecular pathology underpinning HNSCC is beyond the scope of this chapter, and the reader is referred to some of the many reviews in this area.89, 90 Intense investigation is ongoing regarding the complex interplay of cellular and genetic alterations that contribute to carcinogenesis and the metastatic phenotype in HNSCC. The fundamental roles of p53, dysregulated receptor tyrosine kinase signaling, apoptotic resistance, angiogenesis, and chemotherapeutic resistance are under active study.
Anatomy
The term “cancer of the head and neck” refers to a diverse collection of neoplasms arising from the anatomic sites that make up the upper aerodigestive tract (UADT). This chapter, however, deals predominantly with SCC, as it accounts for approximately 90% of malignancies in this region. The UADT consists of a complex mucosa-covered conduit for food and air that extends from the vermilion surface of the lips to the cervical esophagus. In common usage, this terminology has been applied primarily to those cancers arising from the mucosal surfaces of the lips, oral cavity, pharynx, larynx, and cervical esophagus. Included in this designation, however, are other important sites, such as the nose and paranasal sinuses, salivary glands (major and minor), thyroid and parathyroid, and skin (both melanoma and nonmelanoma skin cancers). Some cancers arising in this region are typically excluded from the generic designation of HNC. Examples are tumors of the central nervous system, ocular neoplasms, and primary tumors of lymphatic origin.
Because of the diversity of sites and tissues of origin, the biology of tumor growth, patterns of metastases, and natural boundaries for tumor extension, signs and symptoms of disease are quite varied. The anatomy of the region has also dictated that optimal evaluation, diagnosis, and treatment require specific multidisciplinary expertise, including neurosurgery, otolaryngology, head and neck surgery, oral and maxillofacial surgery, cosmetic and reconstructive disciplines, and specialists in radiology, pathology, radiation therapy, and medical oncology. Although the anatomic structures are only millimeters apart, the low metastatic potential and high curability of vocal cord cancers stand in contrast to the early dissemination and poor prognosis of stage-matched pyriform sinus cancers.91 Clinical differences between cancers in different sites are explained by anatomic factors and major biologic differences. Regrettably, the relatively small number of HNC patients often requires grouping patients for trials. Associated morbidities of disease and treatment involve the special senses to varying degrees, notably speech, mastication and swallowing, smell, and respiratory function, all critically important for social interaction, a good quality of life, and ultimately, survival.
Oral cavity
The oral cavity is defined as starting at the vermilion border of the lips and extending posteriorly to include the lips, buccal mucosa, anterior tongue, floor of the mouth (FOM), hard palate, upper and lower gingiva, and retromolar trigone. The tongue occupies a major portion of the oral cavity and is contiguous with the FOM. The gingival mucosa overlying the mandibular and maxillary alveolar ridges adheres to the underlying periosteum. The hard palate forms the roof of the oral cavity and consists of mucosa overlying the palatine portion of the maxilla extending from the superior alveolar ridge to the junction with the soft palate, which is the anterior border of the oropharynx. Although the delineation between the oral cavity and oropharynx might seem artificial, the distinction is important because of varying natural history and sensitivity to nonsurgical therapy and numerous functional considerations after surgical resection, which is the mainstay of treatment for this subgroup of diseases.
Pharynx
The pharynx is a musculomembranous tube suspended from the skull base to the level of the sixth cervical vertebra, supported by overlapping constrictor muscles and other muscles arising from the styloid process and skull base. This conduit communicates with the oral cavity anteriorly, the nasopharynx superiorly, and the hypopharynx and larynx inferiorly. It is divided into four sites of clinical importance: the tonsillar area, which makes up the major portion of the lateral pharyngeal wall and blends with the tongue base, soft palate and retromolar trigone, the tongue base, and the posterior pharyngeal wall. Innervation of the pharynx is via the pharyngeal plexus, with contributions from the glossopharyngeal (sensory) and vagus nerves (motor and sensory).
The hypopharynx is divided into three distinct regions: the pyriform sinuses; the posterior surface of the larynx (postcricoid area); and the inferior, posterior, and lateral pharyngeal walls. The pyriform sinuses are paired mucosal pouches wrapped around the larynx, which funnel food around the larynx and into the esophagus. They are bounded superiorly by the pharyngoepiglottic folds and inferiorly by the cricoid cartilage. The sinuses come together at the esophageal introitus and cervical esophagus at the level of C6.
Larynx
The larynx consists of a mucosa-covered cartilaginous framework (thyroid and cricoid cartilages) suspended from the hyoid bone above by the thyrohyoid membrane and attached below to the trachea. The opening to the larynx is continuous with the pharyngeal airway. Unlike the rest of the pharynx, the mucosa of the larynx consists largely of columnar, ciliated, respiratory-type epithelium. Stratified squamous epithelium is found on the upper posterior epiglottis, aryepiglottic folds, and true vocal folds. Notably, lymphatics in the upper larynx are sparse in the true vocal folds, or glottis.
The larynx is divided into three anatomic regions: the supraglottic, glottic, and subglottic larynx. The supraglottic larynx includes the epiglottis, aryepiglottic folds, laryngeal surface of the arytenoids, false vocal cords, and ventricles. The glottic larynx consists of both true vocal cords and the mucosa of the anterior and posterior commissures. It extends from the lateral-most apex of the laryngeal ventricle to 1 cm below the free edge of the vocal folds toward the cricoid. It has few, if any, lymphatics. The subglottic larynx consists of the region bounded by the glottis above and the inferior border of the cricoid cartilage. Lymphatic supply to the subglottic larynx is extensive and bilateral. The infraglottic lymphatics drain to the cervical nodes through the cricothyroid membrane, while supraglottic lymphatics drain through the thyrohyoid membrane.
Nose and paranasal sinuses
The term “nose and paranasal sinuses” refers to the region of the UADT that starts at the vestibule of the nose anteriorly, is covered by squamous epithelium, and extends posteriorly to the choana, where the nasopharynx begins. By definition, paranasal sinus malignancy does not include the nasopharynx unless by extension. It does include the paranasal sinuses, specifically, the maxillary, ethmoid, frontal, and sphenoid sinuses. Although the most common malignancy of the nose and paranasal sinuses is SCC, the nose and paranasal sinuses pose a particular set of problems that deserve separate consideration. Moreover, nonsquamous cancers may also occur with distinctive patterns of tumor progression and requirements for effective therapy.
Neck
Anatomic considerations in the treatment of cancers of the head and neck must include a thorough understanding of the neural, vascular, and, especially, the lymphatic structures of the neck. Detailed anatomic studies have described the organization of the lymphatic drainage of the UADT. Specific regions of the head and neck and the tumors that arise therein have lymphatic drainage that is consistent and predictable. There are 12 major groups of lymph nodes (six each bilaterally) in the head and neck (Figure 1),92 although only levels I to V play a major role in HNSCC. Primary and secondary echelons of lymph node drainage have been defined for each major region of the head and neck mucosa. A standard rule of thumb is that the lymphatic drainage for any particular region is predicted by the arterial supply of that region. The lip, cheek, and anterior gingiva drain into the submandibular and submental lymph node groups. In addition, the cheek and upper lip also drain into the inferior parotid and facial nodes, while the posterior gingiva and palate drain into the internal jugular chain and lateral retropharyngeal groups. Lymphatic drainage for the mobile tongue is into the internal jugular, subdigastric, omohyoid, submandibular, and submental nodal groups. Midline lesions often drain bilaterally. Although metastases to the lower neck nodes are infrequent from the oral cavity, generally the more anterior the tumor location in the tongue, the more likely it is that metastases also will spread to lower jugular nodes. FOM drainage is similar to that of the tongue. The upper portion of the pharynx drains directly into the upper cervical lymph nodes along the internal jugular chain. The oropharynx and tonsil drain through the parapharyngeal space into the midjugular region, particularly into the jugulodigastric nodes. Retro and lateral pharyngeal nodes can also be involved. The regions of the hypopharynx and larynx drain primarily along the routes of their vascular supply to either the deep cervical nodes along the midjugular (upper pharynx, larynx) or the deep nodes along the lower jugular and paratracheal region (lower pharynx, larynx).
