Neoplasms of the esophagus
Max W. Sung, MD  Virginia R. Litle, MD
Virginia R. Litle, MD  Steven J. Chmura, MD, PhD
Steven J. Chmura, MD, PhD  Stephen G. Swisher, MD, FACS
Stephen G. Swisher, MD, FACS  David C. Rice, MB, BCh, BAO, FRCSI
David C. Rice, MB, BCh, BAO, FRCSI  Jaffer A. Ajani, MD
Jaffer A. Ajani, MD  Ritsuko K. Komaki, MD
Ritsuko K. Komaki, MD  Mark K. Ferguson, MD
Mark K. Ferguson, MD
Overview
Esophageal cancer remains a leading cause of cancer deaths worldwide, sixth in men and ninth in women. In the past quarter century, esophageal cancer has changed from predominantly squamous-cell carcinoma to adenocarcinoma, but only in North America and Europe. The concurrent increase in cancers of the gastroesophageal junction has led to its currently inclusion in the AJCC TNM (tumor, node, metastasis) staging of esophageal cancer. Treatment options have expanded to include endoscopic mucosal resection for early-stage disease and neoadjuvant chemoradiation followed by surgery for intermediate stage disease. Surgical techniques have also evolved to include less invasive approaches using thoracoscopy and laparoscopy. Advances in systemic chemotherapy have also been reported, with the inclusion of hormonal and targeted therapies, for esophageal adenocarcinomas. A lethal disease when diagnosed in advanced stage, continued progress in therapeutic interventions can be anticipated with early-stage detection in high-risk patients, and the incorporation of molecular strategies in the treatment of advanced stage disease.
Historical perspectives
Esophageal cancer was recognized as early as the twelfth century, and pathologic descriptions were produced in the sixteenth and seventeenth centuries.1 Initial attempts to treat the tumor included resection of a cervical esophageal cancer in 1877 and an intrathoracic cancer in 1913.2, 3 Surgical resection became the mainstay of therapy beginning in the 1940s. Radiotherapy was initially used in the 1920s, but it was not until the development of megavoltage techniques in the 1950s that this modality was used with any frequency. Active chemotherapeutic agents were first identified in the 1960s and have been increasingly incorporated into the treatment of advanced tumors.
Anatomy and histology
The esophagus is a muscular organ that extends from the cricopharyngeus muscle at its cephalad margin to the esophagogastric junction (EGJ). It is divided into regions on the basis of both anatomy and the proclivity for certain neoplasms to develop in specific regions (Figure 1). The cervical esophagus extends from the cricopharyngeus muscle to the thoracic inlet (15–18 cm from incisors). The upper third of the thoracic esophagus extends from the thoracic inlet to the tracheal bifurcation, just below the level of the aortic arch (18–24 cm). The middle thoracic esophagus extends from the tracheal bifurcation to a point midway between the carina and the EGJ (24–32 cm). The lower thoracic esophagus extends from this midway point to the EGJ (32–40 cm). Tumors of the EGJ and gastric cardia are often included in discussions of esophageal cancer because of their pathophysiologic similarities to adenocarcinomas of the distal esophagus.4 Classifications of EGJ tumors have developed that define the different types of tumors according to the epicenter of the mass (i.e., type I, >1 cm above EGJ; type II, 1 cm above to 2 cm below EGJ; type III, >2–5 cm below EGJ).5
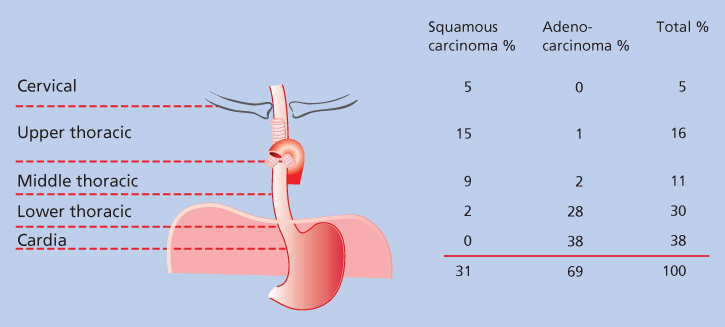
Figure 1 The distribution of malignant neoplasms of the esophagus according to cell type and site of occurrence.
The muscle tissues of the esophagus are arranged in an outer longitudinal layer and an inner circular layer. The proximal one-third to one-half of the muscularis propria is derived from the bronchial arches, making it principally skeletal (striated) muscle and giving it the potential to develop rhabdomyosarcomas (Table 1). The remainder of the esophageal musculature comprises smooth muscle, as does the rest of the foregut, in which leiomyomas or leiomyosarcomas may occur. Fibrous, fatty, and connective tissues interspersed in the wall of the esophagus may also give rise to sarcomas.