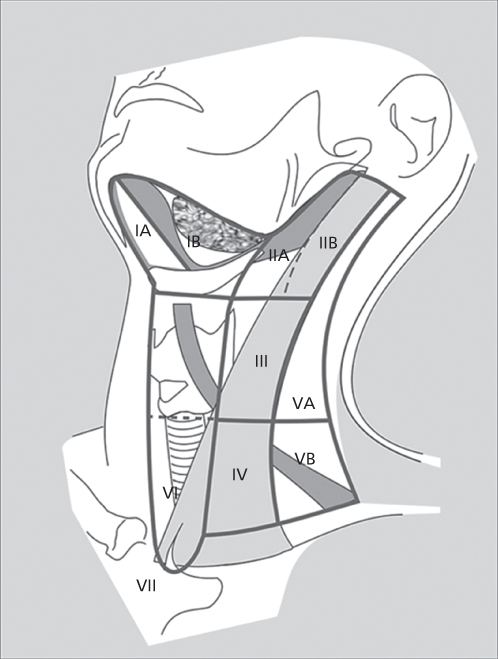
Figure 1 Nodal levels in the head and neck.
For the purposes of local treatment, the various lymph node groups of the neck have been divided into levels. Level I includes the submental group of nodes (IA), located within the triangle bounded by the anterior belly of the digastric muscles and the hyoid bone, and the submandibular group (IB), bounded by both bellies of the digastric muscle and the body of the mandible. Level II nodes consist of the upper jugular lymph nodes located in proximity to the upper third of the internal jugular vein and extending from the skull base to the level of the bifurcation of the carotid artery. The anterior and posterior boundaries are the lateral border of the sternohyoid muscle and the posterior border of the sternocleidomastoid muscle, respectively. Level II is further divided into those lymph nodes located anteroinferior to the vertical plane of the spinal accessory nerve (IIA) and those posterosuperior to the nerve (IIB). Level III nodes include those nodes located adjacent to the middle third of the internal jugular vein from the carotid bifurcation to the plane marked by the omohyoid muscle’s crossing over the jugular vein (the level of the cricoid cartilage). Anterior and posterior boundaries are the same as level II. Level IV nodes include the lower jugular group extending from the omohyoid muscle above to the clavicle below. Level V nodes are those located in the posterior triangle in the region of the spinal accessory nerve and the transverse cervical artery. This level is bounded by the anterior border of the trapezius muscle, the posterior border of the sternocleidomastoid muscle, and the clavicle below. This level is further divided into Va and Vb nodes, with Va nodes being those nodes located above the plane along the inferior edge of the cricoid and including the chain of nodes superior to the spinal accessory nerve posterior to the sternocleidomastoid muscle. Vb nodes are the nodes below the cricoid plane, inferior to the spinal accessory nerve and include the nodes along the transverse cervical artery and all of the supraclavicular fossa.
Diagnosis and staging
Since site and stage of disease at the time of diagnosis are the most important prognostic factors in the treatment of HNSCC, the identification and treatment of early-stage cancers generally correlates with excellent survival. Most dysplastic lesions or in situ carcinomas of the oral mucosa occur as red (erythroplasia) or white (leukoplakia) patches that may be readily apparent on visual examination. In areas less easily visualized directly, such as the larynx and hypopharynx, early lesions cause such symptoms as chronic hoarseness and sore throat and, with progression, referred otalgia or dysphagia. Such symptoms demand visualization of the larynx and hypopharynx usually by fiberoptic approaches.
Dysphagia, odynophagia, otalgia, hoarseness, mucosal irregularities and ulceration, oral or oropharyngeal pain, weight loss, and the presence of an unexplained neck mass are the most common presenting symptoms of invasive HNSCC. The predominant symptoms vary with the site: chronic dysphagia or odynophagia demands thorough visualization of the oropharynx, hypopharynx, and esophagus; chronic hoarseness demands visualization of the larynx; chronic unilateral serous otitis media in an adult may be a result of cancer of the nasopharynx blocking the eustachian tube; and unilateral nasal polyps, nasal obstruction, or epistaxis is a common presenting sign of nasal cavity or paranasal sinus neoplasms. A firm or hard unilateral cervical mass represents malignancy until proved otherwise. In persons older than 20 years, such a mass represents neoplasm more than 80% of the time, and 60% of these neoplasms are due to metastatic spread from an UADT primary.
In patients presenting with a suspicious neck mass, a complete head and neck examination usually reveals the primary malignant tumor (Figure 2). If it does not, a thorough search for occult primary cancers both above and below the clavicles is warranted. Technologic advances in fiberoptics and in flexible and rigid endoscopes now provide excellent upper airway visualization and biopsy capabilities that can be performed routinely in the clinic setting.93 Endoscopic evaluation should include the nasopharynx, oropharynx, hypopharynx, larynx, and upper esophagus. Endoscopic evaluation should be accompanied by chest radiography and axial imaging of the head and neck. If these fail to reveal a primary, then consideration should be given for esophagoscopy as well, since it is more sensitive for mucosal lesions of the esophagus than is computed tomography (CT). Most commonly, occult primaries responsible for neck metastases occur in the nasopharynx, tongue base, tonsil, or hypopharynx. In the absence of an identifiable mass, directed biopsies of these sites are indicated during endoscopic evaluation, and, if present, bilateral tonsillectomies should be performed if a primary is not identified. Metastasis to a solitary left supraclavicular lymph node (Virchow’s node) is occasionally seen with infraclavicular cancer, especially colon cancer. Generally, isolated metastatic supraclavicular masses (level IV) derive from breast, lung, or infradiaphragmatic neoplasms. Thyroid malignancies may also metastasize to this area.

Figure 2 Untreated N3 disease in a patient with HNSCC.
Imaging with CT and magnetic resonance imaging (MRI) is frequently used to supplement the clinical evaluation and staging of the primary tumor and regional lymph nodes. Ultrasonography, when combined with fine-needle aspiration (FNA) technique, is an effective means for staging the neck, thyroid, and salivary glands. Open biopsies should be performed only after attempts by FNA of suspicious nodes are nondiagnostic. If an excisional biopsy is required because FNA is inconclusive or not feasible, then the surgeon and patient should discuss the advisability of neck dissection if the mass should prove to be metastatic SCC. The potential ramifications of false-negative results on FNA are inherently obvious. Accuracy of the cytological interpretation of the aspirate is directly dependent on the skill and experience of the ultrasonographer and pathologist.
Positron emission tomography (PET) imaging has a demonstrated role in management of HNC. Highly elevated primary tumor fluorodeoxyglucose standardized uptake values (FDG SUVs) may predict for more aggressive disease and inferior treatment outcomes.94–96 FDG-PET can provide over 90% sensitivity and specificity for upfront staging of both primary and cervical neck nodal disease,97 can localize occult local primary disease,98, 99 or distant metastases100 not elicited by anatomic imaging or physical examination. Combined FDG-PET/CT imaging may further improve neck staging accuracy results.101, 102 Incremental superiority of FDG-PET for regional staging of the neck relative to CT or MRI alone was confirmed by a meta-analysis of retrospective and prospective studies encompassing over 1200 FDG-PET imaging cases with confirmatory neck dissection pathology.103 Analysis of this dataset revealed FDG-PET to be sensitive (79%) and specific (86%) for this indication. Recent prospective series in early-intermediate T-stage oral cavity and oropharyngeal cancer patients suggest that FDG-PET can potentially guide more appropriate management of clinically N0 patients when directly correlated with CT and sentinel node biopsy104 or with CT/MRI findings.105
Considerable interest has recently focused on FDG-PET monitoring of disease response to radiotherapy or chemoradiotherapy. A number of groups have found that FDG-PET posttreatment restaging provides high negative predictive power;106–108 accordingly, there is now growing acceptance of withholding consolidative neck dissection following radiotherapy in the absence of residual FDG-avid adenopathy,109 although others argue that expert clinical interpretation of serial CT imaging could achieve similar results.61, 110 FDG-PET/CT may eventually prove useful for improving delineation of disease targets for advanced radiotherapy planning.111 However, challenges for this remain, particularly for identification of validated thresholding techniques to precisely distinguish FDG-avid disease from bystander tissues.112, 113 At MD Anderson Cancer Center, we routinely use FDG-PET/CT to supplement anatomic imaging and clinical examination for radiotherapy planning; however, we do not use negative FDG-PET results to defer treatment of suspicious findings identified by examination or conventional restaging techniques after chemotherapy.
Staging criteria for cancers arising in the UADT, paranasal sinuses, and salivary glands have been developed by the American Joint Committee on Cancer (AJCC) (Table 1). The criteria undergo regular reevaluation and modification. The most current version referred to in this chapter is the 7th edition.114 The stage groupings are based on T (primary tumor), N (regional node), and M (distant metastasis) designations. Because of variations in the growth, behavior, and prognosis of HNCs according to site of origin and extent, differences exist in the staging criteria for each anatomic site. However, except for tumors arising in the nasopharynx and those of the thyroid, there is uniformity in the nodal staging criteria and stage grouping (Table 2).
Table 1 AJCC clinical tumor stage and groupings for head and neck cancer
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1 | N1 | M0 | |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| Stage IVA | T4a | N0, N1, or N2 | M0 |
| Any T | N2 | M0 | |
| Stage IVB | Any T | N3 | M0 |
| T4b | Any N | M0 | |
| Stage IVC | Any T | Any N | M1 |
Source: Edge 2010.114 Reproduced with permission of Springer.
Table 2 AJCC clinical tumor staging characteristics for regional lymph nodes and distant metastases
| Regional lymph nodes | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No evidence of regional lymph node metastases |
| N1 | Metastasis in single, ipsilateral regional lymph node <3 cm in greatest dimension |
| N2a | Metastasis in single, ipsilateral regional lymph node between 3 and 6 cm in greatest dimension |
| N2b | Metastasis in multiple ipsilateral regional lymph nodes, none >6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral regional lymph nodes, none >6 cm in greatest dimension |
| N3 | Metastasis to regional lymph node >6 cm in greatest dimension |
| Distant metastases | |
| Mx | Presence of distant metastasis cannot be assessed |
| M0 | No evidence of distant metastasis |
| M1 | Distant metastases are present in one or more locations |
Source: Edge 2010.114 Reproduced with permission of Springer.
Careful documentation of tumor extent and accurate staging classification are crucial for discussions of the results of different treatment approaches. Restaging after treatment or for recurrent cancers must be clearly designated and separate from the primary staging of previously untreated cancers. Postsurgical, or pathologic, staging is important in the primary treatment of HNC because of the increasing use of postoperative radiation therapy (PORT) and/or adjuvant chemotherapy for patients with locally aggressive tumors, ECS into the soft tissues of the neck, close or positive margins, and perineural invasion.
It should be noted, however, that as good as the widely accepted AJCC staging system is for HNC, it still falls short in that it too often fails to distinguish between deeply infiltrative tumors and those that are superficial or exophytic. Experience shows that this distinction is an important one and can have a significant impact on survival. Moreover, the recent emergence of HPV-positive cancers presents an essentially distinctive group of patients for whom some of the older baseline staging data may not be fully applicable.115 Future revisions to the AJCC for HPV status, particularly for oropharyngeal cancers, are anticipated.
General principles of treatment
After a histological diagnosis has been established and tumor extent determined, the selection of appropriate treatment of a specific cancer depends on a complex array of variables, including tumor site and stage, HPV status, prognosis, relative morbidity of various treatment options, patient performance and nutritional status, concurrent health problems, social and logistic factors, therapeutic options for potential recurrences or second primaries, and patient preference. These variables are each considered with respect to the established effectiveness of various treatment regimens available.
The overall management goals in treating patients with HNC are to achieve the highest cure rates at the lowest cost in terms of functional and cosmetic morbidity. The achievement of these goals requires the close cooperation of an interdisciplinary team of practitioners representing surgery, radiation and medical oncology, prosthodontics, dentistry, speech language pathology, social services, dietetics, physical and rehabilitative medicine, pathology, nursing, and often psychiatry.
Effective rehabilitation is an important part of the overall treatment of HNC. Modern advances in surgical reconstruction, microvascular free-tissue transfer, and prosthodontics have significantly improved posttreatment function.116 Rehabilitation concerns must be addressed at initial treatment planning and carefully integrated with the various treatment modalities used. Pretreatment dental evaluations, and speech and swallowing assessments should be routinely performed. Needed dental care and/or extractions should be performed prior to radiation to reduce the risks of dental-associated mucositis and osteoradionecrosis. The overall impact of treatment and rehabilitation on patient quality of life is an important issue that may require specialized social or psychiatric support systems for the patient and family. Furthermore, attention must be paid to nutritional support, and early intervention with the placement of enteral access for gastrostomy feeding should be entertained in selected patients. Contemporary combined approaches of chemotherapy and radiotherapy are often associated with severe mucocutaneous treatment effects that must be addressed. Finally, the prolonged nature of treatment for advanced disease, which may extend over many months, requires consideration of the social and financial impact of treatment decisions on the patient, the family, and the patient’s career.
Biopsies of primary tumors need not be excisional unless the biopsy procedure is sufficient for local control. Oncologic principles of surgical resection must not be compromised by ill-conceived reconstructive efforts or attempts at modifying the necessary resection in order to minimize functional or cosmetic morbidity. Gross residual cancer or positive surgical margins after tumor resection portend high risk for treatment failure. Appropriate management must also include the use of precise modern techniques of conservative surgical resection (e.g., partial laryngectomy and functional neck dissection) that, in selected patients, have cure rates similar to those of more radical techniques.
Oral premalignancy
Appropriate management of leukoplakia and erythroplakia lesions includes a high index of suspicion, particularly in high-risk individuals. Although both lesions are considered premalignant, erythroplasia lesions are of greater clinical concern, since approximately half of these lesions contain carcinoma in situ (CIS) or invasive cancer. Erythroplakia mandates biopsy to rule out invasive cancer. The management of erythroplakia and leukoplakia depends on the location, extent, and histology. The diffuse field effect and multifocal nature of the epithelial carcinogenic process support the need for effective prevention. Various molecular markers, including aneuploidy, loss of heterozygosity (LOH) and podoplanin expression portend a high risk of transformation in dysplastic oral intraepithelial neoplasia (IEN).117–119 White lesions can be confused with mucositis; lichen planus; local tissue irritation from mechanical, thermal, or chemical trauma; histoplasmosis; candidiasis; and other infectious processes.
Topical supravital staining with toluidine blue of suspicious lesions can be helpful in identifying areas for biopsy and in screening high-risk populations. Toluidine blue staining was found to be associated with LOH in dysplastic, minimally dysplastic, or nondysplastic oral IEN, which suggests the potential of toluidine blue for identifying oral IEN with a high risk of cancer and perhaps for helping guide surgical margin widths.120, 121 Lesions that persist despite the removal of local irritating factors, or those that are associated with ulceration, vertical growth, induration, a recent change in size, or pain, should be sampled by biopsy and/or excised. Despite aggressive local therapy, complete surgical resection (as defined by the absence of dysplasia at the margins) does not prevent oral carcinoma development in cases of aneuploid dysplastic leukoplakia.118 In this context, a targeted therapy prevention approach has been tested in a phase II randomized trial (EPOC). This study compared erlotinib, an EGFR inhibitor, versus placebo in patients with high-risk oral premalignant lesions harboring LOH. Despite the negative results for the overall population, there was a trend toward improved oral cancer-free survival in patients who did not have a prior oral cancer.122 Future research in this area should evaluate the roles of optimal surgical margin width and complete resection as confirmed by molecular analyses in reducing the cancer risk associated with molecularly defined high-risk oral IEN.
Overview of natural history and treatment by site
Oral cavity
Both tumor and treatment may significantly compromise speech and deglutition, particularly for those patients in whom cancer involves the tongue, FOM, or mandible. Despite the fact that this region is readily amenable to visual examination and bimanual palpation, more than 50% of patients are diagnosed in advanced stages. The current T staging of oral cavity primaries is presented in Table 3.