Table 1 Neoplasms of the esophagus
Epithelial
|
Nonepithelial
|
The esophagus is lined over most of its length with squamous epithelium, which can give rise to squamous-cell carcinoma (SCC) and is the most common neoplasm of the esophagus in most of the world. These cancers occur most often in the cervical esophagus and in the upper and middle thoracic esophagus. Carcinosarcomas and spindle cell carcinomas, which are subtypes of squamous-cell cancer, infrequently occur in these regions. The distal 2–3 cm of the esophagus and the cardia is lined by columnar epithelium, in which adenocarcinomas may occur.5 The development of intestinal metaplasia, known as Barrett esophagus, more proximally because of gastroesophageal reflux and other factors allows the development of adenocarcinomas in the middle and upper thoracic esophagus in isolated instances. Other cellular elements in the mucosa, submucosal glands, and muscularis propria may give rise to unusual neoplasms, such as small cell cancer, malignant melanoma, granular cell tumors, mucoepidermoid carcinoma, and adenoid cystic carcinoma.6, 7 The histologic types of benign and malignant tumors of the esophagus are given in Table 1.
Etiology
In most of the world, dietary and nutritional factors are the most common etiologic agents and are associated with the development of predominantly SCCs. Among the most frequently cited carcinogens are nitrosamines, which have been found to be in high concentrations in foods in endemic areas of esophageal cancer in northern China.8 Contamination of food by fungi that reduce nitrate to nitrite may further aggravate this situation. Mechanical factors that have been cited include drinking beverages at excessively high temperatures and consumption of foods containing silica or other substances, such as crushed seeds, that directly irritate the esophagus.8, 9 Deficiencies of folic acid, vitamins A and C, and riboflavin, molybdenum, and selenium also have been implicated in the development of esophageal neoplasms.10, 11, 12a Higher intake of fruits and vegetables, on the other hand, has been reported in a meta analysis of observational studies to be associated with a reduced risk of esophageal SCC.12b
In the western hemisphere, social factors figure more prominently in the development of esophageal cancer. Heavy alcohol consumption increases the risk of cancer 10–25 times, depending on the concentration of alcohol in the beverage.13 Cigarette smoking has been linked to the development of both squamous-cell cancers and adenocarcinomas.14 The combined exposure to low levels of tobacco and alcohol increases the risk of esophageal cancer by a factor of 10–20, whereas the synergistic effect of exposure to high levels of both alcohol and tobacco increases the risk by a factor of over 100.15 Chronic esophageal injury due to gastroesophageal reflux has also been shown to be a risk factor for the development of adenocarcinoma, with severe, longstanding reflux symptoms increasing the risk of cancer by a factor of 40.16 Chronic gastroesophageal reflux is believed to be etiologically related to the development of Barrett esophagus, which occurs primarily in white males and is associated with a 40-fold increase in the risk of adenocarcinoma of the esophagus.17 A relationship between reflux and the development of squamous-cell cancers has also been suggested in patients who consume a diet high in linoleic acid.18 The lifetime risk of squamous-cell cancer of the esophagus is 5–10% in patients with esophageal achalasia, a 15-fold increase in incidence that is likely due to chronic irritation from retained food.19–21
Race and gender are associated with varying incidences of cancer of the esophagus in the western hemisphere. Men are more commonly affected than are women, blacks develop squamous-cell cancers more often than do whites, and white males develop adenocarcinomas more often than do females or individuals of other race groups.22 However, none of these increased frequencies have yet been linked to genetic factors, and most have been explained by variations in socioeconomic status and the attendant social habits described earlier. The single proven genetic abnormality that is associated with a 25% lifetime incidence of squamous-cell cancer of the esophagus is tylosis A, the late-onset, familial form of palmar, and plantar hyperkeratosis.23 A number of genetic alterations are associated with neoplasms of the esophagus, including allelic losses at chromosomes 3p, 5q, 9p, 9q, 13q, 17p, 17q, and 18q. Abnormalities of TP53, Rb, cyclin D1, and c-myc have also been associated with esophageal cancer development.24
Infectious agents, including human papillomavirus (HPV), have been implicated in the development of neoplasms of the esophagus. Transforming proteins from high-risk HPV subtypes 16, 18 cause loss of function of the tumor suppressor genes TP53 and Rb, resulting in abnormal proliferative states.25 HPV has been documented in up to 50% of patients with squamous-cell cancers of the esophagus and appears to be more common in areas in which esophageal cancer is endemic.26 These findings have not been universally reproducible, however.27a A systematic review and meta-analysis of 66 case–control studies suggests an association between HPV infection and esophageal SCC.27b There are significant variation and lack of consistency between studies; the International Agency for Research on Cancer (IARC) has concluded that the epidemiologic evidence of the association is inadequate.27c
Epidemiology
The most common esophageal neoplasm worldwide is SCC (90%); in the United States and Europe, the incidence of adenocarcinoma has been rising and has surpassed SCC (Figure 2). The incidence of esophageal cancer varies more worldwide than any other cancer. In the United States, the incidence of esophageal cancer is approximately seven cases per 100,000 people, whereas in high-risk areas in China, Iran, and Russia, it can be more than 100 per 100,000 people.27d In rural Linxian, China, esophageal cancer is the leading cause of death.29, 30a These geographical variations imply a strong role for local environmental carcinogens in esophageal carcinogenesis. The long-term survival rate for patients with esophageal cancer, regardless of histology, is less than 10%, which is due, in large part, to the advanced stage at which these cancers are detected. Worldwide, the incidence and mortality for esophageal cancer are estimated to be 455,800 and 400.200, respectively, for 2012.30b For the United states, incidence and mortality for 2015 (excluding EGJ tumors) were estimated at 16,890 and 15,590, respectively. 31 The age-adjusted mortality rate from esophageal cancer in the United States has increased by 11% between 1975 and 2012.32
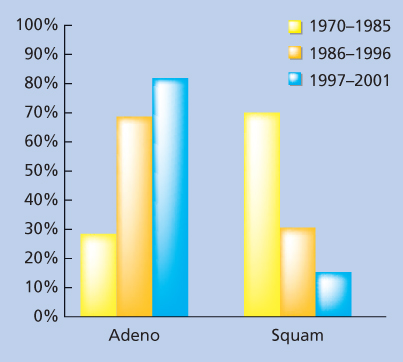
Figure 2 The proportion of adenocarcinoma and distal esophageal tumors has increased over time. Abbreviations: Adeno, adenocarcinoma and squam, squamous-cell carcinoma.