Table 3 Primary tumor staging characteristics for oral cavity and oropharynx carcinoma
| Tx | Primary tumor cannot be assessed (as occurs after excisional biopsy) |
| T0 | No evidence of primary (as in unknown primary tumors) |
| Tis | Carcinoma in situ |
| T1 | Tumor is 2 cm or less in greatest dimension |
| T2 | Tumor is between 2 and 4 cm in greatest dimension |
| T3 | Tumor is >4 cm in greatest dimension |
| T4 | A: Moderately advanced local disease: Tumor invades adjacent structures (through cortical bone, maxillary sinus, skin, tongue musculature, deep tissue, nerves) |
| B: Very advanced local disease: Oral cavity: tumor invades masticator space, pterygoid plates, skull base, encases carotid artery Oropharynx: tumor invades prevertebral fascia, encases carotid artery, or involves mediastinal structures |
Lips
SCCs of the mucosal surface of the lips are the most common oral cavity cancers. An important distinction must be made with cancers of the skin surrounding the lips, which are considered cutaneous malignancies. Over 90% occur on the lower lip, usually on the exposed vermilion border, midway between the midline and the oral commissure.123 Well-differentiated and verrucous cancers rarely metastasize. Poorly differentiated and spindle cell varieties tend to grow aggressively and metastasize commonly. Perineural infiltration of large nerves is indicative of aggressive disease and often requires combined therapies.
Considerations in the treatment of lip cancers include (1) oncological control of the disease, (2) a functional oral sphincter with oral competence, and (3) acceptable cosmetic outcome. These goals may be achieved with either primary radiation or surgery when the tumors are less than 2 cm in size or very superficial. Larger or deeply invasive lesions, however, are best treated with surgical resection and reconstruction, which allow for greater accuracy in evaluating the extent of tumor and nerve or lymphatic involvement. Frequently, adjacent precancerous changes are present, which can also be treated with surgery (lip shaving and advancement) to prevent recurrences or the development of second primary tumors.124, 125 For larger lesions, primary reconstruction with local, regional, and sometimes free-tissue flaps avoids defects that result from tissue loss with radiotherapy, provides for future reconstructive and treatment options, and decreases the risk of osteoradionecrosis of the mandible. Lesions demonstrating extensive infiltration, bone involvement, or lymphatic metastases should be managed with combined surgery and PORT.
Radiation therapy techniques for management of lip cancers include external irradiation, interstitial implants, and combinations of both. Local tumor control rates with irradiation exceed 80%,126, 127 with determinant survival at 5 years (including surgical salvage) in excess of 95%. Similar tumor control and survival rates are reported with primary surgical excision.128 Regional metastasis decreases the survival rates to approximately 55%.129, 130 The 5-year survival rates for patients with carcinomas of the upper lip are lower than for those with similar lower lip lesions and range from 40% to 60%.131 Involvement of both lips and the lateral commissure is uncommon. The prognosis for commissure lesions is not as good as for cancers of other areas of the lip.
Tongue
Tongue cancers account for approximately 25% of oral cavity SCCs and most commonly arise in the anterior two-thirds of the tongue on the lateral or ventral surface. Infiltration of the underlying tongue musculature occurs early. The biologic aggressiveness of T1 and T2 (<4 cm) tongue cancers is noteworthy and is reflected in higher rates of occult regional metastases than those of similarly staged lesions arising from other oral sites. Occult nodal metastases are present in 30–40% of early lesions.132 Bilateral nodal involvement can occur with cancers of the tip or the midline of the tongue. Locoregional recurrence in patients with tongue cancer accounts for 60–70% of cancer deaths. Distant metastases account for 10–15% of deaths, and second primaries in the UADT account for approximately 20–40%.132, 133 The management of carcinomas of the tongue has been significantly influenced by an increased appreciation of the aggressiveness of seemingly small but deeply infiltrative lesions, the high rate of occult lymph node metastases, and improvements in soft tissue and bony reconstruction. Although surgical excision alone has been the mainstay of treatment, combined surgery and adjuvant radiation therapy to include the primary site and regional nodes is commonly used for advanced cancers (stages III and IV) and is being used increasingly for small stage II cancers that exhibit pathologic indicators of lymph node metastasis or perineural invasion (Figure 3). Postoperative chemoradiotherapy is indicated for adverse pathologic findings of perineural invasion, nodal ECS, or close surgical margins.134
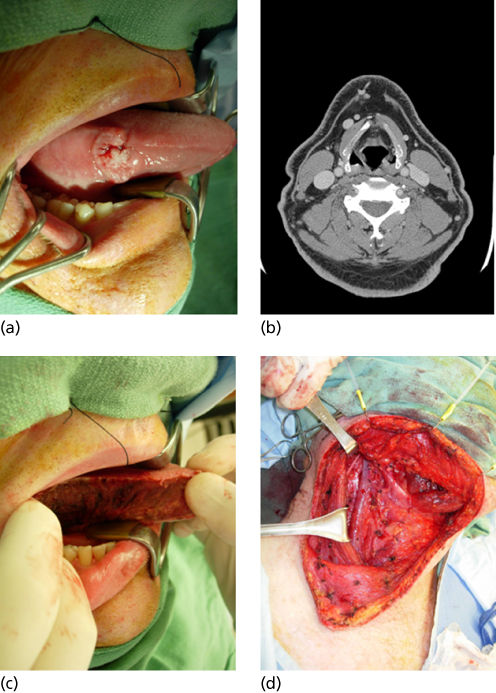
Figure 3 (a) T1 N0 SCCA of the oral cavity. (b) CT scan revealing no lymphatic metastasis. (c) Hemiglossectomy resection. (d) Staging supraomohyoid neck dissection.
For stage I cancers, surgical excision is effective and expeditious, with excellent preservation of function. For stage II lesions that are infiltrative, hemiglossectomy or partial glossectomy achieves excellent tumor control rates and should be combined with dissection of neck nodes at risk (supraomohyoid dissection) to provide accurate information about staging and determine the need for PORT. Free-tissue transfer reconstruction can significantly offset the morbidity of hemiglossectomy.
Extension of cancer to the FOM or the mandible may necessitate partial mandibulectomy or segmental mandibular resection. Modern reconstructive techniques with vascularized composite bone and soft tissue free flaps, titanium metal prostheses, and dental implants have improved the functional and cosmetic results of major mandibular resections. An elective neck dissection is recommended for lesions with >4 mm of invasion owing to the risk of occult nodal disease.
For more advanced primary lesions (stages III and IV), surgery and postoperative external beam radiation are generally used. No prospective controlled trials have proved the superiority of combined therapy over surgery alone for disease without nodal metastases, but retrospective studies indicate improved locoregional control rates.135–138 These improvements have generally been offset, in part, by an increased frequency of distant metastases and second primaries. Surgical management generally consists of partial glossectomy and neck dissection, with the mandible being spared unless directly involved. In instances with limited periosteal invasion, partial mandibular resections that spare mandibular continuity and maintain function can be performed. When tumors extend to the midline or involve the tongue base, subtotal or total glossectomy may be necessary. Continued advances in reconstructive techniques have improved the functional results of these aggressive resections. Provision for temporary tracheostomy and prolonged enteral nutrition should be made. Total glossectomy or sacrifice of both hypoglossal nerves frequently necessitates permanent feeding gastrostomy. Current experience indicates that total glossectomy can, in highly select patients, be accomplished without the need for laryngectomy, although prolonged or even permanent parenteral feeding will likely be required.139 PORT is generally administered within 4–6 weeks of surgery. High-risk surgical margins or ECS can be treated to a high-dose or with concomitant chemoradiotherapy. For advanced oral cavity cancers, both ipsilateral and contralateral necks are irradiated, with the dosage determined by the extent of disease. Close surgical margins are often treated to high doses (66–70 Gy) because of the difficulty in eradicating even small amounts of tumor in the tongue after glossectomy. Even with combined therapy, estimated 2-year disease-free survival (DFS) and OS rates for advanced disease are about 50%. The 5-year survival rates range from 50% to 70% for stages I and II to 15–30% for stages III and IV.140–144
Floor of mouth (FOM)
FOM cancers occur with a frequency similar to that of tongue cancer. Early spread to adjacent areas (gingiva and periosteum of the mandible) is common. The periosteum of the mandible is a natural barrier to invasion. Fixation of the tongue is a sign of deep invasion. The tumor may extend to or through the mylohyoid muscle, which serves as a natural barrier to direct spread below the hyoid bone. Lymph node metastases at presentation are seen in approximately 40% of patients. The occult metastatic rate increases with the T stage of the primary: T2 tumors have a 40% and T3 tumors a 70% occult metastasis rate.145, 146
First-echelon nodes of lymphatic drainage include the submandibular and jugulodigastric lymph nodes (levels I and II). Evaluation for early mandibular involvement is facilitated by palpation. since fixation to the mandible indicates periosteal involvement and direct bone invasion is present in 50–60% of such tumors. This distinction is often aided with bone windows on CT.
Small cancers (T1, T2) are generally treated effectively by wide resection. Lateral FOM tumors can often be resected transorally and the resection defect closed with the advancement of adjacent mucosa, skin grafts, or secondary intention. Sialodochoplasty of the severed submandibular duct can be performed for superficial lesions. An elective selected neck dissection is performed for T1 tumors with more than 4 mm of invasion and for all T2-4 cancers. Bilateral neck dissections should be performed for anterior FOM lesions, as both necks are at risk for occult metastasis due to the nature of lymphatic drainage in this region. If nodal metastases are present, therapeutic neck dissection is indicated. Surgery remains the mainstay of treatment of early FOM malignancies, achieving excellent functional and curative results.
More advanced FOM cancers (T3, T4) are generally treated with resection combined with similar approaches to that described for oral tongue cancers in the prior section (Figure 4). Again, mandibular continuity-sparing procedures with cortical resections can often be employed. In these instances, we have found fasciocutaneous flaps to offer excellent FOM and tongue reconstructive potential. Large mucosal and soft tissue surgical defects are typically reconstructed with free-tissue transfers, and contemporary management of mandibular defects entails bony reconstruction with either a fibula or scapula free flap.
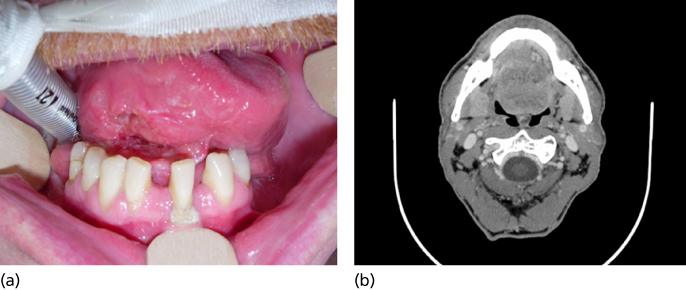
Figure 4 (a) T3 N0 floor of mouth SCCA. (b) Deep infiltration of the intrinsic tongue musculature on axial imaging.
Treatment results are influenced by the size of the primary tumor, presence of lymph node metastases, degree of mandibular involvement, and adequacy of resection. The 5-year survival rates for stage I and II FOM carcinomas range around 80%.146 Cancers that cross the midline or involve the tongue or the mandible are associated with 5-year survival rates of 50–60%.147 Survival rates for more advanced lesions (stages III and IV) are less than 50%. The major advantage of combined treatment (radiation and surgery) in these patients is improved control of neck disease. Recurrence in the untreated, clinically negative neck is the most frequent site of failure in patients treated only with surgery.148 For patients with multiple nodal metastases, induction chemotherapy (IC) is under study149 as there is high risk for later development of distant metastases. Continuing surveillance for metachronous second primary cancers in the head and neck, esophagus, or lungs is advised.150, 151
Gingival and buccal mucosa
Gingival cancers occur most commonly (80%) in the lower gingiva, posterior to the bicuspids. For both sites, trismus is an ominous sign indicating extension to the masseter or pterygoid muscles. Occult nodal metastases have been documented in as high as 30% of buccal cancers and elective neck dissection recommended in all but the earliest of cancers.152 Exophytic tumors tend to be papillary or verrucous in appearance and can be confused with benign hyperkeratosis.
Small, superficial gingival cancers can be effectively treated with surgical resection transorally with excellent preservation of function. Even larger lesions requiring partial maxillectomy or alveolectomy can be resected without external incision. For larger lesions (T3 and T4), segmental mandibulectomy and/or maxillectomy is required, and adjuvant radiation is frequently recommended (Figure 5). Elective neck dissection should be performed for advanced lesions of the mandibular gingival, as these lesions tend to have occult metastases. Limited data are available on the behavior of maxillary ridge and hard palate cancers, but these lesions can metastasize to the lateral neck nodes, and thus elective management of the neck is strongly encouraged, whether with neck dissection or neck irradiation.153 Clinically positive neck nodes warrant neck dissection at the time of the resection of the primary tumor.
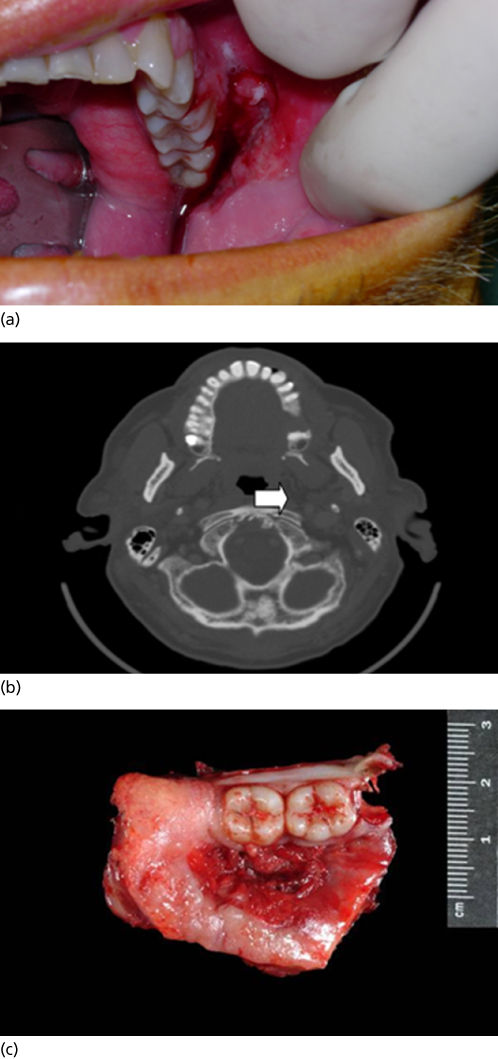
Figure 5 (a) T4 N0 SCCA of the oral cavity (buccal mucosa). (b) CT scan demonstrating bony destruction of the hard palate. (c) Specimen resected during infrastructure maxillectomy.
OS rates for gingival and buccal cancers depend on tumor size, bone involvement, and node metastases. Surgical results are clearly superior to those of radiation when bone involvement is present.152, 154
Retromolar trigone
Cancers arising in the retromolar trigone are rarely confined to that gingiva, but often involve adjacent buccal mucosa, anterior tonsillar pillar, the FOM, and/or posterior gingiva. The risk of clinically positive and occult lymph node metastases is higher than with other gingival cancers. Frequent involvement of periosteum mandates partial (rim or marginal) mandibulectomy as part of the surgical management, even for small lesions. Primary radiation therapy is reserved for superficial lesions that cover a large surface area, such as extension to the soft palate or buccal mucosa, and remain mobile. Moderately advanced or deeply invasive lesions are best treated with surgical resection (mandibulectomy and neck dissection), followed by postoperative adjuvant therapy, as indicated.