Source: Hofsetter 2002.28a Reproduced with permission of Wolters Kluwer.
Besides geographical differences, there are other important differences that have been described in the Western hemisphere. Esophageal carcinoma is more common in men than in women regardless of the histologic subtype, and squamous-cell cancers tend to occur more frequently in blacks than in whites. In addition, there has been a striking increase in the incidence of adenocarcinoma in the United States and Europe, whereas SCC has remained stable.33 Adenocarcinoma of the distal esophagus and cardia has risen by more than 350% among white males since the mid-1970s, increasing at a more rapid rate than any other solid tumor (Figure 2).22, 34–38 The reasons for these dramatic changes in the Western hemisphere are not clear but may include increased Barrett esophagus, gastroesophageal reflux, obesity, and over-the-counter medications as well as changing smoking and alcohol use.16 In other areas of the world (outside Europe and North America), SCC of the esophagus predominates and adenocarcinoma has not increased.
Patients usually present because of complaints of dysphagia, which requires either the involvement of the entire circumference of the esophagus by the neoplasm or the growth of a large, polypoid obstructing mass. Dysphagia first develops in response to dense solid foods and progresses to result in difficulties with soft foods and then liquids. Accompanying vomiting and regurgitation are common. Symptoms of heartburn or gastroesophageal reflux (40%) are often associated and occur more frequently in patients with adenocarcinoma.39 The most common symptom in the absence of dysphagia is pain (25% of cases).39 It may be related to swallowing (odynophagia) or local extension of the tumor into adjacent structures such as the vertebral bodies, pleura, or mediastinum. In some instances, it may be due to bony metastases from systemic spread. Weight loss is noted in more than 70% of patients and is due to the inability to swallow or systemic manifestations of the disease.39 Patients with weight loss have a significantly worse prognosis in many series.40, 41
The histology of the tumor depends in large part on its location. Adenocarcinoma is located predominantly in the lower esophagus, whereas SCC predominates in the cervical, upper, and middle esophagus (Figures 1 and 2). Unfortunately, because of the distensible nature of the esophagus, these symptoms often do not occur until the tumors are quite large and no longer localized to the esophagus.
In contrast to patients who present because of symptoms of dysphagia, a smaller subset of earlier stage adenocarcinoma patients are increasingly being identified in North America and Europe with endoscopic abnormalities noted during endoscopic surveillance for gastroesophageal reflux symptoms or Barrett’s esophagus. These patients tend to present with smaller, earlier stage tumors that are more likely to be localized and amenable to treatment. In addition, in some areas of China where squamous-cell cancer is endemic, routine cytologic screening is performed, and if results are diagnostic or suspicious, follow-up endoscopy is performed. These mass screening efforts have led to early diagnosis in many areas of China, with 5-year survivals of more than 90%.30a The low incidence of esophageal cancer in most areas of the world, however, makes this type of mass screening impractical and cost ineffective from a public health standpoint.
Treatment overview
Patients who present with suspected esophageal cancer, because of either the above-mentioned symptoms or mass screening efforts, initially require pathologic confirmation of malignancy (see section titled “Diagnosis”). Once the pathologic diagnosis has been obtained, patients are assessed for therapy by determining the clinical stage (see the section titled “Staging Evaluation”) of the tumor and the physiologic status of the patient (see section titled “Pretreatment Assessment”). With this information, an informed decision about treatment can be made that optimizes the chance to cure or palliate the disease while minimizing the treatment-related morbidity (see section titled “Therapy”) (Figure 3).