Oropharynx
The clinical staging of oropharyngeal cancers is similar to the staging of oral cavity cancers (Table 3). Tumors arise most commonly from the palatine arch, which includes the tonsillar fossa and base of the tongue. The recent identification of HPV as the major etiological factor in the development of OPSCC has led to a recognition of a distinct disease phenotype. Many of these patients are younger and without any history of smoking, and molecular analysis of these tumors reveals the presence of HPV DNA. Clinically, HPV-associated tumors are small, but the nodal burden is more robust.37 Aside from a cervical mass of unknown etiology, the most common presenting symptom is chronic odynophagia (often unilateral) and referred otalgia. Change in voice, dysphagia, and trismus are late signs. Regional lymphatic metastases occur frequently and are related to the depth of tumor invasion and tumor size. Upper cervical nodes are generally first involved, but lower nodes can become clinically involved with skipping of the upper first-echelon nodes. Bilateral lymphatic metastases can occur, particularly with cancers of the soft palate, tongue base, and midline pharyngeal wall. The retropharyngeal lymph nodes are also common sites of metastasis and warrant evaluation when planning treatment.
Management of oropharyngeal cancers is very challenging, given the essential role this anatomic site plays in breathing, speech, and swallowing. Therefore, the goal of treatment is to not only achieve oncologic cure but also to preserve the multimodal function of the oropharynx. Traditional surgical approaches to the oropharynx are associated with significant morbidity, which prompted a shift toward nonsurgical modalities in the 1990s, specifically utilizing radiation or chemoradiation, which have been the mainstay therapeutic approaches for the past 15–20 years.155, 156 However, recent technological innovations have led to a resurrection of surgical options through a transoral robotic approach (TORS), which allows for adjuvant therapy to be modified on the basis of pathological findings of disease. This novel paradigm can reduce radiation doses and may theoretically decrease long-term side effects. These advantages become imperative considering the emerging population of young patients with HPV-related cancer.
Tumor-related contraindications for TORS include unresectable cervical lymphadenopathy, mandibular invasion, pharyngeal wall or tongue base involvement requiring resection of greater than 50% of these sites, radiologic evidence of carotid involvement, and fixation to the prevertebral fascia. The other limitation of TORS is access, and therefore a thorough preoperative assessment of the patient and tumor characteristics is essential. This should include an evaluation of the dentition, presence of trismus or tori, tongue size, degree of neck extension, sequelae of previous treatment, and the tumor extent. The oncologic outcomes for robotic surgery have been favorable thus far, with OS and DFS rates ranging 82–100% and 86–96%, respectively. Although the role of TORS is still being determined, early oncologic results from several case series are comparable to the outcomes observed with radiation or concurrent chemoradiation.157
Tonsil
The traditional treatment of stage I and II tonsillar neoplasms is radiation therapy as a single modality. Transoral wide local excision of small, superficial lesions may be locally effective, but does not address the high potential of occult lymph node metastasis. While surgery and primary radiation offer comparable locoregional control for small tumors, patients with HPV-negative tumors may more often require PORT.158 For patients with early-stage HPV-positive tumors, TORS with neck dissection offers excellent local-regional control, without the need for adjuvant radiotherapy.157 Surgical management of advanced cancers results in poor patient function and, therefore, combined chemotherapy and radiotherapy approaches are utilized (Figure 6). For patients with nodal disease (N1-2), the addition of PORT to TORS is associated with excellent oncological and functional outcomes, and spares the need for concurrent chemoradiotherapy. There is a growing rationale for avoiding XRT, particularly among patients with p16-positive (HPV-related) small tumors (T1–2) and small volume neck disease (N1), and this approach is currently under investigation in a multi-institutional clinical trial (ECOG 3311).
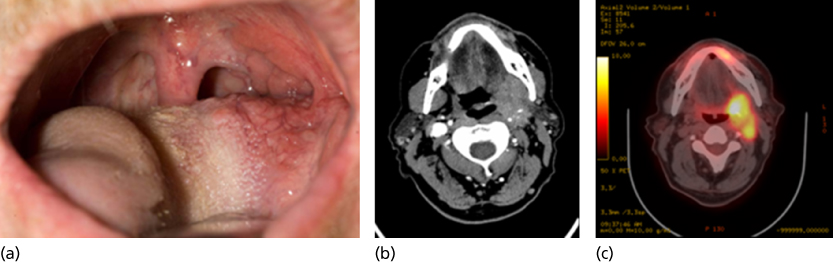
Figure 6 (a) T2 N2b SCCA of the oropharynx, clinically small lesion. (b) Deep infiltration into the parapharyngeal space is evident on CT scanning. (c) On PET-CT imaging, two distinct lesions are evident, the primary tumor and a posterior lymphatic metastasis.
Alternatively, radiation for early tonsillar cancers offers the advantage of treating upper echelon lymph nodes along with the primary tumor. Treatment is usually unilateral unless extension to the tongue base or midline soft palate is present.159 Ipsilateral treatment portals allow sparing of the contralateral mucosa and salivary glands. Modern treatment techniques, such as IMRT, permit conformal dose delivery which can reduce the potential morbidity of radiation treatment, particularly by reducing radiation-related xerostomia. Initial institutional reports have indicated encouraging treatment outcomes with the use of IMRT in oropharyngeal cancer patients;160–164 this is discussed in greater detail in the section titled “Radiotherapy.”
Survival rates for patients with advanced (stage III/IV) tumors vary according to HPV/p16 expression status and smoking history. Patients with HPV-positive tumors and less than 10 pack-year smoking history (low-risk) treated with the combination of chemotherapy and radiotherapy have a 3-year survival rate of 93%; while a smoking history of more than 10 pack-years and HPV-/p16-negative tumor undergoing chemoradiation have a 3-year survival rate of 46.2%.88 In general, surgery is rarely recommended for advanced tonsillar tumors unless the mandible is grossly invaded. When surgery is planned, postoperative concurrent therapy should be anticipated in the properly selected patient.
Tongue base
Cancers of the base of the tongue pose a more difficult therapeutic problem than do tonsillar carcinomas, particularly for HPV-negative malignancies. Most patients with HPV-negative tumors present with advanced primary site disease due to the silent nature of these tumors, resulting in frequent regional metastases, greater treatment morbidity, and poor patient survival. Due to the aggressive nature of HPV-negative tumors, most are treated with definitive radiation with or without chemotherapy, although a role for TORS is currently under clinical investigation. Owing to the rich network of lymphatics present in the base of the tongue, 75% of patients will present with stage III or IV disease (Figure 7). Understaging of the primary tumor is common because these cancers tend to be diffusely infiltrative beyond their clinical appearance.165
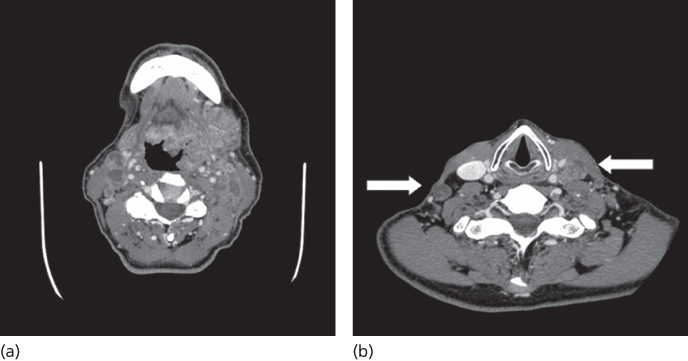
Figure 7 (a) Massive T4 N2c SCCA of the oropharynx (left base of tongue). (b) Multilevel bilateral nodal metastases present.
The results of radiation therapy alone as definitive treatment of small primary tumors (T1, T2) are better for exophytic than for deeply invasive tumors.166 Radiation alone is generally reserved for those patients without clinical nodal metastases, but can be combined with planned neck dissection for patients with clinically positive nodes that persist after the completion of radiation-based approaches.
Surgical management of early primary tongue-base tumors (T1–2) achieves results similar to those from radiation alone. Advances in robotic surgery have prompted the application of this technology in the management of tongue-base cancers, and is now a well-established approach in the HPV-positive population.167 Elective neck dissection can serve as a staging procedure, thereby providing a rationale for adjuvant radiation therapy. To date, no prospective randomized trial data that compare surgery alone with combined surgery with either preoperative or postoperative radiation are available.