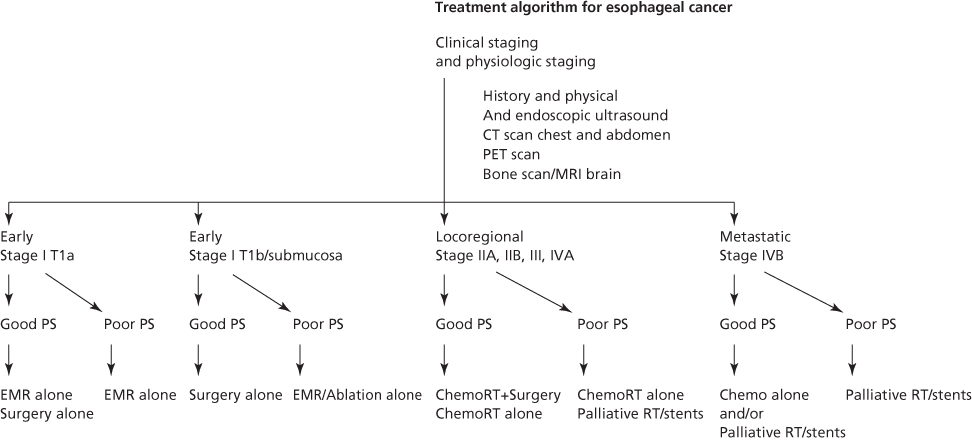
Figure 3 Current treatment recommendations for esophageal cancer based on performance status and clinical stage. Abbreviations: Chemo, chemotherapy; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; PS, performance status; and RT, radiotherapy.
Diagnosis
Symptomatic patients or patients in whom an esophageal mass is diagnosed by screening require endoscopy to enable biopsy for histologic examination and/or brushing for cytologic examination. The overall accuracy of histologic diagnosis of esophageal cancer using flexible endoscopy with biopsy is about 80%.42, 43 Endoscopically directed cytologic brushings have a diagnostic accuracy in excess of 90%. Combining these techniques yields an overall diagnostic accuracy of 98%.42 If flexible endoscopic diagnostic techniques fail, rigid endoscopy with biopsy, which has a diagnostic accuracy close to 100%, should be considered.43 In selected patients who have symptoms or findings on physical examination suggestive of metastatic disease, biopsy of the suspected metastatic site provides both a tissue diagnosis and a confirmation of stage.
Pretreatment assessment
In addition to a careful history and physical examination, the overall assessment of a patient with esophageal cancer focuses on specific concerns. Factors that are associated with treatment-related morbidity and mortality include patient age, a recent history of alcohol or tobacco abuse, body weight, recent weight loss, nutritional status, performance status, hepatic dysfunction, and renal dysfunction. Specific preoperative risk factors have been identified for esophageal resection and are therefore included in the physiologic assessment. Pulmonary complications are predicted by age, preoperative arterial oxygen tension, abnormal chest radiograph, and forced expiratory volume in one second (FEV1).44 Operative mortality is predicted by age, mid-arm circumference, history of smoking, incentive spirometry, performance status, and the frequency with which the operation is performed in an institution.44, 45 Any symptoms of cardiac disease necessitate careful evaluation with electrocardiography (ECG), echocardiography, stress tests, and coronary arteriography as indicated. Patients with significant coronary artery disease are at increased risk of morbidity and mortality. Cardiac intervention may be warranted before surgical treatment. These physiologic assessments allow patients to be classified as good or poor performance status, which then allows the aggressiveness of treatment to be tailored to their specific risks (Figure 3).
Staging evaluation
Assessment of stage permits medical practitioners to discuss the status of individual patients with accuracy, allows informed recommendations about therapy, and gives patients and their families necessary information about prognosis. The typical assessment for most patients often includes upper gastrointestinal endoscopy, contrast radiography of the esophagus, computed tomography (CT) of the chest and abdomen, endoscopic ultrasonography (EUS), and positron emission tomography (PET). Other examinations are selected on the basis of specific findings in individual patients, such as neurologic symptoms (magnetic resonance imaging [MRI] of brain), musculoskeletal symptoms (bone scan), or supraclavicular nodes (neck ultrasonography and biopsy). Staging techniques allow patients to be accurately placed into groups in which risk can be assessed as well as the optimum type of therapy selected.
Presentation
Contrast radiography of the esophagus
A contrast study of the esophagus is often the initial diagnostic examination obtained in patients with dysphagia. It allows confirmation of mucosal irregularity and serves as guide for subsequent endoscopy. It also allows evaluation of the esophagus and stomach distal to an area of stenosis, which cannot also be assessed by endoscopy if tight strictures exist.
Endoscopy
Upper gastrointestinal endoscopy allows a pathologic diagnosis to be obtained in the majority of patients. The gross appearance of the tumor can be categorized as advanced or superficial, and the extent of the tumor can be accurately determined. Additional unsuspected malignancies that may be present in up to 20% of patients with squamous-cell cancer may also be identified. Endoscopy also permits assessment of the mobility of a tumor, indicating whether it is fixed within the mediastinum.
Endoscopic ultrasonography
EUS improves the staging accuracy of primary tumors and regional and some nonregional lymph nodes. It has become an essential tool to help identify patients with early-stage carcinoma who may not need multimodality treatment (Figure 3). EUS depicts the normal esophagus as five alternating hyperechoic and hypoechoic layers representing the mucosa and lamina propria, muscularis mucosa, submucosa, muscularis propria, and adventitia. Depth of tumor invasion is determined by assessing the level to which the tumor extends. EUS is also useful for assessing whether there is involvement of the aorta, but airway invasion is not accurately determined because of interference of the ultrasound signal with the intratracheal air column. The accuracy of EUS determination of primary tumor stage is related to the pathologic tumor stage, being more accurate for more advanced stages of disease, with an overall accuracy of about 80%.46–48 EUS is substantially less accurate in staging primary tumors after chemoradiotherapy is administered, primarily because of overstaging. The technique is not able to distinguish between treatment-induced fibrosis and residual tumor, leading to a mean overall accuracy in this setting of 45%.49a,b
In assessing lymph nodes with EUS, three criteria are used: size, border characteristics, and internal architecture. Lymph nodes that are enlarged, have a well-defined external border, and are characterized by relatively uniform, hypoechoic internal architecture are more likely to be malignant. Using these criteria, the overall accuracy of lymph node staging by EUS is about 75%.47, 50 After chemoradiotherapy, the accuracy of EUS for staging lymph nodes decreases to just over 50%.49a Development of fine needle aspiration techniques has allowed pathologic confirmation of enlarged lymph nodes in both regional and nonregional sites.48–51
CT of the chest and abdomen
CT has become a standard technique for esophageal cancer staging since its inception in the late 1970s because it has allowed better identification of patients with metastatic (M1b) and locally invasive tumors (T4). CT is not very accurate for determining the depth of the primary tumor (T) status, but it is helpful in identifying patients who might have direct invasion of local structures, such as the aorta or major airways, either of which precludes surgical intervention.46 Aortic invasion is suspected when more than 25% of the aortic circumference is effaced by an esophageal cancer.
CT does not accurately determine lymph node (N) status as normal lymph nodes often vary in size according to their location in the mediastinum and abdomen, and a single size limit for nodes is not possible to establish. In addition, lymph nodes involved by metastatic spread are often not enlarged.52–55 The sensitivity for CT detection of involved lymph nodes is therefore poor (30–60%), and the overall accuracy of nodal detection by CT is <60%.56–58
CT is most useful for detecting distant often unsuspected metastatic (M) disease. The most common sites for metastatic spread, aside from nonregional lymph nodes, are (in decreasing order of frequency) the liver, lung, peritoneum, adrenal gland, bone, and kidney. CT of the thorax and abdomen evaluates almost all these regions. Accuracy of the CT detection of liver metastases is in excess of 90%.52, 57, 59, 60
Bronchoscopy
Bronchoscopy should be performed for all patients who are candidates for surgical therapy and whose tumors are adjacent to the trachea or mainstem bronchi, which typically are the mid-esophageal SCCs. This permits direct assessment of tumor invasion into the airway lumen or submucosa, which would be a contra-indication to surgical resection.
Magnetic resonance imaging
As a method for routine staging of esophageal neoplasms, MRI offers no advantages compared with CT and is therefore seldom used as it is a more difficult and expensive test to obtain. Both techniques have similar specificities, sensitivities, and overall accuracy for determining resectability with regard to direct invasion of the aorta and airway.53, 54, 57 Neither test provides much useful information about regional or metastatic lymph nodes, and MRI offers no improvements over CT in evaluating the liver for metastatic disease. Whether advances in MRI technology will offer improved staging capabilities remains to be seen.