Radiation therapy is a standard approach for definitive treatment for the oropharynx which combines the goal of an oncological cure with organ preservation. The current XRT regimens are the results of several large randomized trials that have demonstrated favorable OS and DFS,168 although permanent dysphagia and gastrostomy tube usage rates remain substantial.156 Total radiation dosages and overall treatment times are regarded as important variables for oncologic response and for tissue toxicity. Several studies have shown that altered fractionation improved the locoregional rate, and a meta-analysis of 15 trials demonstrated a survival advantage with altered fractionation regimens.169 Since cancers of the oropharynx are adjacent to several critical structures, the ideal dose distributions would tightly conform to the target area and spare the nonaffected surrounding tissue, which has led to the use of IMRT as a standard of care for HNSCC.170
The classic pattern of relapse after RT in OPSCC has been locoregional recurrence, attributed to the development of radioresistent tumor cells that persist after treatment. To overcome this resistance, chemotherapy has been added to sensitize the tumor cells to the damaging effects of ionizing radiation. For patients with locally advanced oropharyngeal cancer, a pivotal randomized phase III trial by the French GORTEC group revealed an improvement in both progression-free survival (PFS) and 5-year OS for patients receiving combined modality therapy (42% and 51%) versus radiation therapy alone (22.4% and 15.8%) and improvement in locoregional control rates in the chemoradiation arm (66%) versus radiation therapy alone (42%). Despite these benefits, similar rates of distant metastases were observed in both arms (11%) and more significant side effects, including hematologic toxicities and grades 3 and 4 mucositis were observed in the chemotherapy arm.171, 172 These toxicities led to a higher rate of temporary gastrostomy tube usage in the combination arm compared to the radiation therapy alone arm. Taken together, these results suggest that the addition of chemotherapy concurrently to radiation therapy improves locoregional control, which translates into both a PFS and OS benefit for these patients, at the expense of more acute toxicities.
The risk of recurrence for locally advanced OPSCC following surgical resection has traditionally been high. For patients with high-risk features, including perineural invasion, multiple positive nodes, and advanced T stage, PORT has been shown to decrease locoregional recurrences. Furthermore, it has been shown that the addition of chemotherapy given concurrently with radiation can improve locoregional control rates in patients with high-risk features, particularly when surgical margins are positive or ECS is present.134, 173 Thus, patients who undergo surgical resection for OPSCC should be considered for adjuvant chemoradiotherapy when adverse pathological features are present. Whether this treatment paradigm is necessary for HPV-positive patients is currently under investigation.
Soft palate and pharyngeal wall
Cancers of the soft palate and pharyngeal wall are less common than other oropharyngeal neoplasms. Many soft palate and posterior wall cancers tend to be superficial. Advanced lesions with deep invasion have ready access to the prevertebral fascia, infratemporal fossa, and skull base and can be associated with extensive submucosal spread with clinical skip areas.
Radiation-based approaches as curative treatment are preferred in most cases, even for T3 primary tumors. Resection of most soft palate lesions is associated with severe functional disability. The rates of occult regional metastases are difficult to determine because elective irradiation of bilateral nodal groups is included as part of primary treatment and must include the retropharyngeal lymphatics. Clinically positive lymph nodes at presentation occur in 30% of patients.174 Small primary tumors with positive nodes can be effectively treated with definitive radiation to the primary tumor and neck. Neck dissections should be performed if disease in the neck persists at 6 to 8 weeks following the completion of XRT. Pharyngeal wall cancers or palate cancers with extension to the tonsil and those cases with advanced regional metastases are usually treated with combined chemoradiotherapy approaches unless gross mandibular involvement is noted. Overall 5-year survival rates for soft palate and faucial pillar cancers are 60–70% and range from 80% to 90% for T1 and T2 lesions to 30–60% for stages III and IV lesions.175
Hypopharynx
The hypopharynx represents one of the most lethal sites for HNSCC. Lymph node metastases are clinically evident at time of diagnosis in 70–80% of patients.176 Primary tumor extension beyond the hypopharynx is common.177 Hypopharyngeal cancers are characterized by a propensity to spread submucosally to involve the oropharynx or esophagus. Ulcerated deep infiltration and skip areas are anticipated. This leads to difficulties in adequately assessing the margins of the tumor and contributes to poor local tumor control, even with the addition of adjuvant radiation. More than 75% of hypopharyngeal cancers arise in the pyriform sinus, while 20% occur in the posterior pharyngeal wall (Figure 8). Postcricoid cancers are rare (<5%). Because of the locale of hypopharyngeal cancers, their growth patterns and proximity to the larynx, surgical management often entails total laryngopharyngectomy. Extension to the esophagus will necessitate a cervical esophagectomy.
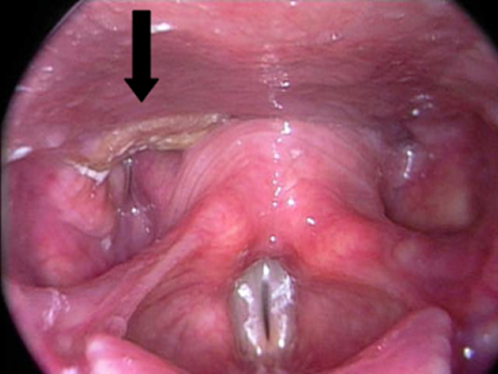
Figure 8 T1 SCCA of the hypopharynx, involving the posterior pharyngeal wall and extending into the esophageal inlet.
The staging of hypopharyngeal cancer is based on the subsite involved, the size of the tumor, the presence of vocal cord fixation, and the extent of lymph node metastases.114 Staging is critical for treatment planning and must include endoscopic evaluation.178
Because of the necessity to remove the larynx as part of the surgical treatment of most hypopharyngeal cancers, radiation therapy for early T1 and T2 and in combination with chemotherapy for T3 disease has been investigated.179 Retrospective analyses have consistently demonstrated that survival rates are lower and locoregional failure rates higher with radiation alone as compared with surgery or surgery and radiotherapy.180, 181
Nevertheless, the functional implications of primary surgery for even the earliest of hypopharyngeal cancers have brought about the primary role of radiation-based approaches. For small (T1) cancers of the hypopharynx, and particularly for superficial posterior pharyngeal wall lesions, radiation therapy alone has been used effectively, with surgery reserved for salvage.182 Radiation therapy offers the advantage of treating bilateral occult lymph node disease, including that of retropharyngeal nodes, which are frequently involved when cancer arises from the posterior pharyngeal wall. Most patients, however, present with advanced primary tumors (T3–4) and positive lymph nodes. In such patients, local control rates with radiation alone decrease to 50% and salvage surgery is rarely successful. Thus, surgical management remains the mainstay of treatment of most advanced hypopharyngeal cancers. This is especially true when function is poor at diagnosis.
Tumors arising in the lower hypopharynx or postcricoid mucosa often spread to involve the esophagus. Distal submucosal spread into the esophagus can be extensive and requires partial or total esophagectomy. Reconstruction with transposition of the stomach (gastric pull-up), jejunal free graft, or tubed fasciocutaneous free flap is currently recommended.183–185 With improved locoregional control following the advent of total laryngopharyngectomy and PORT, disease recurrence more commonly occurs in distant sites (i.e., the lung). Treatment approaches with combined preoperative or postoperative radiation have dramatically improved the control of locoregional disease, but survival rates have not improved as substantially over those with surgery alone because of the increased rates of distant metastases. Postoperative radiation is currently preferred to preoperative radiation because of its lower local recurrence rates, fewer complications, and less difficulty in accurately assessing tumor margins.180, 186 The presence of lymph node metastases, extracapsular lymph node involvement, and direct extension of the primary tumor into the soft tissues of the neck are adverse prognostic factors and are indications for postoperative chemoradiotherapy.187, 188
Overall 5-year survival rates range from 10% to 30% for posterior pharyngeal wall cancers and from 20% to 40% for pyriform sinus cancers. The rates of distant metastases range from 20% to 50%123, 127 and increase with the extent of lymph node disease.176, 189–191
Larynx
Because of the prominent role the larynx plays in communication, swallowing, respiration, and protection of the lower airway, the treatment of cancer of the larynx presents formidable dilemmas regarding functional consequences in addition to the intrinsic threat to life posed by these cancers. More so than with any other site of HNC, quality-of-life issues have been incorporated into treatment decisions and have echoed throughout the management strategy of the other head and neck sites.