Positron emission tomography
PET scanning to detect involved lymph nodes and sites of metastatic disease through increased metabolism with 18F-fluorodeoxyglucose was introduced as an investigational staging technique for esophageal cancer in the mid-1990s. PET is most useful in helping identify patients with unsuspected metastatic disease. Given the possibility of false positive results from inflammation, biopsies are still required to definitively confirm metastatic disease in PET positive patients. The PET scan often serves as a guide to help identify suspected areas of metastatic disease for confirmatory biopsy. Another potential role for PET may be in determining response to chemotherapy and radiation therapy although false positives induced by chemotherapy and radiation-induced inflammation remain a problem.61–63 PET may also have a role in identifying recurrent esophageal cancer by allowing targeting of unsuspected areas of increased metabolic activity.64
Bone scintigraphy
Bone scans have been used for decades for staging patients with esophageal neoplasms; however, PET-CT scan has essentially replaced bone scans in the work-up of esophageal cancer. In patients without bone pain or other evidence for metastatic disease, the overall likelihood of identifying skeletal metastases is <5%.65
Neck ultrasonography
Cervical and supraclavicular lymph nodes are affected by metastatic spread in up to 30% of patients with neoplasms of the thoracic esophagus, and most are not detectable on physical examination. The use of routine ultrasound examination of the neck in patients without palpable lymph nodes yields unsuspected nodal metastases in over 10% of patients.66 The overall accuracy of cervical and supraclavicular nodal assessment with ultrasonography is about 90%.67, 68a The addition of routine needle aspiration under ultrasound guidance for cytology may improve the yield of this potentially valuable technique.68a At the present time, however, this is not a commonly used screening technique and is reserved for cases where nodes are palpable to confirm metastatic disease
Minimally invasive surgical staging
Laparoscopy has been used since the early 1980s, and thoracoscopy has been used since the early 1990s in an effort to improve staging of esophageal neoplasms. Video-assisted thoracoscopic surgery (VATS) staging was studied in a CALGB study in 1995 and was found to be feasible and 88% accurate.68b,c With the current standard and accurate staging modalities of PET-CT and EUS, surgical staging is not routinely done except in conjunction with placement of a laparoscopic jejunostomy tube for nutritional support before chemoradiation therapy.
Biologic staging
A variety of biologic markers have been investigated for their utility in estimating prognosis in patients with esophageal neoplasms. These include growth factors (epidermal growth factor [EGF], transforming growth factor [TGF]-β, platelet-derived growth factor [PDGF]), oncogenes (c-myc, int-2, hst-1, cyclin D, EGFR, HER-2/neu, h-ras), tumor suppressor genes (Rb,TP53, p73, APC, MCC, p27), the cell adhesion molecule E-cadherin, the oncodevelopmental marker CEA, and deoxyribonucleic acid (DNA) content and ploidy.69–71 To date, none of these techniques have become generally accepted staging techniques except in isolated referral centers.
TNM (tumor, node, metastasis) staging system
These pretreatment staging evaluations allow patients to be clinically staged by a cTNM (tumor, node, metastasis) staging system that can help determine the optimum therapy (Figure 3). The current staging system for esophageal cancer includes epithelial tumors of the cervical, thoracic, and intra-abdominal esophagus, as well as the EGJ (Table 2).72a,b Tumors are staged according to clinical findings from noninvasive tests and pathologic findings resulting from any invasive staging procedures. It is useful to specify lymph node locations during biopsy and resection because their location determines whether they are considered regional nodes or nonregional metastatic nodal disease (Figure 4). The prognosis of patients with esophageal cancer is determined by the depth of penetration of the primary tumor (transmural vs nontransmural), whether there is lymph node involvement, the relative number of lymph nodes involved, and whether distant metastases are present.28, 72, 74–87 Nonanatomic classifications have been added, including histopathologic cell type (adenocarcinoma, SCC), histology grade (G1-4), and cancer location. The long-term survival in patients with esophageal neoplasms correlates well with the pathologic stage and histopathologic cell type (Figure 5).74, 80 The ability of the clinical cTNM staging system to accurately predict the pTNM status has improved with time as the use of endoscopic ultrasound, CT of the chest and abdomen, and PET scan has increased.28a
Table 2 TNM staging system for esophageal neoplasms
| Primary tumor (T) | |||||
| TX | Primary tumor cannot be assessed | ||||
| TO | No evidence of primary tumor | ||||
| Tis | High-grade dysplasia | ||||
| T1 | Tumor invades lamina propria or submucosa | ||||
| T2 | Tumor invades muscularis propria | ||||
| T3 | Tumor invades adventitia | ||||
| T4a | Resectable cancer invades adjacent structures such as pleura, pericardium, and diaphragm | ||||
| T4b | Unresectable cancer invades adjacent structures such as aorta, vertebral body, and trachea | ||||
| Regional lymph nodes (N) | |||||
| Any periesophageal lymph node from cervical nodes to celiac nodes | |||||
| NX | Regional lymph nodes cannot be assessed | ||||
| NO | No regional lymph node metastases | ||||
| N1 | 1–2 positive regional lymph nodes | ||||
| N2 | 3–6 positive regional lymph nodes | ||||
| N3 | ≥7 positive regional lymph nodes | ||||
| Distant metastasis (M) | |||||
| MX | Distant metastasis cannot be assessed | ||||
| MO | No distant metastasis | ||||
| M1 | Distant metastasis | ||||
| Additions of nonanatomic cancer characteristics | |||||
| Histopathologic cell type | |||||
| Adenocarcinoma | |||||
| Squamous-cell carcinoma | |||||
| Histologic grade | |||||
| G1 | Well differentiated | ||||
| G2 | Moderately differentiated | ||||
| G3 | Poorly differentiated | ||||
| G4 | Undifferentiated | ||||
| Cancer location | |||||
| Upper thoracic | 20–25 cm from incisors | ||||
| Middle thoracic | >25–30 cm from incisors | ||||
| Lower thoracic | >30–40 cm from incisors | ||||
| Esophagogastric junction | Includes cancers whose epicenter is in the distal thoracic esophagus, esophagogastric junction, or within the proximal 5 cm of the stomach (cardia) that extend into the esophagogastric junction or distal thoracic esophagus (Siewert III). These stomach cancers are stage grouped similarly to adenocarcinoma of the esophagus | ||||
| Adenocarcinoma stage groupings | |||||
| Stage 0 | Tis | N0 | M0 | G1 | |
| Stage IA Stage IB | T1 T1 | N0 N0 | M0 M0 | G1–2 G3 | |
| Stage IIA | T2 T2 | N0 N0 | M0 M0 | G1–2 G3 | |
| Stage IIB | T3 T1–2 | N0 N1 | M0 M0 | GAny GAny | |
| Stage IIIA | T1–2 T3 T4a | N2 N1 N0 | M0 M0 M0 | GAny GAny GAny | |
| Stage IIIB | T3 | N2 | M0 | GAny | |
| Stage IIIC | T4a T4b TAny | N1–2 NAny N3 | M0 M0 M0 | GAny GAny GAny | |
| Stage IV | TAny | NAny | M1 | GAny | |
| Squamous-cell carcinoma stage groupings | |||||
| Stage | T | N | M | G | Location |
| Stage 0 | Tis | N0 | M0 | G1 | Any |
| Stage IA Stage IB | T1 T1 | N0 N0 | M0 M0 | G1 G2–3 | Any Any |
| Stage IIA | T2–3 T2–3 | N0 N0 | M0 M0 | G1 G1 | Lower Upper, middle |
| Stage IIB | T2–3 T2–3 T1–2 | N0 N0 N1 | M0 M0 M0 | G2–3 G2–3 GAny | Lower Upper, middle Any |
| Stage IIIA | T1–2 T3 T4a | N2 N1 N0 | M0 M0 M0 | GAny GAny GAny | Any Any Any |
| Stage IIIB | T3 | N2 | M0 | GAny | Any |
| Stage IIIC | T4a T4b TAny | N1–2 NAny N3 | M0 M0 M0 | GAny GAny GAny | Any Any Any |
| Stage IV | TAny | NAny | M1 | GAny | Any |
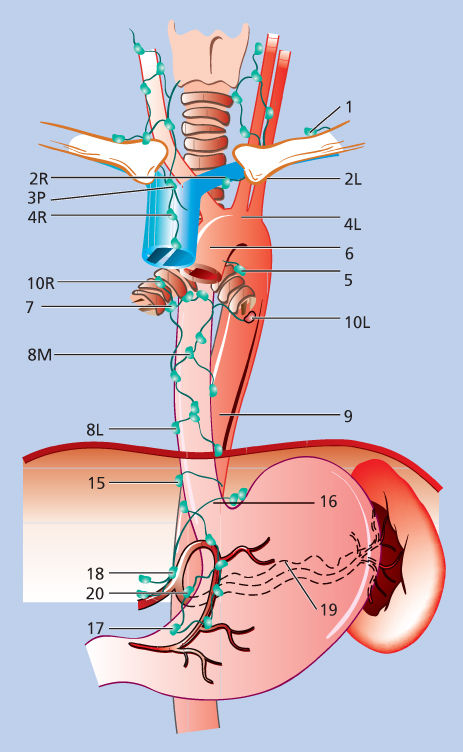
Figure 4 Lymph node staging map for neoplasms of the esophagus.
Source: Ferguson 2010.73 Reproduced with permission of Elsevier.
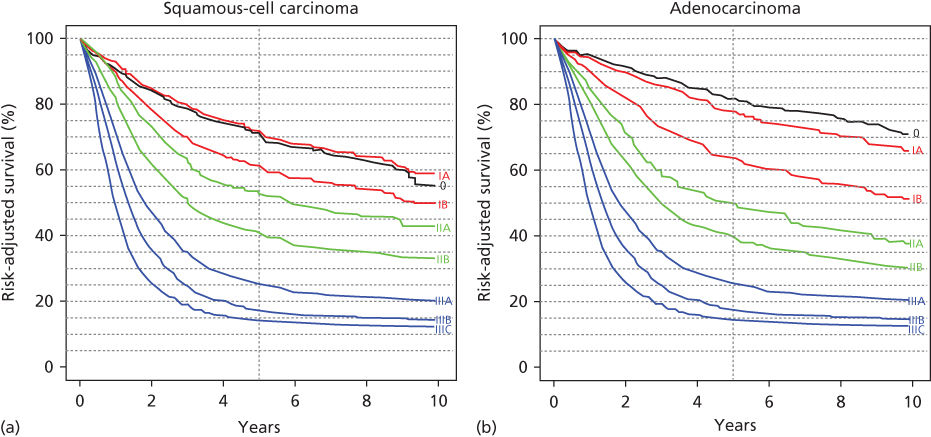
Figure 5 (a) Risk adjusted survival for squamous-cell carcinoma according to the ANCC Cancer Staging Manual, seventh edition. Source: Rice 2010.72a Reproduced with permission of Springer. (b) Risk-adjusted survival for adenocarcinoma according to the ANCC Cancer Staging Manual, seventh edition.
Source: Rice 2010.72b Reproduced with permission of Springer.
Therapy
Standard curative treatment options for esophageal cancer include surgical resection, external beam radiotherapy, chemotherapy, or combinations of two or three of these options. The selection of appropriate therapy is often challenging because few comparisons of these options have been performed in prospective, randomized manner. In addition, these trials have often been performed over a long time period with an inadequate number of patients during which significant changes in histology and types of treatment have occurred (i.e., different radiation equipment, dosages and fields, and different surgical techniques and chemotherapy agents). Clinical staging techniques have also evolved over time, leading to stage migration and poor correlation with pathologic stage, especially from studies before CT scan, endoscopic ultrasound, and PET.28a These problems make comparisons between different trials or treatment arms difficult. Treatment strategies have therefore evolved over time based on regional experiences and biases. In an effort, to guide current treatment strategies, we have included an esophageal treatment algorithm that incorporates different treatment strategies based on the physiologic status and clinical stage of the patient (Figure 3). This algorithm reflects the treatment biases of the authors and is meant only to give some guidance in an otherwise confusing therapeutic arena.
In esophageal cancer, local control of the disease, as well as cure, are the primary objectives of therapy because of the debilitating effects of dysphagia caused by progressive tumor growth and esophageal obstruction. Early-stage cancers (stage 0, I) traditionally have been treated by resection in good performance patients and by radiotherapy with or without radiation-sensitizing chemotherapy in patients who cannot tolerate surgery. Recently, newer less invasive modes of treatment include endoscopic mucosal resection (EMR) and other ablative therapies. Most patients, however, present with locoregionally advanced esophageal cancer because of the long period of asymptomatic tumor growth. These patients (stages II, III, and IVa) tend to have poor outcomes when treated with a single modality such as surgery or radiation therapy because of the development of metastatic and locoregional recurrences. Attempts have therefore focused on treating these patients with multimodality approaches combining locoregional (surgery/radiation) therapies with systemic (chemotherapy) treatments. Although controversial, many oncologists in North America currently recommend treating these patients with definitive chemoradiation alone or preoperative chemoradiation and surgery. The data to unequivocally support these approaches are lacking as demonstrated in the often conflicting randomized trial results (Table 3). Some centers have argued that more aggressive en bloc resection and three-field lymphadenectomies are also important. Surgery and chemotherapy are often avoided in poor performance status locoregionally advanced patients because of treatment-related morbidity. In these patients, locoregional palliation can often be achieved with palliative stents and/or radiation. For patients who are initially recognized to be in advanced (metastatic) stages of disease, chemotherapy with or without palliative radiotherapy has been the mainstay of treatment. The development of endoesophageal stents has provided additional locoregional palliation and has limited the need for palliative surgical bypass even in patients with tracheoesophageal fistulas (Figure 3).
Table 3 Results of randomized trials of definitive chemoradiotherapy for locoregionally advanced esophageal cancer
| Author | Year | Treatment | Technique | Histology | Patients | Median survival | p Value |
| Chemo/RT versus RT alone | |||||||
| Roussel et al.88 | 1989 | Methotrexate + 56 Gy | C ∼ RT | S | 77 | 9 mo | NS |
| 56 Gy | 73 | 8 mo | |||||
| Araujo et al.89 | 1991 | 5-FU, bleomycin, mito + 50 Gy | C/RT | S | 28 | 18 mo | NS |
| 50 Gy | 31 | 16 mo | |||||
| Hatlevoll et al.90 | 1992 | Cisplatin, bleomycin + 53 Gy | C ∼ RT | S | 46 | 6 mo | NS |
| 53 Gy | 51 | 6 mo | |||||
| Slabber et al.91 | 1998 | Cisplatin, 5-FU + 40 Gy | C/RT | S | 34 | 6 mo | NS |
| 40 Gy | 36 | 5 mo | |||||
| Smith et al.92 | 1998 | Mitomycin, 5-FU + 40 Gy | C/RT | S > A | 60 | 15 mo | .04 |
| 40 Gy | 59 | 9 mo | |||||
| Cooper et al.93 | 1999 | Cisplatin, 5-FU + 50 Gy | C/RT | S > A | 61 | 13 mo | .01 |
| 64 Gy | 62 | 9 mo | |||||
| Chemo/RT (high dose) versus chemo/RT (low dose) | |||||||
| Minsky et al.94 | 2002 | Cisplatin, 5-FU + 50 Gy | C/RT | S > A | 10 | 18 mo | NS |
| Cisplatin, 5-FU + 64y | C/RT | 9 | 13 mo | ||||
Chemo
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||||