The larynx is divided into three subsites: supraglottic, glottic, and subglottic. Each site is associated with differences in patterns of local spread, risks of lymphatic metastasis, and control rates. Anatomic studies of the vascular and lymphatic compartments of the larynx have defined natural anatomic barriers to cancer spread within the larynx and have contributed to the development of select surgical procedures for partial laryngeal resections of certain cancers.192, 193
The true vocal cords present an effective boundary between supraglottic and subglottic lymphatic spread within the larynx. This anatomic barrier can be compromised by tumors involving the anterior or posterior commissures and with deeply invasive tumors that extend vertically across the true and false vocal cords (transglottic cancers). Normally, the inner perichondrium of the thyroid cartilage also presents an effective barrier to cancer spread. However, cancer involvement of the anterior commissure or transglottic extension is associated with invasion of the thyroid cartilage in 40–60% of cases.194
Early diagnosis is critical for achieving high survival rates and larynx preservation. Most cancers that are diagnosed at an early stage arise in the glottic larynx. This is because minimal changes of the vibrating vocal cord from tumor growth result in dysphonia or hoarseness. Supraglottic cancers are usually more advanced than glottic cancers at the time of diagnosis because they do not generally produce early symptoms of hoarseness. Rather, the earliest symptoms of a supraglottic cancer are usually sore throat, dysphagia, referred otalgia, or the development of a neck mass representing regional metastasis. Airway compromise may be an early symptom with subglottic cancer.
Modern clinical evaluation of laryngeal cancers includes fiberoptic laryngoscopy, direct laryngoscopy, CT, or MRI of the larynx and neck, as well as videostroboscopic analysis (Figure 9). Radiological imaging is of value in assessing direct extension to the preepiglottic and paraglottic spaces, detecting cartilage invasion, and evaluating the soft tissues and lymph nodes of the neck. However, the precise evaluation of tumor extent still requires direct laryngoscopy under anesthesia. With large, obstructive tumors, tracheostomy may be required. In some patients, debulking the tumor mass at the time of direct laryngoscopy can obviate the need for tracheostomy and thereby reduce the potential risk of tumor seeding of the tracheostomy site. Even with precise clinical evaluation, inaccurate estimation of tumor extent (usually underestimation) occurs in 30–40% of cases.195, 196
Figure 9 (a) Normal larynx. (b) T3 left glottic SCCA, with obliteration of the normal vocal cord anatomy. Extension onto the supraglottis and onto the arytenoid is evident.
Supraglottic primary tumors account for approximately 35% of all laryngeal cancers.8 The staging of supraglottic cancers is based on the subsite or region of the supraglottis involved. Subsites include the false vocal cords, arytenoids, lingual and laryngeal surfaces of the epiglottis, and aryepiglottic folds. The epiglottis itself is also subdivided into the region extending above the plane of the hyoid and that below the hyoid. Suprahyoid tumors tend to have a better prognosis than infrahyoid, with the exception of those invading the aryepiglottic fold to involve the pyriform sinus. This, again, is due to the richer network of lymphatics in the infrahyoid portion of the epiglottis. Early cancers (T1 and T2) can involve one or more subsites but have normal vocal cord motion. Those cancers that cause fixation of the arytenoid or involve the postcricoid region, medial wall of the pyriform sinus, or preepiglottic space are staged T3. Those that extend beyond the larynx or invade thyroid cartilage are staged T4.
Functional and anatomic features also determine the staging of glottic carcinomas. Cancers limited to the true vocal cords are T1, those with extension to an adjacent site or with impaired cord mobility are T2, arytenoid fixation and vocal cord immobility upstages a lesion to a T3. Those tumors with cartilage involvement or extension outside the larynx are T4.
True subglottic cancers that are limited to the subglottic region (T1) or to the subglottis and true vocal cords (T2) are early cancers. Fixation of the vocal cord (T3) and cartilage invasion or extension outside the larynx (T4) is associated with a worse prognosis. The nodal classification for staging is the same as for other HNSCC sites.
Although controversy exists regarding the optimal treatment for larynx cancer, at the University of Texas MD Anderson Cancer Center, curative radiotherapy is generally the treatment of choice for early-stage laryngeal lesions. For moderately advanced lesions, one must consider the trade-offs between definitive radiotherapy with salvage surgery held in reserve versus definitive surgery or combined chemotherapy and radiation therapy approaches. A number of factors, aside from stage, warrant consideration in determining the optimal treatment strategy, including patient’s age, medical comorbidities, laryngeal function, and rehabilitative potential. Advanced T4 lesions are treated with surgery and PORT.
Radiation therapy remains the management of choice for early glottic cancers. Nevertheless, in some instances, patients may choose conservation laryngeal surgery, including endoscopic laser excision of localized lesions, or partial laryngeal surgery. Both require frozen-section analysis of margins if the patient and tumor factors support such an approach. In addition, conservation laryngeal surgical salvage is effective in those 10–20% of cases in which external beam therapy has been unsuccessful for stages I and II cancers.
The treatment of more advanced laryngeal cancers (T3 and T4) has historically included surgery with or without radiation therapy. Prospective randomized studies have shown convincingly that chemotherapy and radiation therapy (including surgical salvage) are equally effective in the long-term survival of patients with T3 laryngeal cancers as compared with surgery with or without radiation therapy. It is important to note that approximately 60% of patients may preserve their larynx, and thus quality of life has significantly improved.197–199 Speech communication profiles are clearly better in the group of patients randomized to the larynx preservation arm, but there was no determination of swallowing function.200 However, local control was poorer for patients with T4 lesions. Current standard of care argues that laryngeal preservation approaches or protocols be considered in treating such patients, with the corollary that patients with poor function at diagnosis will likely have poor laryngeal function after conservation treatment. Thus, a primary surgical approach should be strongly considered for patients with significant aspiration based on pretreatment swallowing studies. In addition, due to the toxicity of combined chemoradiotherapy regimens, those with pulmonary and cardiac comorbidities that may limit treatment intensity may be best managed with surgery and radiotherapy.
Many surgical procedures for laryngeal carcinoma involve the creation of a tracheal stoma. This area is at significant risk of tumor recurrence, which is most likely associated with paratracheal nodal metastases. For this reason, bilateral paratracheal dissections should be performed in T4 glottic cancers and radiation therapy provided postoperatively if metastases to this echelon of nodes are found pathologically. Once a stomal recurrence has developed, the prognosis is grave regardless of salvage treatment, with a reported 5-year survival of 17%.201 If risk factors for stomal recurrence are present, then the tracheal stoma should be irradiated as part of the initial management.
Supraglottic cancers
Important factors in selecting therapy for supraglottic cancers are tumor location, cord fixation, and pre-epiglottic extension. Tumors limited to the suprahyoid epiglottis are amenable to radiation with fields that encompass neck regions at risk of lymphatic metastases. In addition, some proponents of limited surgical interventions recommend endoscopic laser excision separate from management of the neck. Tumors involving the aryepiglottic folds, pyriform sinuses, or infrahyoid epiglottis tend to be more aggressive, are deeply infiltrative, and frequently involve the pre-epiglottic space. Radiation alone is less effective than surgery, resulting in more frequent local recurrences that require surgical salvage. The addition of systemic concurrent chemotherapy will positively impact the outcomes of patients with these tumors. Persistent postradiation edema of the supraglottic larynx is not uncommon and contributes to difficulty in detecting recurrence, which occurs in 40–50% of cases.202, 203 Most patients who have recurrence will ultimately require a salvage total laryngectomy.
In any patient undergoing partial laryngectomy, preoperative consent should be obtained for total laryngectomy in case the surgical findings dictate that more extensive surgery is needed. Approximately 20% of patients require prolonged tracheostomy, and this is usually related to edema secondary to PORT. The rates of persistent swallowing difficulties are low, however, and the need for completion laryngectomy for persistent aspiration ranges only from 0% to 5%.204, 205
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree