Ana M. Rodriguez, MD, MPH, FACOG
Overview
Cervical cancer is the third most common cancer among women worldwide and the fourth leading cause of cancer deaths in females, with an estimated 529,800 new cases and 275,100 deaths in the year 2008. The incidence is declining in the United States with 12,900 new cases and 4100 deaths in 2015. Squamous cell carcinoma is the most common histology with the human papilloma virus (HPV) being the most common etiology. Cervical cancer can be easily prevented with pap smears, HPV testing, and hopefully the HPV vaccine. Early stage cervix cancer can be treated with surgery including now fertility sparing surgery and there are high cure rates. Local advance cervical cancer is treated with combination of chemotherapy and radiation therapy with high survival rates but there is room for improvements with advancing stage. Systemic chemotherapy can be used for treatment of both recurrent and metastatic disease, but careful attention should be paid to balancing benefit and toxicity. The key to success will be the transportation of success seen in the developing countries to success in areas of the world where advanced-stage invasive cervical cancer is most common.
Epidemiology
Incidence and mortality
Cervical cancer is the third most common cancer among women worldwide and the fourth leading cause of cancer deaths in females, with an estimated 529,800 new cases and 275,100 deaths in the year 2008.1 The incidence of cervical cancer in developing countries remains nearly twice that in developed countries, with the highest rates observed in Africa, South-Central Asia, and South America (29 per 100,000 per year) and the lowest in Western Asia, Australia/New Zealand, and North America (7.5 per 100,000 per year).2 In the United States, it is estimated that there will be 12,900 new cases of cervical cancer in 2015, with 4100 related deaths.3 Cervical cancer is frequently seen in the Hispanic population (10.5% of all cases of cervical cancer) followed by the African-American population (10.2%), American Indians (9.5%), whites (7.1%), and Pacific Islanders/Asian (6.4%).3
In the past 40 years, primarily because of the introduction of screening with the Pap smear, the incidence and mortality rates for cervical cancer have declined in most developed countries.4 In the United States; incidence rates have declined nearly by 70% during this period. Rates of invasive cancer per 100,000 females have declined from 10.2 in 1998 to 8.5 in 2002.5 In developing countries, however, cervical cancer continues to be a significant health problem owing to suboptimal screening programs and a lack of therapy for precancerous conditions.
There are no reliable data on the prevalence and incidence of precancerous cervical lesions. In the United States, the National Cancer Institute (NCI) estimates that each year approximately 300,000 women are found to have premalignant cervical lesions, whereas the American Cancer Society reported that in 2001, an estimated 65,000 women were found to have carcinoma in situ (CIS) of the cervix. From the SEER data, the overall incidence of squamous cell CIS among whites is 41.4 per 100,000 woman-years from 1991 to 1995.6
Risk factors for cervical neoplasia
Human papillomavirus and other sexually transmitted agents
Epidemiologic evidence has long suggested a sexually transmitted etiology for cervical neoplasia. Supporting this hypothesis, several measures of sexual behavior (including multiple sexual partners, early age at first sexual intercourse, and sexual habits of male partners) have consistently been associated with an increased risk of cervical neoplasia.7 In the mid-1970s, the hypothesis of a causal relationship between human papillomavirus (HPV) and cervical neoplasia was first proposed.8 Since then, a large body of experimental, clinical, and epidemiologic research has accumulated supporting an etiologic role for some types of HPV.9
Of the more than 78 types of HPV that have been described, in excess of 35 types are associated with anogenital disease, and 30 or more are associated with cancer.10 Similarly, HPV DNA has been detected by polymerase chain reaction (PCR) in up to 94% of women with preinvasive lesions [cervical intraepithelial neoplasia (CIN)] and in up to 46% of women with cytologically normal tissue.9, 11
HPV types classified as intermediate and high risk have been identified in about 77% of high-grade squamous intraepithelial lesion s (HGSILs) (CIN 2 and 3) and in 84% of invasive lesions.12 In the series studied by Bosch and colleagues, HPV types 16, 18, 31, and 45 were detected in approximately 80% of the cases.13 HPV 16 is by far the most prevalent HPV type in women with cervical neoplasia, present in up to 50% of HGSILs and invasive lesions, and it is the most common HPV type identified in cytologically normal women.12, 14, 15
The association between cervical neoplasia and HPV is independent of study population, study design, and HPV detection method.13 Higher risk has been associated with specific HPV types (16, 18, 31, 33, 35, and 45), increasing viral load, and concurrent infection with multiple HPV types.16, 17 An increased risk of high-grade CIN ranging from 16- to 122-fold has been reported among women whose test results were positive for HPV of any type.17 The percentage of cases of CIN attributed to HPV has been estimated to range from 60% to 92%.11 In addition, adjustment for HPV status appears to account for most of the associations between cervical neoplasia and number of sexual partners and other characteristics of sexual behavior.11, 16, 17
Although a strong and consistent association between HPV and cervical neoplasia has been clearly established, the discrepancy between HPV prevalence and the incidence of cervical neoplasia suggests that other cofactors are necessary for the development and progression of the disease.
Numerous studies have addressed the association between HIV and cervical neoplasia.7 The Centers for Disease Control and Prevention added invasive cervical cancer to the list of conditions related to AIDS in 1993.15 HIV-positive women have been reported to have higher rates of cervical abnormalities, larger lesions, higher grade histology, and higher recurrence rates than HIV-negative women. In addition, HIV-positive women have been reported to have higher HPV prevalence and HPV persistence rates than HIV-negative women. A meta-analysis by Mandelblatt and colleagues concluded that HIV is a cofactor in the association between HPV and cervical neoplasia, and this association seems to vary with the level of immune function.18
Other molecular markers
Other specific genetic abnormalities may also play an important role in carcinogenesis and the aggressiveness of cervical tumors, although, to date, the role of most of these genetic abnormalities in cervical cancer does not appear as important as the role of HPV. Most studies report a 32–34% incidence of c-myc activation in cervical cancers, predominantly through amplification.19, 20 Amplification has been related to tumor size and nodal status as well as a risk factor for relapse.21 Mutations have been reported in the K-ras and H-ras genes in cervical cancer at a rate of only 10–15%.22 One report found that increased ras p21 expression correlated with risk of lymph node metastasis.23
Epidermal growth factor receptor (EGFR) is expressed not only in a large proportion of cervical carcinomas but also in normal and premalignant epithelia. The prognostic role of EGFR in cervical carcinoma remains controversial, although two studies found EGFR prognostic for overall survival (OS) and disease-specific survival (DSS) in patients with invasive cervical cancer.24, 25
The apoptosis inhibitor Bcl2 prevents apoptosis. Two studies have shown that Bcl2 is overexpressed in 61–63% of all cervical cancer and correlates inversely with OS,26, 27 whereas other studies have found no correlation with survival.28
Angiogenesis is critical for the progression of most cancers. One angiogenic factor, VEGF, has recently been associated with cervical cancer,29, 30 but the precise role that angiogenic factors play in the development and progression of cervical cancer requires further elucidation.
Sexual behavior
Although previous studies report a strong and consistent association between cervical neoplasia and some characteristics of sexual behavior of women and their male sexual partners, a weaker association has been found in more recent studies in which HPV infection has been taken into account.16, 31 This, characteristics of sexual behavior may be only a proxy measurement for infection with HPV and other infectious agents that may be causally related to cervical neoplasia.
The association between early age at first sexual intercourse and increased risk has been less consistent. After controlling for HPV and other risk factors, a statistically significant association between age at first sexual intercourse and cervical neoplasia has remained in some studies, but in others, no association has been observed.16, 17, 32 The association between cervical neoplasia and early age at sexual intercourse may indicate a period of higher susceptibility of the cervical tissue, a higher likelihood of exposure, or a longer period of exposure to carcinogenic factors. Establishing age at first sexual intercourse as an independent effect is, however, difficult because of its high correlation with number of sexual partners.
An association between factors related to male sexual partners and an increased risk of cervical neoplasia has also been suggested.33 Among men, the prevalence of HPV has been associated with the number of sexual partners and sexual contact with prostitutes.34 In addition, higher HPV prevalence has been reported among males in geographic areas with higher rates of cervical cancer incidence than among males in geographic areas with lower rates, which supports a possible contribution of male partners to cervical carcinogenesis in their female sex partners.34 Male circumcision has been associated with a reduced risk of penile HPV infection and, in the case of men with a history of multiple sexual partners, a reduced risk of cervical cancer in their current female partners.35
Reproductive factors
No consistent relationships have been established between cervical neoplasia and menstrual or reproductive characteristics, including age at menarche or menopause, parity, number of spontaneous or induced abortions, age at first pregnancy, first live birth, or last birth, and number of vaginal deliveries or Cesarean sections. There is an association among increased risk of cervical neoplasia and higher parity, early age at first birth, higher number of live births, and vaginal deliveries.16, 17, 35, 36 Repeated trauma to the cervix during childbirth could be an etiologic factor.35
Smoking habits
Several epidemiologic studies have provided evidence supporting an approximately twofold increased risk among smokers and a dose–response relationship with duration and intensity of smoking.37, 38 Some support an independent effect of smoking, whereas others do not.16, 17, 39 High levels of nicotine, cotinine and tobacco-specific N-nitrosamines have been detected in the cervical mucus of active and passive smokers. DNA damage has been found in cervical tissue and exfoliated cells of women smokers. The local cell-mediated immune response is impaired in smokers. Furthermore, reduction of cervical lesion size has been documented among women participating in smoking cessation intervention.40 Although the mechanism of smoking-induced carcinogenesis in cervical tissue is not fully understood, current biologic, epidemiologic, and clinical studies suggest that cigarette smoking may be a risk factor for cervical neoplasia.
Risk factors for cervical adenocarcinoma
Adenocarcinoma of the cervix accounts for more than 20% of all cervical cancers. However, in most developing countries, the incidence is increasing, particularly among younger women. Between the early 1970s and mid-1980s, the incidence of adenocarcinoma more than doubled among women under 35 years of age.41 Adenocarcinoma is associated with a higher likelihood of HPV-16 and HPV-18, which is present in more than 80% of cases. HPV 18 accounts for approximately 50% of adenocarcinoma of the cervix but only 15% of squamous cell carcinoma.42 Adenocarcinoma has been linked to several other risk factors more commonly associated with endometrial cancer, including obesity43 and nulliparity.6
Summary
Cervical neoplasia continues to be a major health problem worldwide. Higher incidence and mortality rates are observed in developing countries. Among more developed countries, a significant decline in incidence and mortality has been observed in the past 50 years, which has been attributed to the introduction of screening programs. Current epidemiologic data support a strong role for HPV infection in the etiology of cervical neoplasia. This association satisfies all criteria for causality in epidemiologic research: strength, consistency, and specificity of the association; dose–response and temporal relationship; and biologic plausibility.11 HPV infection appears to explain many of the established risk factors for cervical neoplasia, including sexual behavior and cigarette smoking. Nonetheless, the high prevalence of HPV infection in young healthy women compared with the low incidence of cervical neoplasia and the low progression rate of untreated CIN lesions support the existence of other cofactors in cervical carcinogenesis.44 Future epidemiologic studies will need to further assess the role of these cofactors and their interaction with HPV. In addition, the role of viral factors such as HPV persistence and HPV variants in the progression of cervical neoplasia as well as of the determinant factors of HPV persistence will require further evaluation.45 Similarly, the impact of recent trends in environmental factors such as smoking, exogenous hormones, and dietary factors deserves further attention.7
Histologic classification of epithelial tumors
The histologic classification of epithelial tumors of the uterine cervix by the World Health Organization (WHO) separates them into three main groups: squamous cell carcinomas, adenocarcinomas, and other epithelial tumors (Table 1).46, 47
Table 1 Modification of the WHO histologic classification of epithelial tumors of the uterine cervix
| Squamous cell carcinoma |
| Microinvasive squamous cell carcinoma |
| Invasive squamous cell carcinoma |
| Verrucous carcinoma |
| Warty (condylomatous) carcinoma |
| Papillary squamous cell (transitional) carcinoma |
| Lymphoepithelioma-like carcinoma |
| Adenocarcinoma |
| Mucinous adenocarcinoma |
| Endocervical type |
| Intestinal type |
| Signet-ring type |
| Endometrioid adenocarcinoma |
| Endometrioid adenocarcinoma with squamous metaplasia |
| Clear cell adenocarcinoma |
| Minimal-deviation adenocarcinoma |
| Endocervical type (adenoma malignum) |
| Endometrioid type |
| Serous adenocarcinoma |
| Mesonephric carcinoma |
| Well-differentiated villoglandular adenocarcinoma |
| Other epithelial tumors |
| Adenosquamous carcinoma |
| Glassy cell carcinoma |
| Mucoepidermoid carcinoma |
| Adenoid cystic carcinoma |
| Adenoid basal carcinoma |
| Carcinoid-like tumor |
| Small cell carcinoma |
| Undifferentiated carcinoma |
Source: Reproduced with permission from Carcinoma and other tumors of the cervix. In: Blaustein’s Pathology of the Female Genital Tract, 4th ed.
Squamous cell carcinoma
The majority of cervical carcinomas are squamous cell carcinomas, which are classified as either large cell nonkeratinizing or large cell keratinizing. Nonkeratinizing carcinoma is characterized by squamous cells with somewhat hyperchromatic nuclei and a moderate amount of cytoplasm growing in discrete nests separated by stroma (Figure 1). In the center of some of the nests, the squamous cells appear to differentiate and degenerate. Keratinizing carcinoma is characterized by cells with very hyperchromatic nuclei and densely eosinophilic cytoplasm growing in irregular invasive nests. Many of these nests have central “pearls” that contain abundant keratin. The average age of patients with squamous cell carcinoma is 51.4 years. Selected variants of squamous cell carcinoma are described in the following paragraphs.
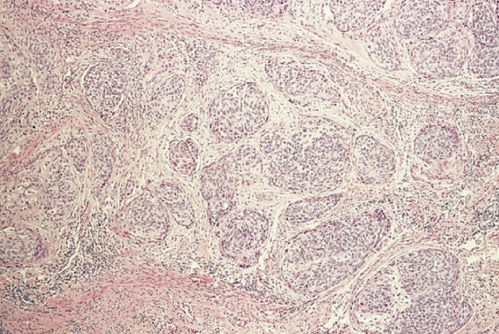
Figure 1 Squamous cell carcinoma, nonkeratinizing.
Verrucous carcinoma
Verrucous carcinomas are exophytic with frond like papillae and macroscopically resemble condylomas. They rarely metastasize, but local invasion can be extensive. Death usually occurs because of ureteral obstruction, infection, or hemorrhage.
This tumor rarely goes to the nodes, therefore, for early-stage disease, the treatment of choice is a type II modified radical hysterectomy without lymphadenectomy.
Papillary squamous cell carcinoma
Papillary squamous cell carcinomas of the uterine cervix with transitional or squamous differentiation often resemble transitional cell carcinomas of the urinary tract (Figure 2). Urinary tract transitional cell carcinomas have a cytokeratin profile strongly positive for cytokeratin 20, whereas primary genital tract transitional cell carcinomas stain positive for cytokeratin 7.48 Invasive papillary transitional cell carcinomas of the uterine cervix are potentially aggressive carcinomas. It is important to distinguish these carcinomas from benign squamous papillomas and condyloma acuminata.49 Biopsy material must include the underlying stroma to permit identification of invasion.
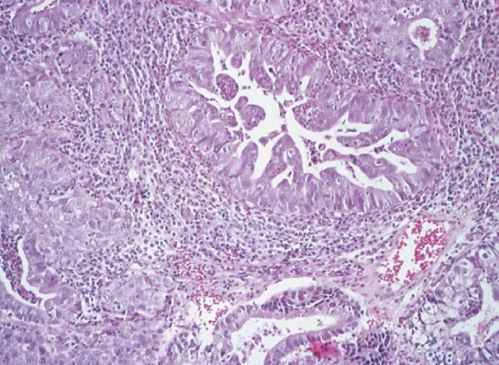
Figure 2 Papillary squamous cell (transitional) carcinoma.
Lymphoepithelioma-like carcinoma
Lymphoepithelioma-like carcinomas are histologically similar to lymphoepitheliomas arising in the nasopharynx and salivary glands (Figure 3). These carcinomas are usually well circumscribed and composed of undifferentiated cells. The cancer cells are surrounded by inflammatory infiltrates composed of lymphocytes, plasma cells, and eosinophils.50 Hasumi and colleagues reported 39 cases from the Cancer Institute Hospital in Tokyo. Their patients, 72% of whom were younger than 50 years of age, were treated with radical hysterectomy and pelvic lymphadenectomy. Two patients had positive lymph nodes. At the time of the report, 38 of the 39 patients were alive. The single death occurred 5 months after surgery and was due to serum hepatitis.
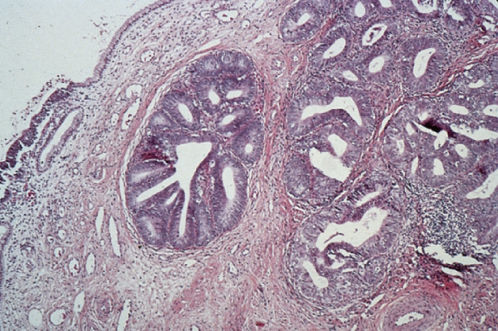
Figure 3 Lymphoepithelioma-like carcinoma.
Adenocarcinoma
Adenocarcinomas represent 20–25% of cervical carcinomas today, whereas from 1950 to 1960, they represented only 5%.51 This change in prevalence is a worldwide phenomenon.52 The mean age at diagnosis for patients with invasive adenocarcinoma is between 47 and 53 years. Selected variants of adenocarcinoma are described in the following paragraphs.
Mucinous adenocarcinoma is the most common type of cervical adenocarcinoma.53 In the WHO classification, the first type of mucinous adenocarcinoma is composed of cells that resemble the columnar cells of the normal endocervical mucosa and is referred to as the endocervical type (Figure 4). The second type is termed the intestinal type because it is composed of cells similar to those present in adenocarcinomas of the large intestine. A third type is composed of signet-ring cells and designated the signet-ring type. Frequently, mucinous adenocarcinomas are a mixture of these cell types.
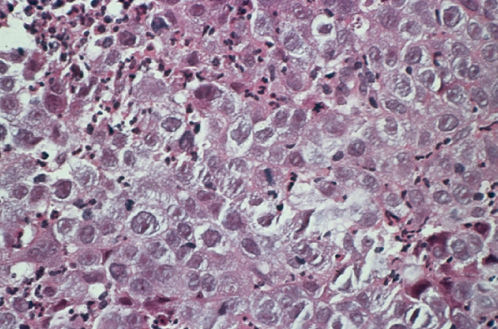
Figure 4 Mucinous adenocarcinoma, endocervical type.
Endometrioid adenocarcinoma is the second most common type of primary endocervical tumor, accounting for 30% of all primary endocervical tumors. Endometrioid adenocarcinomas resemble typical endometrioid adenocarcinoma arising from the endometrial cavity (Figure 5). Identification of the site of origin (i.e., whether the primary tumor is in the endocervix or endometrium) may be difficult, but proper identification is important as the site or origin significantly influences therapy.
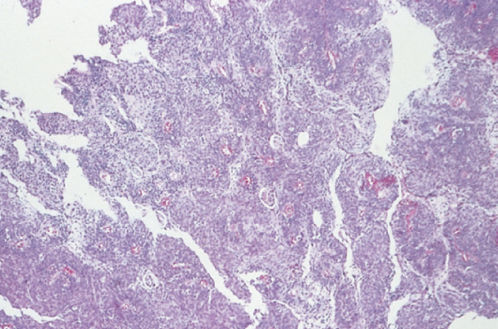
Figure 5 Endometrioid adenocarcinoma.
Adenoma malignum is difficult to distinguish cytologically from normal endocervical glands (Figure 6) and is referred to as minimal-deviation adenocarcinoma. A distinguishing feature of adenoma malignum is a bizarre and irregular glandular branching pattern. These irregular glands invade deeply into the stroma, and diagnosis requires a large tissue specimen from a cone biopsy or hysterectomy specimen. Adenoma malignum is extremely rare and is sometimes associated with Peutz–Jegher syndrome.54 The survival rate is poor if the well-differentiated pattern leads to under treatment.
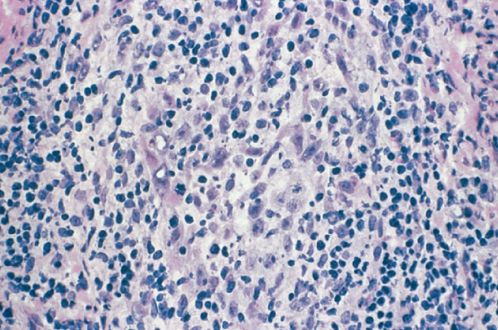
Figure 6 Mucinous adenocarcinoma, endocervical type (adenoma malignum).
Other epithelial tumors
Adenosquamous carcinoma is defined as a cancer that contains an admixture of histologically malignant squamous and glandular cells.55 Adenosquamous carcinomas account for 5–25% of the cervical carcinomas in some series.56, 57 These carcinomas are similar in their clinical presentation, epidemiology, and pattern of spread to squamous cell carcinomas and adenocarcinomas. The poorly differentiated form of adenosquamous carcinoma can be made up of large uniform polygonal cells with a finely granular cytoplasm of the ground-glass type, hence the term “glassy cells” (Figure 7). Similar to other undifferentiated tumors, glassy cell carcinomas spread early and are aggressive.57 The mucoepidermoid carcinoma, also placed in this category, contain large cell nonkeratinizing or focally keratinizing squamous carcinoma, which stains positive for mucin but lacks recognizable glands. The mucinous component includes goblet or signet-ring-type cells localized in a nest of squamous cells. These carcinomas represent 20% of the carcinomas in some series if mucin is measured.
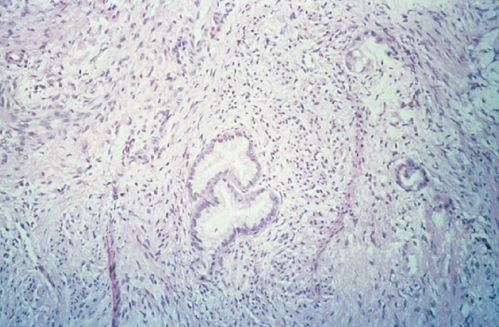
Figure 7 Glassy cell carcinoma.
Small cell neuroendocrine carcinoma contains small anaplastic cells with scant cytoplasm (Figure 8). These highly aggressive cancers diffusely infiltrate the cervical stroma.58 Staining reveals neuroendocrine markers in most cases. Women with small cell carcinoma are likely to be 10 years younger than those with squamous cell carcinoma. Small-cell carcinomas are frequently associated with widespread metastasis to multiple sites, including bone, liver, skin, and brain. These tumors should not be confused with small squamous cell carcinomas, which are associated with a better prognosis. Efforts to treat these cancers with approaches typically used for small cell carcinomas of the lung have had mixed results.
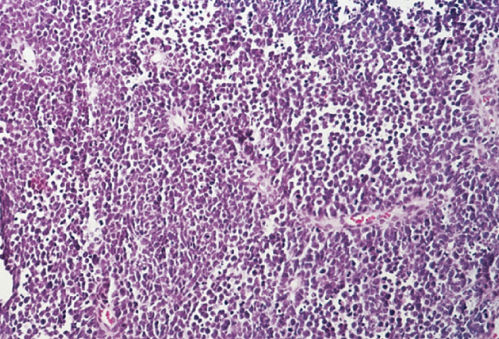
Figure 8 Small cell carcinoma.
Non-small cell neuroendocrine carcinoma
Nonsmall cell neuroendocrine carcinomas of the cervix have been reported, but they are not listed in the current WHO classification of cervical tumors.59 The tumors contain intermediate to large cells, high-grade nuclei, and eosinophilic cytoplasmic granules of the type seen in neuroendocrine cells. A trabecular pattern is frequently evident, with or without glandular differentiation (Figure 9). Tumors are usually immunoreactive for chromogranin. Reported survival rates for patients with these aggressive carcinomas are similar to those for patients with small cell carcinoma.
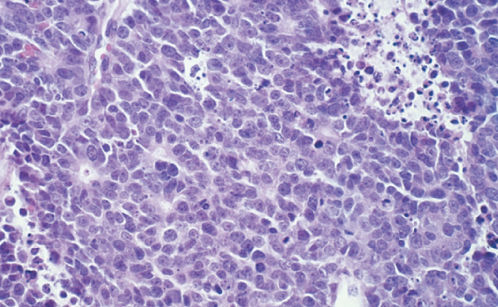
Figure 9 Non-small-cell neuroendocrine carcinoma.
Diagnosis and treatment of precancerous lesions
The most recent guidelines for screening for cervical cancer are listed in Figure 10.60 CIN is an increasingly common finding among sexually active young women. Since the introduction of the Bethesda system, the proportion of Pap smears identified as having low-grade cytologic abnormalities, minimal or ambiguous cytologic changes classified as atypical squamous cells of undetermined significance (ASCUS), or low-grade squamous intraepithelial lesions (LGSIL)s has increased.44 Approximately 50 million Pap smears are done yearly in the United States, and 5–10% of these smears are reported as having low-grade cytologic abnormalities. Although near consensus exists regarding the evaluation and management of HGSILs and carcinoma detected on Pap smears, controversy continues regarding appropriate management of ASCUS and low-grade abnormalities.61 Issues include the risk of progression of the disease, the anxiety caused to the patient, the risk of overtreating patients with minor disease, and, more recently, the financial implications of prompt intervention and treatment.62

Figure 10 Screening guidelines for the early detection of cervical cancer.
As more studies of women with mildly atypical Pap smears were published, data showed that 5–20% of women presenting with a single mildly atypical Pap smear were at risk of HGSILs or other more severe lesions.63 In addition, it has recently been estimated that more than one-third of the HGSIL cases in a routine screening population are proceeded by a cytologic diagnosis of ASCUS.64 This has led some clinicians to suggest that it is safer and more expeditious to perform colposcopy in women with a finding of ASCUS.65 However, given the relatively high frequency of low-grade cytologic abnormalities in the absence of significant disease and the high financial and emotional cost of colposcopy, some have argued that these women should be evaluated by repeat cytology rather than colposcopy. The American College of Obstetricians and Gynecologists and a NCI consensus panel have acknowledged that managing a single mildly atypical Pap smear by repeating the test is an acceptable practice.61, 66
The main goal in managing ASCUS and low-grade cytologic abnormalities is to identify those women at higher risk of HGSILs, primarily women more than 25 years old who cannot be relied on to return for long-term follow-up, who are suspected of having or known to have a history of abnormal cytologic findings or treatment of cervical neoplasia, who may be promiscuous, and who have no history of adequate screening.67 Currently, two strategies are recommended for managing these lesions: the physician may repeat the Pap smear and perform a colposcopic evaluation, or, for patients with an ASCUS cytologic diagnosis or LGSILs without clinical evidence of cervical disease and without risk factors, the physician may repeat the Pap smear without performing colposcopy (Figure 11).61, 67 The American Society for Colposcopy and Cervical Pathlogy (ASCCP) also emphasizes HPV testing when triaging with ASCUS or LGSIL PAPs (Papanicolaous).
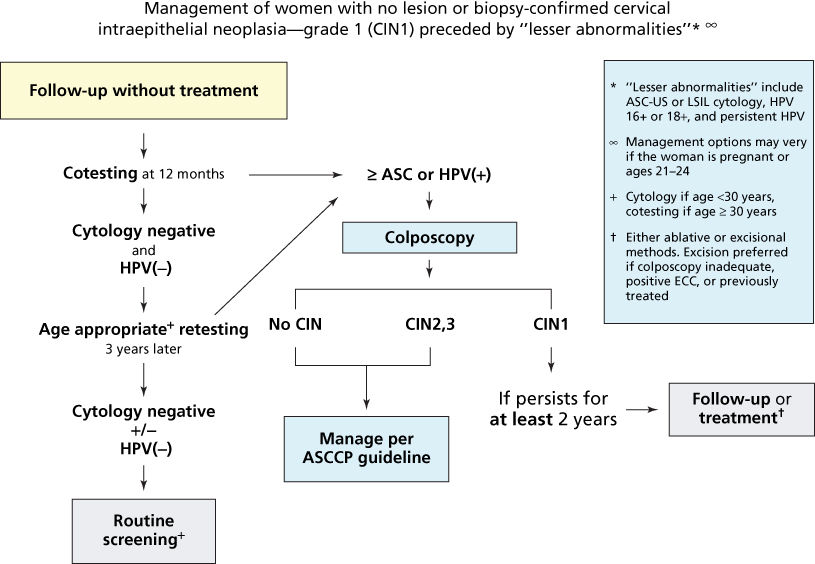
Figure 11 Management of women with biopsy-confirmed cervical intraepithelial neoplasia-grade 1 (CIN 1).
Source: Massad et al. 2013.67 Reproduced with permission of the American Society for Colposcopy and Cervical Pathology.
Management of low-grade cytologic abnormalities
Low-grade cytologic abnormalities are usually treated first with antibiotics and repeating the smear several months later. If the smear result regressed to normal, the patient is scheduled for an annual Pap smear screening, whereas patients whose smears remained abnormal are referred for colposcopic evaluation, and Pap test every 4–6 months for 2 years. After three consecutive negative smears in the 2-year follow-up period, patients can be monitored using a routine cervical cancer screening protocol.68, 69 For patients with clinical suspicion of cancer or persistent abnormal Pap smears during the 2-year follow-up, colposcopic evaluation is recommended. Recommended management form ASCCP for women with ASCUS or LGSIL Paps is based on age. For women aged 21–24 years with ASCUS or LGSIL, the recommendation is to repeat cytology at 12 months. For older women with LGSIL Pap and no HPV testing or positive HPV testing, recommendation is to proceed with colposcopy (Figure 12).67
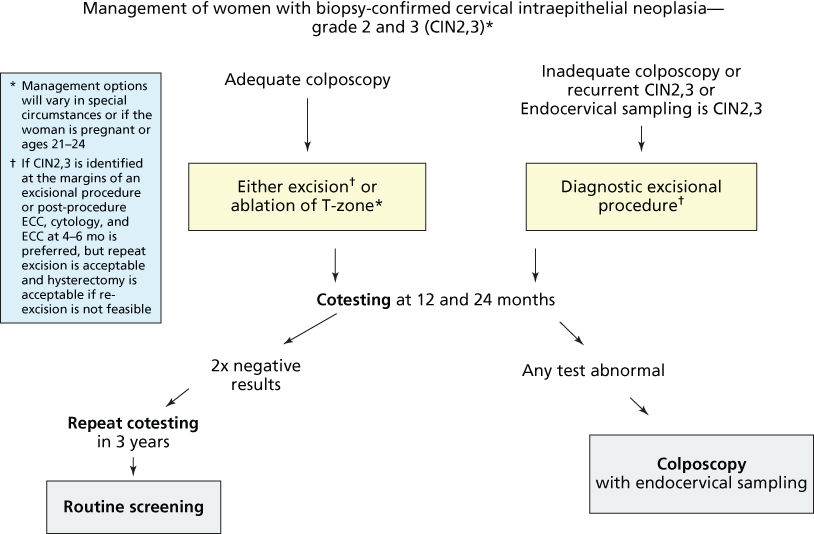
Figure 12 Management of women with biopsy-confirmed cervical intraepithelial neoplasia grades 2 and 3 (CIN 2.3).
Source: Massad et al. 2013.67 Reproduced with permission of the American Society for Colposcopy and Cervical Pathology.
In view of the costs, both emotional and economic, associated with evaluating women with low-grade cytologic abnormalities, considerable interest has arisen in developing novel cost-effective triage strategies for these patients. HPV DNA testing, automated cytology screening, and cervicography are being evaluated as adjunct methods in the assessment and triage of patients with low-grade cytologic abnormalities. Most research has focused on the assessment of HPV testing.
Several researchers have assessed the value of HPV DNA testing as a specific and economical alternative for triage of low-grade cytologic abnormalities and as an adjunct to cervical cytology in primary screening.70–73 With second-generation HPV DNA detection methods, including PCR-based methods and the hybrid capture HPV DNA assay, both of which have a higher sensitivity and detect a broader range of HPV types with high oncogenic risk than earlier methods, results have been more consistent, supporting the role of HPV typing in the triage of patients with low-grade cytologic abnormalities and as an adjunct to cytology in primary screening.70–73
New data on HPV primary screening from the ATHENA trial were presented to the FDA in the spring of 2014 supporting the evidence that HPV testing alone is an excellent alternative screening strategy.74 More experience and data analysis pertaining to this new strategy will permit a more formal ACS evaluation in the future.
The preferences of clinician and patient and cost considerations should dictate the choice of treatment of CIN, but proper management of LGSILs is expected to remain a fiercely debated question. Although management guidelines have increasingly included nontreatment of LGSILs as an option for patients who may be reliable for long-term follow-up, concerns over legal responsibility for progressive lesions continue to drive intensive follow-up protocols, particularly in the United States, that may not be cost-efficient.75 Guidelines recently published may be helpful to follow.
Management of high-grade cytologic abnormalities
Optimal management of HGSILs includes colposcopic evaluation and biopsy or in women older than 24 years of age to proceed to a loop electrical excision procedure (LEEP) without performing colposcopic evaluation. The consensus is that HGSILs should be treated once diagnosed.76 For biopsy-proven HGSILs with negative findings on endocervical curettage (ECC), a satisfactory colposcopy examination, and congruent Pap smear and biopsy results, ablation of the transformation zone has been the standard of care for several decades. Three outpatient therapies are used in the United States for treating these lesions: cryotherapy, laser ablation, and LEEP. For patients with unsatisfactory colposcopic examination findings, a Pap smear result more severe than the biopsy findings, the presence of an adenomatous component, suspicion of invasive cancer, or positive findings on ECC, a cone biopsy is indicated. Cone biopsies (talked about later in the chapter) remove tissue to a depth of 20–30 mm and up to 30 mm in diameter, including the transformation zone.
The three outpatient therapies of cryotherapy, laser vaporization, and LEEP have been the focus of controversy. Safety, efficacy, and cost issues have dominated the debate. Cryotherapy, introduced in 1972, was the first outpatient treatment of CIN and remains a dependable treatment because of its reliability, low complication rate, ease of use, and low cost.77 Another advantage of cryotherapy is that leaving a large dead viral HPV load within disrupted cells may improve the immune response to the causative agent of CIN. Major disadvantages include lack of ability to tailor treatment to the size of the lesion, lack of a tissue specimen, and the risk of treatment of undetected invasive lesions. Eligible for cryotherapy are patients with satisfactory findings on colposcopic examination, negative findings on ECC, and small lesions (2.5–3.0 cm in diameter) that allow the entire lesion and transformation zone to be covered by the cryotherapy probe.
Laser vaporization was introduced in 1977. It has the advantage of being easily tailored to lesion size, but the cost of the equipment and the lack of a tissue specimen are major disadvantages.77 In addition, laser vaporization requires more training and skills than the other two procedures and is associated with more serious safety issues (eye injuries and inadvertent burns). Candidates for this procedure are patients with large CIN lesions, young women with suspicious or invasive lesions or adenocarcinoma in situ in whom preservation of fertility is desired, and patients unwilling to undergo LEEP under local anesthesia.
LEEP was introduced in 1989, and it is currently the technique of choice for the treatment of HGSILs. LEEP is reliable and easy to use. It can be tailored to lesion size and provides a tissue specimen.77 The advantage of this last characteristic is underscored by the finding of unsuspected adenocarcinoma in situ and microinvasive squamous cell carcinoma in 2–4% of LEEP specimens.67, 77 LEEP, however, has the potential to result in unintentional removal of excessive cervical stroma and removal of disease-free tissue (more frequent when LGSILs are treated). Other disadvantages include its high cost and the increased risk of bleeding and infection. Bleeding after LEEP has been reported in 2–7% of cases.77 The high rates of overtreatment observed with LEEP have been related to misdiagnosis of abnormality and multiple punch biopsies of small lesions before treatment.67 The use of LEEP in see-and-treat protocols has been shown to improve patient compliance with treatment when patient selection is adequate. This strategy has been suggested as having the greatest potential benefit for populations with poor treatment compliance.67
No statistically significant differences in success rates (based on recurrence and persistence) between cryotherapy and laser vaporization have been reported, but variability in these rates from study to study is striking.77, 78
Prevention
Chemopreventative management
One of the more exciting research areas in therapy for CIN is the use of chemopreventative agents. These therapies involve ingestion of an agent that reverses precancerous changes, returning the tissue to normal. Laboratory data have shown that retinoids can induce apoptosis in dysplastic cervical cells, suggesting that these compounds may be active in cell-cycle control.79 Women with HGSIL and colposcopically evident lesions are the target population for chemoprevention studies because they have a higher risk of persistent disease or progression to cancer. α-Difluoromethylornithine and retinoids (vitamin A derivatives) are two drugs currently receiving attention in the United States and China.80, 81
Vaccine development
Papillomaviruses are epitheliotropic agents that induce benign papillomas of the skin and mucous membranes. In contrast to hepatitis B virus (HBV), there are more than 100 HPV genotypes (types). A subset of HPV types that are almost always transmitted sexually is the main cause of human cervical cancer. Infection with these HPV types is a strong risk factor for cervical cancer, and HPV DNA from one or more of these types is found in virtually all cervical tumors.82, 83 The virus encodes oncoproteins that appear to be required for both the induction and the maintenance of the cancer.
Because papillomaviruses contain oncogenes, and a prophylactic vaccine would be directed toward healthy young individuals, efforts to develop a prophylactic vaccine have emphasized a subunit approach, analogous to that used for HBV vaccine. Indeed, constitutive high-level expression of the L1 major structural viral protein, even in nonmammalian cells, leads to its efficient self-assembly into virus like particles (VLPs) that resemble authentic viral capsids structurally and antigenically. Preparative amounts of VLPs can be synthesized in insect cells or yeast. Such VLPs are suitable immunogens that, as is true of authentic virions, possess the immunodominant conformational epitopes capable of raising high titers of neutralizing antibodies.
Two vaccines have been developed: Gardasil, a quadrivalent vaccine targeting HPV 6, 11, 16, and 18,84 and Cervarix, a bivalent vaccine that targets HPV 16 and 18.85 These vaccines are now licensed in more than 55 countries worldwide and are expected to be an enormous asset to cervical cancer prevention efforts. The FUTURE (Female United to Unilaterally Reduce Endo/Ectocervical Disease) II study group performed a randomized, double-blind, placebo-controlled trial in order to evaluate a quadrivalent vaccine (Gardasil) against HPV types 6, 11, 16, and 18 for the prevention of high-grade cervical lesions associated with HPV 16 and 18. After 3-year follow-up from the first dose of the vaccine, the efficacy of the vaccine in preventing the primary endpoint was 98% in the per-protocol susceptible population and 44% in an intention-to-treat population of all women who had undergone randomization. The study concluded that the quadrivalent vaccine was highly effective in preventing HPV 16- and 18-related high-risk cervical lesions, and that widespread immunization of girls may result in a substantial decrease in cervical cancer resulting from infection with HPV 16 and 18.84
The interim analysis of the randomized double-blind-controlled trial of the Papilloma Trial against Cancer In young Adults (PATRICIA) looking at the efficacy of prophylactic administration of bivalent vaccine against infection with HPV 16 and 18 was published in June 2007.85 This vaccine has also shown evidence of cross-protection against HPV 45 and 31. The vaccine had an efficacy of 90.4%, and there were no significant safety profile differences between the study groups.85
HPV vaccines are presently recommended for all 11–12-year-old girls, though vaccination can be started as early as 9 years, and women between the age of 13 and 26 should also be vaccinated if they are not yet sexually active. Recent data from the United States suggest that HPV vaccination and biennial cervical screening from the age of 24 years will reduce the annual total PAP test volume by 43% and result in the reduction in the workload at sexually transmitted disease clinics.86 The Markov model of incorporation of the HPV vaccination into a UK national cervical screening program predicts a 76% reduction in cervical cancer deaths and 66% reduction in high-grade lesions.86 However, there are problems in world widespread distribution of the vaccinations including price of the vaccine, accessibility of the vaccines in countries due to lack of immunization infrastructure, and opposition by conservative groups to the vaccination of young girls against what is perceived to be an sexually transmitted disease.87
Diagnosis and treatment of invasive lesions patterns of spread
During the transition from in situ to invasive carcinoma, tumor cells penetrate the epithelial basement membrane and enter the underlying cervical stroma. Once the cervical stroma is invaded, the lymphatics and blood vessels are accessible, and dissemination beyond the cervix is possible.
The cervical, vaginal, and uterine lymphatic channels coalesce to form major drainage pathways. The major lymphatic trunks are the utero-ovarian (infundibulopelvic), parametrial, and presacral, which drain into the paracervical, obturator, hypogastric, external iliac, common iliac, inferior gluteal, presacral, and lower aortic lymph nodes. A series studying the incidence and distribution pattern of retroperitoneal lymph node metastases in 208 patients with stages 1B, IIA, and IIB cervical carcinomas who underwent radical hysterectomy and systemic pelvic node dissection reported that 53 patients (25%) had node metastasis.88 The obturator lymph nodes were the most frequently involved, with a rate of 19% (39 of 208), and the authors proposed them as sentinel nodes for cervical cancers. In fact, finding negative obturator nodes may be an indication that the pelvic lymph node dissection can be limited.
Cervical cancers of similar size may have very different metastatic potentials, depending on their intrinsic aggressiveness and histologic cell type. Cervical carcinomas also invade directly. As the cancer grows, disease may extend to the lateral pelvic walls, into the bladder or rectum, or into the vagina.
The incidence of lymph node metastasis at diagnosis for each of the squamous cell carcinoma stages designated by the International Federation of Gynecology and Obstetrics (FIGO) has been well defined by surgical series.89–91 Pelvic node involvement occurs in 10–25% of stage I carcinomas, 25–30% of stage II carcinomas, and 30–45% of stage III and IV carcinomas. Stage I carcinomas are more likely to metastasize to nodes once they reach 3 cm.92, 93 Because most patients with large bulky adenocarcinomas are treated with radiation therapy, the incidence of positive nodes by tumor size is not as well defined for adenocarcinoma as it is for squamous cell carcinoma. The incidence of positive nodes for poorly differentiated squamous cell carcinoma and for poorly differentiated adenocarcinoma is higher than that for the better-differentiated carcinomas.
Carcinoma of the cervix spreads in an orderly manner. Nodes adjacent to the cervix are usually the first to be involved, and “skip” metastases are uncommon. Patients with positive para-aortic nodes usually have positive pelvic nodes. The incidence of positive para-aortic nodes in 978 patients with stage IB and IIA carcinoma whose aortic nodes were sampled before radical hysterectomy was 4.7% and 8.4%, respectively. The incidence of positive nodes in patients with adenocarcinomas is probably equal to that in patients with squamous cell-carcinomas when cancer size, histologic differentiation, and extent of tumor or FIGO stage are similar. Many large series report poorer survival rates for patients with adenocarcinomas than for patients with squamous cell carcinomas, especially those who have bulky lesions.94, 95 Small cell carcinoma and some of the carcinomas classified as other epithelial tumors are particularly aggressive. Carcinomas of the cervix, regardless of histology and size of the primary tumor, may contain highly malignant clones of cells that can prove unpredictable and spread extensively.
Clinical symptoms
The clinical symptoms of carcinoma of the cervix are vaginal bleeding, discharge, and pain. The growth pattern of the carcinoma plays a role in the development of symptoms. Exophytic carcinomas bleed earlier in a sexually active patient (because of contact) than lesions that expand the cervix. Lesions that expand the endocervix in a barrel-shaped configuration may leave the squamous epithelium of the exocervix intact until the lesions exceed 5 or 6 cm in transverse diameter; therefore, carcinomas with this growth pattern may be silent and grow large before the patient bleeds. Cytologic findings may be negative unless the endocervix is sampled with a brush device. Ulcerative lesions that destroy the exocervix bleed early, and necrosis and infection induced by the cancer’s outgrowing its blood supply result in a foul-smelling vaginal discharge.
Severe pelvic pain experienced during the pelvic examination may indicate salpingitis. Tubal infections require management before radiation therapy. Patients with an adnexal mass need surgical treatment before radiation therapy is started.
Paracervical extension of a carcinoma may remain silent until fixation to the pelvic wall occurs. Fixation with or without nodal involvement may obstruct a ureter. Ureteral encroachment is usually a silent process. Patients may present with bilateral ureteral obstruction with impending renal failure and report no history of urinary system complaints. Direct invasion of branches of the sciatic nerve roots causes back pain, and encroachment on the pelvic wall veins and lymphatics causes edema of a lower extremity. The triad of back pain, leg edema, and a nonfunctioning kidney is evidence of an advanced carcinoma with extensive pelvic wall involvement.
The anatomic position of the bladder, so closely adjacent to the cervix, favors contiguous spread from the cervix to the bladder. Urinary frequency and urgency are early manifestations of such spread; patients with advanced disease may present with hematuria or incontinence, suggesting direct extension of tumor to the bladder. Cystoscopy and biopsy should confirm the cause of hematuria or incontinence.
In contrast, posterior extension to the rectum and disruption of the rectal mucosa is an unusual pattern of disease spread in untreated patients. The deep cul-de-sac provides anatomic separation of the rectum and cervix. In patients who present with rectal mucosal involvement, there is usually extensive involvement of the posterior vaginal wall with direct extension to the rectum. For staging and treatment planning, cystoscopy and sigmoidoscopy are essential.
Metastatic carcinoma in para-aortic nodes may extend through the node capsule and directly invade the vertebrae and adjacent nerve roots. Back pain owing to involvement of the lumbar vertebrae and psoas muscles may be a manifestation of massive nodal disease; however, hematogenous spread to the lumbar vertebrae and involvement of the psoas muscle without significant nodal disease may occur.
Diagnosis
The diagnosis of cervical carcinoma is made by pathologic examination of a tissue specimen. A biopsy sample taken from the periphery of a tumor is more likely to contain morphologically intact neoplastic cells that are best able to represent the tumor pathologically. A biopsy specimen taken from the center of a tumor mass may include necrotic tumor debris; the result of hypoxia induced by the tumor’s outgrowing its blood supply. Therefore, to rely on these dead and distorted cells is to compromise the accuracy of the histologic interpretation.
The endocervix should be curetted if no lesion is visible or if the cervix is enlarged, nodular, or hard. Older patients with adenocarcinoma require an endometrial biopsy. It may be difficult to distinguish an endocervical primary tumor from an endometrial primary tumor involving the lower uterine segment.
Patients with an abnormal Pap smear and no visible lesion require colposcopy and biopsy. The tissue specimen may be a simple colposcopy-directed biopsy specimen, an endocervical specimen obtained with a curette, or a conization specimen.
It is the current recommendation of FIGO to classify as stage IA any invasive cancer that can be identified only with a microscope. All gross lesions, even with superficial invasion, are at least stage IB cancers. Vascular space involvement, either venous or lymphatic, should not alter staging. Many clinicians prefer to use the term microinvasion and use criteria recommended by the Society of Gynecologic Oncologists instead of using the FIGO staging system. Microinvasion is invasion limited to 3 mm in depth, is measured from the base of the squamous or glandular epithelium of origin, and does not encompass lymphatic or vascular space involvement. A simple punch biopsy is inadequate for making the diagnosis of microinvasion: a conization specimen, containing the entire neoplastic process, is necessary. Additional tissue is required from patients with positive cone margins because an occult, frankly invasive carcinoma may lie adjacent to a positive margin.
Evaluation and staging
Successful therapy planning requires detailed evaluation of the patient’s general medical condition and the size and extent of the carcinoma. Medical illness must be stabilized and anemia must be corrected before treatment commences. Patients with anemia, which has been extensively studied, have a higher local relapse rate than patients with a normal hemoglobin (Table 2).96, 97 The patient’s surgical history is important, and operative notes may describe the status of the abdominal organs as well as report abdominal and pelvic operations. Diagnoses of importance to therapy planning include ulcerative bowel disease, diverticulitis, and pelvic inflammatory disease. Such inflammatory conditions induce adhesions and fix loops of the intestines to each other, the adjacent organs, and the peritoneal surfaces.
Table 2 Relapse rates for patients with stage IIB or III cervical cancer
| Hemoglobin (gm/dL) | Patients (number) | Relapse rate (%) | ||
| Local | Distant | Total | ||
| <10 | 29 | 46 | 18 | 49 |
| 10–11.9 | 319 | 29 | 24 | 47 |
| 12–13.9 | 578 | 20 | 16 | 33 |
| ≥14 | 129 | 20 | 18 | 33 |
Relapse rates for patients with stage IIB or III cancer of the cervix according to average hemoglobin level during radiation therapy. p Values: p = 0.002 (local), p = 0.1 (distant), p = 0.0007 (total).
Source: Bush 1986.96 Reproduced with permission of Elsevier.
Patients with small stage I carcinomas should undergo chest radiography, a complete blood count, urinalysis, and blood chemistry analysis before treatment. Patients with advanced carcinomas require cystoscopy and proctoscopy. It is important to apply the FIGO rules for clinical staging (Table 3).98 It is clearly stated in the FIGO guidelines that for staging purposes, the following examinations are permitted: cystoscopy, inspection, colposcopy, ECC, hysteroscopy, proctoscopy, intravenous pyelography, chest radiography, and skeletal radiography.99 Findings from examinations such as lymphangiography, laparotomy, laparoscopy, computed tomography (CT), magnetic resonance imaging (MRI), and other examinations unnamed by FIGO should not be the basis for changing the clinical stage, despite the fact that such examinations or procedures can provide valuable information for planning therapy. The tumor-nodes-metastasis (TNM) staging categories have also been accepted by FIGO.100 Lymph node status is not addressed in the FIGO staging system for carcinoma of the cervix, but three radiologic imaging techniques are available to evaluate lymph node status: CT, MRI, and fluorodeoxyglucose-positron emission tomography (FDG-PET) detect abnormal lymph nodes more lymphangiography.
Table 3 Modified FIGO staging
| Stage | Description |
| I | The carcinoma is strictly confined to the cervix (extension to the corpus should be disregarded) |
| IA | Invasive cancer identified only microscopically. All gross lesions, even with superficial invasion, are stage IB cancers. Invasion is limited to measured stromal invasion with a maximum depth of 5 mm and a width no >7 mm |
| IA1 | Measured invasion of stroma ≤3 mm in depth and ≤7 mm in width |
| IA2 | Measured invasion of stroma >3 and ≤5 mm in depth and ≤7 mm in width |
| IB | Clinical lesions confined to the cervix or preclinical lesions larger than stage IA |
| IB1 | Clinical lesions ≤4 cm |
| IB2 | Clinical lesions >4 cm |
| II | The carcinoma extends beyond the uterus but has not extended onto the pelvic wall or to the lower third of vagina |
| IIA | Involvement of up to the upper 2/3 of the vagina. No obvious parametrial involvement |
| IIA1 | Clinical visible lesion ≤4 cm |
| IIA2 | Clinical visible lesion >4 cm |
| IIB | Obvious parametrial involvement |
| III | The carcinoma has extended onto the pelvic wall. On rectal examination, there is no cancer-free space between the tumor and the pelvic wall. The tumor involves the lower third of the vagina. All cases with hydronephrosis or a nonfunctioning kidney should be included unless they are known to result from another cause |
| IIIA | No extension onto the pelvic wall but involvement of the lower third of the vagina |
| IIIB | Extension onto the pelvic wall or hydronephrosis or nonfunctioning kidney |
| IV | The carcinoma has extended beyond the true pelvis or has clinically involved the mucosa of the bladder or rectum |
| IVA | Spread to adjacent organs |
| IVB | Spread to distant organs |
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Source: From Ref. 98.
The best radiologic imaging technique for detecting lymph node metastases is unclear. CT and MRI are good in identifying enlarged nodes; however, the accuracy of these techniques in the detection of positive nodes is compromised by their failure to detect small metastases, and many enlarged nodes are not due to metastases, but inflammation associated with advanced disease. The accuracy of MRI in the detection of lymph node metastases (72–93%) is similar to that of CT; however, when compared with surgical findings, MRI is superior to CT, clinical examination, and sonography in the evaluation of tumor location, tumor size, depth of stromal invasion, vaginal extension, and parametrial extension of cervical cancer.101–104 Furthermore, studies suggest that MRI is a cost-effective method of evaluating cervical cancers.104 Figure 13 shows an MRI of a patient with a cervical tumor.
Figure 13 Magnetic resonance image of a patient with cervical tumor.
PET or PET/CT is the rapidly expanding modality in oncologic imaging (Figure 14). In a study of 101 patients with carcinoma of the cervix, Grigsby and colleagues reported that CT demonstrated enlarged pelvic lymph nodes in 20% and enlarged para-aortic lymph nodes in 7%, while PET demonstrated abnormal FDG uptake in pelvic lymph nodes in 67%, abnormal FDG uptake in para-aortic lymph nodes in 21%, and abnormal FDG uptake in supraclavicular lymph nodes in 8%.105 The 2-year progression-free survival rate (PFS), based solely on para-aortic lymph node status, was 64% in CT-normal and PET-normal patients, 18% in CT-normal and PET-abnormal patients, and 14% in CT-abnormal and PET-abnormal patients. The authors concluded that often CT and that the findings on PET are a better predictor of survival than those on CT in patients with carcinoma of the cervix. Further studies are needed to confirm these results and determine if PET imaging is better than surgical staging particularly for determining if patients have positive para-aortic nodes.
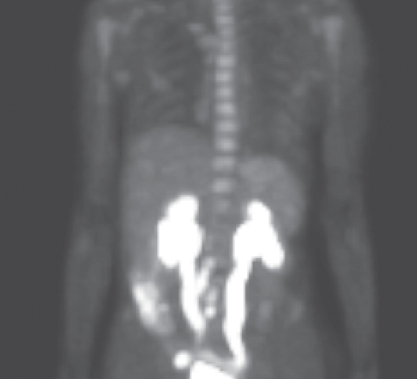
Figure 14 Positron emission tomography scan of a patient with cervical tumor showing positive nodes.
In some patients, surgical examination of the lymph nodes is warranted. The risk of occult para-aortic metastases is highest in patients with grossly involved pelvic nodes, and these patients may be the best candidates for operative exploration. These patients also may benefit from removal of the grossly enlarged nodes, which may be difficult to control with radiation alone.106 When lymph node metastases are sought surgically, the extraperitoneal approach is currently the preferred technique.107 It is preferred because high complication rates resulted from using a transperitoneal approach followed by radiation therapy.108 Lymph node exploration and dissection may also be performed using a laparoscopic approach, which is associated with a shorter postoperative recovery time and probably less late radiation morbidity than open transperitoneal staging.109
Prognostic factors
FIGO stage correlates with survival and control of pelvic disease in patients with cervical cancer; however, prognosis is also influenced by other factors, including tumor characteristics and patient characteristics that are not included in the FIGO staging system.
Tumor size and local extent
Tumor size is one of the most important predictors of local recurrence and death in patients with cervical cancer treated with surgery or radiation therapy (Figure 15). The FIGO staging classification for stage I disease was recently modified to include tumor diameter (i.e., ≤4 cm, stage IB1; >4 cm, stage IB2).98 For patients with more advanced disease, other estimates of tumor bulk that correlate with prognosis include presence of medial versus lateral parametrial involvement in FIGO stage IIB disease and unilateral versus bilateral pelvic wall involvement in FIGO stage IIIB disease.111, 112
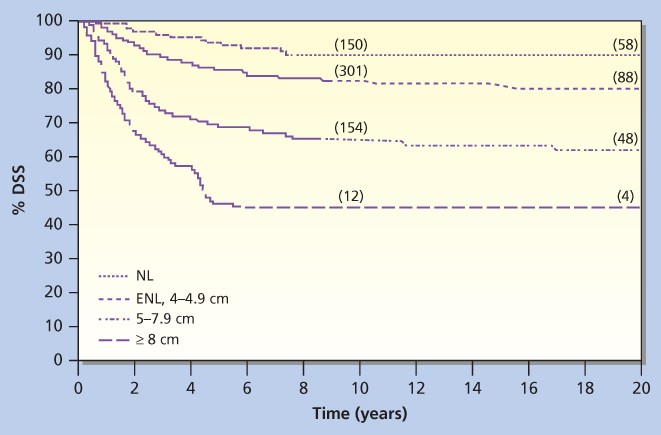
Figure 15 Disease-specific survival (DSS) is indicated for patients grouped according to size of cervix (NL, cervix of normal size; ENL, enlarged cervix, 4–4.9 cm).
Source: Lai et al. 1999.110 Reproduced with permission of Wolters Kluwer Health.
In patients who have had a radical hysterectomy, histologic evidence of extracervical spread (≥10 mm) and deep stromal invasion (>70% invasion) are associated with a poorer prognosis, as is parametrial extension, which is associated with higher rates of lymph node involvement, local recurrence, and death from cancer.91, 113, 114, 115 Uterine body involvement is associated with an increased rate of distant metastases in patients treated with radiation or surgery.116
Lymph node involvement
Lymph node metastasis is another important prognostic factor for survival that is not part of the FIGO staging system. In several surgical series, after a radical hysterectomy, patients with positive pelvic lymph nodes had a 35–40% lower 5-year survival rate than patients with negative nodes.89, 91 However, recent studies suggest that post-operative chemoradiation improves these results.117 Several authors have reported a correlation between size of the largest node involved or higher number of nodes involved and decreased survival rates. Patients with positive para-aortic nodes have a survival rate that is about half that of patients with similar-stage disease and negative para-aortic nodes.89, 91, 114, 118, 119 With extended-field radiation therapy, patients with early-stage disease and positive para-aortic nodes have a cure rate of approximately 40–50%.
There is a strong correlation between positive lymph nodes in patients with cervical neoplasms and positive lymph-vascular space invasion (LVSI) in the tumor specimen. However, LVSI may be an independent predictor of prognosis, as a number of large series of patients treated with radical hysterectomy have demonstrated.113, 114, 120, 121 Roman and colleagues reported a correlation between the percentage of histopathologic sections containing LVSI and the incidence of lymph node metastasis.122 In patients with adenocarcinoma of the cervix, there is a strong correlation between LVSI and outcome.123, 124
Histologic type
There is controversy regarding whether adenocarcinomas of the cervix are associated with outcome similar to that seen with squamous carcinomas of the cervix. In several retrospective studies, investigators found that patients with adenocarcinomas of the cervix had outcome similar to that of patients with squamous carcinoma of the cervix treated with radiation therapy.125, 126 However, other investigators have come to an opposite conclusion. Among patients treated surgically, they found that patients with adenocarcinoma had unusually high relapse rates compared with the rates in patients with squamous cell carcinoma and among patients treated with surgery or irradiation, they found that patients with adenocarcinoma had poorer survival rates than the rates seen in patients with squamous cell carcinoma.110, 127, 128 Eifel et al.,127 in an analysis of 1767 patients treated with radiation for FIGO stage IB disease, reported that patients with adenocarcinoma had a significantly higher risk of recurrence and death from disease. This finding was independent of age, tumor size, and tumor morphology. There was no difference in the rate of pelvic recurrence between patients with bulky adenocarcinoma (≥4 cm) and patients with squamous cell carcinoma; however, the rate of distant metastasis was almost twice as high in patients with adenocarcinoma as in patients with squamous cell carcinoma. Although the prognostic significance of histologic grade for squamous carcinomas has been disputed, there is a clear correlation between the degree of differentiation and the clinical behavior of adenocarcinomas.123, 124, 129
Other tumor factors
Pretreatment SCCAg levels have been shown to correlate with tumor bulk, stage, histology, grade, type of tumor (i.e., exophytic vs infiltrative), microscopic depth of invasion, and risk of lymph node metastases in patients with early-stage disease.130–132 The most important property of the pretreatment SCCAg level, however, is its ability to predict clinical outcome. Several authors have reported significantly lower survival rates in patients with very elevated values compared with patients with normal baseline levels, independent of stage.132–135 Monitoring of tumor response using SCCAg needs further investigation, especially studies designed to determine how often SCCAg measurement should be done, the level of SCCAg that is significant, which patients would benefit from this monitoring and the cost-effectiveness of using SCCAg as a tool for monitoring patients after treatment.136
Several authors have reported a correlation between HPV subtype and prognosis.137–139 In two studies of patients with histologically negative lymph nodes, investigators reported higher rates of disease recurrence when findings on PCR assay of the lymph nodes were strongly positive for HPV DNA.140, 141
Other molecular markers that have recently been evaluated for predictive power in cervical carcinoma are EGFR and cyclooxygenase-2.142 Studies have small populations and are presently too difficult to interpret. Other biologic features that have been investigated for their predictive power, with variable results, include inflammatory response in cervical stroma, peritoneal cytology, tumor vascularity, and DNA ploidy or S-phase fraction.143, 144
Patient factors
Several investigators have reported correlations between low hemoglobin level before or during treatment and poor prognosis.111, 145 It has been speculated that the poor prognosis of anemic patients is cause in part by hypoxia-induced radiation resistance. Other patient-related factors that have been shown to correlate with prognosis include age, platelet count, socioeconomic status, and smoking.93, 146, 147, 148, 149, 150 Kucera et al.149 reported that smokers with cervical cancer had a poorer 5-year survival rate than nonsmokers, and this relationship was statistically significant in patients with stage III disease (5-year survival rate 20.3% vs 33.9%, p < 0.01).
Surgical treatment options
Sentinel lymph nodes
A biopsy of sentinel lymph node was originally a process meant to simplify the surgical procedure and decrease morbidity by removing just one or a few nodes instead of systematic lymphadenectomy. However, the concept may have other advantages including a more reliable detection of key nodes in atypical localizations, detection of small metastasis, and intraoperative triage of patients, thanks to identification of key nodes for pathologic evaluation. The first large, multicenter study on sentinel lymph nodes for cervical cancer was published in 2008 and found a sensitivity of only 77%, which was a major setback for this procedure in cervical cancer.151 However, there were multiple problems with this study including the size of tumors included, lack of surgeon proficiency assessment, and pathologic ultrastaging. Subsequent studies have shown a very high sensitivity in women with small tumors undergoing lymphatic mapping including a recent large French SENTICOL study that has reported a sensitivity of 92% and a negative predictive value of 98%.152 Presently, sentinel lymph node biopsies is a useful intraoperative triage for patients with small tumors where the false-negative rate is lower.
Cervical conization and LEEP
Cervical conization is a procedure that removes or destroys the transformation zone and can be diagnostic, therapeutic, or both. A conization specimen is conical, as the name implies, and its size varies according to the area in question. The cone is shallow when an exocervical lesion is removed. The cone is deeper when the endocervix is being investigated. Patients requiring conization usually have one of the following: normal colposcopy findings and an abnormal Pap smear or positive ECC specimens; abnormal colposcopy findings in the form of failure to visualize the entire squamocolumnar junction or failure to define the extent of the lesion; microinvasive carcinoma in a biopsy specimen; adenocarcinoma in situ in a biopsy or ECC specimen; or a lack of correlation between cytologic (Pap smear), colposcopic, and histologic interpretations.
Cone biopsy is designed to completely remove the squamocolumnar junction and the lower portion of the endocervical canal in women with very small cervical cancers. The surgical specimen should include the entire lesion, as this permits measurement of both depth of invasion and extent of lateral spread.
Another surgical technique that can be used for conization is LEEP. In LEEP, a thin wire loop electrode is used to excise the lesion in patients with HGSIL. LEEP is an outpatient procedure. Although destructive techniques (such as laser ablation and cryotherapy) can provide effective treatment of suspicious lesions; the preferred technique is one that provides an appropriate histologic specimen, such as cervical cold knife conization (CKC) LEEP, or laser conization. Recently, Linares and colleagues compared CKC, laser conization, and LEEP and found that LEEP was associated with fewer complications and a shorter operating time than the other two procedures.153 The only drawback of the LEEP procedure was a slightly shorter cone depth and a slightly higher risk of lesion recurrence.153
Patient selection
Conization as sole treatment of early cervical cancers is a relatively recent concept. For women who have very limited risk of lymph node spread and who have a strong desire to maintain fertility, conization may be an option.154, 155 MD Anderson Cancer Center recommends that conization be considered only for patients with squamous cell lesions that invade <3 mm, have no LVSI, and have uninvolved resection margins.156 There is very little information on which to base a recommendation regarding the use of conization as therapy for women with nonsquamous cervical cancer.157
Complications of CKC include hemorrhage, pelvic cellulitis, cervical stenosis, and incompetent cervix.158 In addition, because this procedure requires general anesthesia, there is the additional burden of possible complications and cost of anesthesia.
Complications of LEEP are similar to but not the same as those of CKC: Infection, bleeding, burns to the vagina, cervical stenosis, cervical incompetence, and recurrence of dysplasia. With LEEP, however, stenosis is rare (occurring in 1% of patients) and is seen primarily in nulliparous, perimenopausal, or postmenopausal patients. Cervical incompetence is usually only a complication of multiple procedures. The other advantage of LEEP is that it does not require general anesthesia. As mentioned earlier, when three conization techniques (CKC, laser conization, and LEEP) were compared, LEEP was associated with fewer complication as well as decreased operative time.153
Radical trachelectomy
Recently, for early stage disease, several groups have tried to preserve the uterus and child-bearing capability by treating patients with a radical vaginal trachelectomy. This technique involves a laparoscopic pelvic lymphadenectomy followed by vaginal resection of the cervix, the upper 1–2 cm of the vaginal cuff, and the medial portions of the cardinal and uterosacral ligaments. The cervix is transected at the lower uterine segment, and a prophylactic cerclage is placed at the time of surgery. Several investigators recommend that this procedure be limited to patients with a tumor not exceeding 2 cm.159, 160
Extrafascial hysterectomy
The extrafascial technique permits removal of the intact uterine fundus and cervix, leaving the parametrial soft tissues and a portion of the upper vagina. Extrafascial hysterectomy can be accomplished through an abdominal incision, transvaginally, or using a combination of laparoscopic and transvaginal techniques.
Simple extrafascial hysterectomy is the standard definitive treatment option for women with stage IA1 cervical cancers and is sometimes performed following radiation therapy for bulky endocervical carcinomas. For patients with stage IA2 disease, there is some controversy regarding the most appropriate surgical procedure. These patients have 3–5% incidence of lymph node metastases and a higher rate of vaginal recurrence than patients with IA1 disease. So, although some data suggest that these IA2 lesions can be effectively resected with extrafascial hysterectomy, many American gynecologic oncologists limit this operation to women with IA1 tumors.161, 162 There is uniform consensus that conization-only therapy should not be offered to women with stage IA2 disease.
Radical hysterectomy
Radical hysterectomy involves the en bloc removal of the uterus, cervix, parametrial tissues, and upper vagina. In the early twentieth century, Wertheim of Vienna described the radical hysterectomy for the treatment of cervical cancer. In the 1940s, Meigs of the United States championed the procedure, to which he added a pelvic lymphadenectomy. Today, radical hysterectomy with pelvic lymphadenectomy is the standard treatment in the management of stage IA2-IIA tumors.
The type II (modified radical) hysterectomy is a less extensive version of the type III (radical) hysterectomy. The primary indication for type II hysterectomy is early invasive carcinoma, tumors <2 cm diameter. The type II operation ensures an adequate paracervical specimen and a vaginal cuff of 2–3 cm. In the type II operation, the medial half of the parametrium and upper one-third of the vagina are included in the surgical specimen. This is accomplished by exposing the ureters and taking the medial half of the cardinal and uterosacral ligaments instead of taking these ligaments where they attach to the pelvic wall and pelvic floor. The posterior approach is the surgeon’s choice. The incidence of bladder and ureteral complications is lower with the type II operation than with a type III procedure. The type II hysterectomy can be performed with a modified or complete pelvic lymphadenectomy.
The type III (radical) hysterectomy is the classic Wertheim-Meigs radical hysterectomy. This operation is reserved for patients with stage IB and selected stage IIA carcinomas. The vaginal extension for stage IIA patients should be limited to no more than 1 cm. The parametrium, cardinal, and uterosacral ligaments are severed at the pelvic wall, and half of the vagina is removed. The uterine vessels are taken at their origin from the internal iliac vessels. The ureters are taken out of their tunnel and reflected laterally. This dissection of the distal ureters sacrifices the blood supply from the uterine and superior vesicle arteries. Reflection of the ureters clears the way for applying instruments across the parametrium along the pelvic wall. Complete removal of the cardinal ligaments and the rectal pillars and uterosacral ligaments at their base results in a greater risk of bladder atony, and loss of the distal-ureter blood supply results in a greater risk of fistulae. This operation produces an excellent cure rate in properly selected patients. In young patients, the ovaries are spared.
Intraoperative and immediate postoperative complications of radical hysterectomy include blood loss (average, 0.8 L), ureterovaginal fistula (occurring in 1–2% of patients), vesicovaginal fistula (<1%), pulmonary embolus (1–2%), small bowel obstruction (1–2%), and postoperative fever secondary to deep vein thrombosis, pulmonary infection, pelvic cellulitis, urinary tract infection, or wound infection (25–50%).163 Subacute complications include lymphocyst formation and lower extremity edema, the risk of which is related to the extent of the node dissection. Lymphocysts may obstruct a ureter, but hydronephrosis usually improves with drainage of the lymphocyst.164 The risk of complications may be increased in patients who undergo preoperative or postoperative irradiation.
Although most patients have transient decreased bladder sensation after radical hysterectomy, with appropriate management severe long-term bladder complications are infrequent. However, chronic bladder hypotonia or atony occurs in approximately 3–5% of patients despite careful postoperative bladder drainage.165 Bladder atony probably results from damage to the bladder’s innervation and may be related to the extent of the parametrial and paravaginal dissection.166 Radical hysterectomy may be complicated by stress incontinence, but reported incidences vary widely and may be influenced by the addition of postoperative radiation therapy.167 Patients may also experience constipation and, rarely, chronic obstipation after radical hysterectomy.
Criteria for selecting patients who are appropriate candidates for radical hysterectomy include factors affecting the patient’s suitability for major surgery as well as tumor characteristics, including tumor volume and lymphatic involvement. Patient factors play a very important role in the selection of primary radical surgery versus primary radiation therapy in patients with early-stage disease. The ability to preserve ovarian function as well as a more pliable vagina is important to young women facing this decision. Just as important, for women with significant medical problems, including obesity, primary radiation therapy may be the better treatment option.
In patients with early-stage (IA-IB1) disease, tumor characteristics play a very important role in treatment selection. Patients with high-risk factors may benefit from up-front definitive radiation therapy plus possible chemotherapy. Multiple studies have found that morbidity increases in patients who receive both radical surgery and radiation therapy.168 High-risk tumor characteristics include large tumor size, which is associated with lymph node spread, increased chance of recurrence, and decreased survival rates. Eifel et al.164 found that DSS decreased significantly in patients with stage IB carcinoma if the tumor diameter was >5 cm (88% vs 69%, p < 0.0001). Other high-risk factors include lymph node metastases, parametrial involvement, and positive surgical margins.
Outcomes after surgical treatment
Reported 5-year survival rates for women with stage IB cervical cancer treated with radical hysterectomy and pelvic lymphadenectomy are approximately 80–90% (Table 4).89, 90, 91, 168, 169, 170, 171, 172, 173, 174, 175, 176 Patients with positive surgical margins or positive lymph nodes are at the highest risk of recurrence and poor outcome. Delgado et al.,91 in a large prospective study, reported 3-year DSS rates of 85.6% in patients with negative nodes and 50–74% in patients with positive nodes. In the group with positive nodes, increasing number of positive nodes and involvement of common iliac nodes correlated with decreased survival. A randomized study showed that postoperative chemoradiation improved survival in patients with positive lymph nodes, positive surgical margins, or tumor present in the parametrium.117
Table 4 5-Year survival rates for stage IB-IIA cervical cancer patients after radical hysterectomy and bilateral pelvic lymphadenectomy
| First author (references) | Stage | Year | n | Survival (%) |
| Sall169 | IB-IIA | 1979 | 219 | 90.0 |
| Kenter170 | IB-IIA | 1989 | 213 | 87.3 |
| Lee171 | IB-IIA | 1989 | 343 | 87.2 |
| Ayhan90 | IB-IIA | 1991 | 270 | 80.7 |
| Hopkins129 | IB | 1991 | 213 | 92.5 |
| Alvarez172 | IB | 1991 | 401 | 85 |
| Averette89 | IIB-IIA | 1993 | 726 | 90.1 |
| Landoni163 | IB-IIA | 1997 | 172 | 83 |
Abbreviation: N, number of patients.
Radiation therapy
The management of invasive carcinoma of the cervix with primary radiation therapy involves a combination of external beam radiation therapy (EBRT) plus either low dose rate (LDR) or high dose rate (HDR) intracavitary irradiation. The goal of treatment is to balance these two elements in a way that optimizes the ratio of tumor control to treatment complications. The required dose varies according to the tumor burden in the cervix, paracervical sites, and regional nodes. Factors that influence the tolerable dose of radiation include the patient’s vaginal and uterine anatomy, the degree of tumor-related tissue destruction and infection, and patient characteristics (e.g., body habitus, comorbid illnesses, and smoking habits).
Treatment options EBRT
EBRT is used as initial treatment in patients with bulky tumors. The usual plan is to give 40–45 Gy to the whole pelvis. This gives a homogeneous distribution to the central mass plus the regional lymph nodes. Such treatment reduces the primary tumor and any regional lymph nodes harboring disease, and it destroys microscopic foci in lymph-vascular spaces adjacent to the tumor. The shrinkage of the primary tumor allows better dose distribution from intracavitary irradiation.
High-energy photons (15–18 MV) are usually preferred for pelvic treatment because they spare superficial tissues that are unlikely to be involved with tumor. Simulation films, as well as CT, MRI, PET scan, and lymphangiography, guide the radiation oncologist in selecting boundaries for the portals. Typical radiation therapy fields are shown in Figure 16. A standard course of radiation therapy is 40 to 45 Gy given with external beam radiation tberapy (EBRT). Patients with grossly positive pelvic nodes within the 40- to 45-Gy field require a boost with a small field (Figures 16 and 17). The dose to the boost area is 8–10 Gy. The total dose to the positive nodes, including a 1–2 cm margin, is 60–66 Gy, which includes the contributions from brachytherapy intracavitary systems. Intensity-modulated radiation therapy (IMRT) may be used to boost the dose to large pelvic nodes (up to approximately 66 Gy) in a very tightly defined volume.
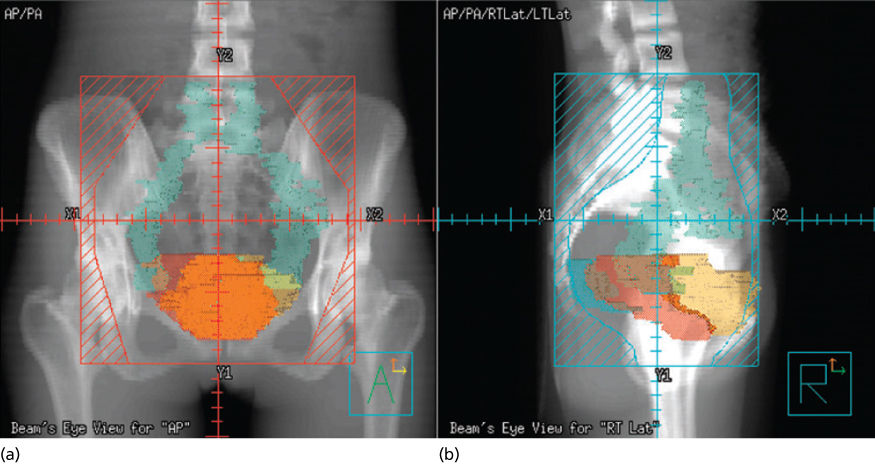
Figure 16 Typical radiation fields for a patient with cervical cancer. (a) Anterior field; (b) lateral field.
There has been a recent interest in using IMRT in the treatment of cervical carcinoma. Unlike standard treatment, IMRT allows one to dose paint tissues in the area of treatment, that is, allowing higher doses to tumor, while giving less dose to normal tissues especially small bowel (Figure 17). Mundt et al.177 have published reports showing a decrease in both acute and chronic178 bowel toxicities in patients with endometrial or cervical carcinoma treated postoperatively to the pelvis with IMRT compared to standard treatment. However, the highly conformal dose distributions achieved by IMRT also increases the room of error. In particular, great attention needs to be given to internal organs motion (especially the bladder and the rectum) and tumor regression, and therefore at this time IMRT is still considered the investigation therapy, especially in the field of intact cervix. Several multi-institutional trials are underway evaluating the use of IMRT for cervical cancer.
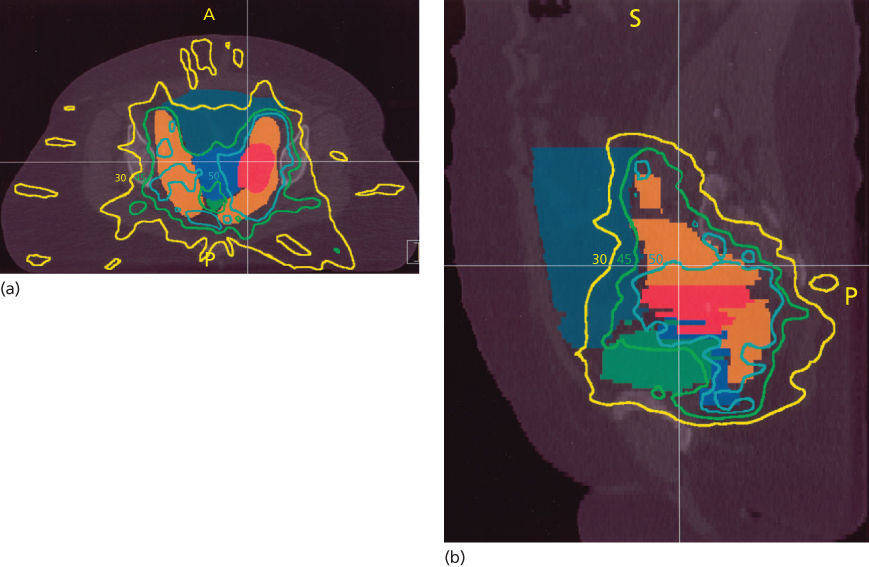
Figure 17 Dose distribution of a typical intracavitary system; it is important to note how quickly the dose falls off the further one gets from the system. (a) Anterior view; (b) lateral view.
Intracavitary radiation therapy (ICRT)
The importance of ICRT (intracavitary radiation therapy) for cervical cancer should not be underestimated because, although EBRT plays a critical role in sterilizing pelvic wall disease and improving tumor geometry, too much reliance on external beam irradiation will compromise the chance for central disease control and increase the risk of complications.
Brachytherapy is usually delivered using afterloading applicators that are placed in the uterine cavity and vagina. Ideal placement of the intrauterine tandem and vaginal ovoids produces a pear-shaped radiation distribution, delivering a high radiation dose to the cervix and paracervical tissues and a reduced dose to the rectum and bladder. Several systems have been used throughout the world to determine dose rates and doses for cervical cancer. In the United States, the paracentral doses are most frequently expressed at a single point, point A, which is defined earlier. Point A bears no consistent relationship with the tumor or target volume but lies approximately at the crossing of the ureter and uterine artery. Other systems of dose specifications include the International Commission on Radiation Units and Measurements (ICRU) reference points based on ICRU Report 38 (1985) and the mg-h system. Whichever system of dose specification is used, emphasis should always be placed on optimizing the relationship between the intracavitary applicators and the cervical tumor and other pelvic tissues. Source, strength, and position should be carefully chosen to provide optimal tumor coverage with exceeding safe doses to normal tissue (Figure 18).
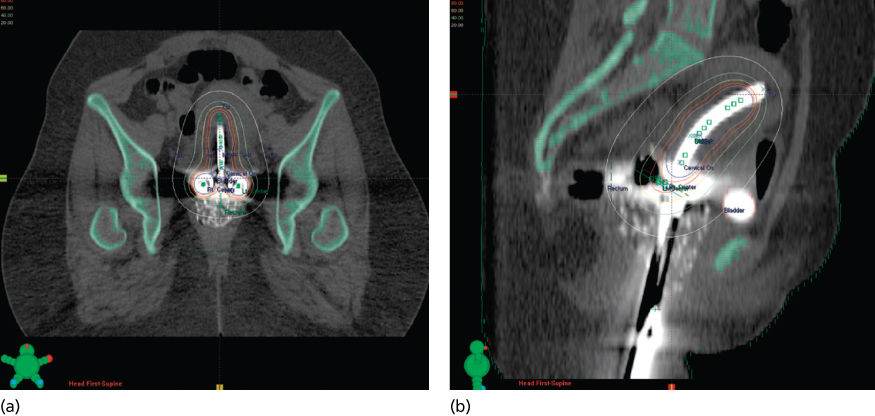
Figure 18 A patient with cervical cancer being treated with radiation therapy after a hysterectomy. The areas that need to be covered are the nodes and vagina. This is an IMRT plan that conforms to the area that needs to be covered while sparing the small bowel. Most of the small bowel receives <30 Gy.
In the past, clinicians regarded the radiobiological advantages of LDR intracavitary treatment (usually delivery of 40–60 cGy/h to point A) as a major factor contributing to the success of cervical cancer treatment. These LDRs permit repair of sublethal cellular injury, preferentially sparing normal tissues and optimizing the therapeutic ratio. During the past two decades, computer technology has made it possible to deliver brachytherapy at very HDRs (<100 cGy/min) using a high-activity cobalt 60 or iridium 192 source and remote afterloading. HDR intracavitary therapy is now become the standard of care in most parts of the world. Clinicians have found this approach attractive because it does not require that patients be hospitalized and may be more convenient for the patient and the physician. Multiple randomized and nonrandomized studies have suggested that survival rates and complications rates with HDR treatment are similar to those with traditional LDR treatment.179 In 2012,180 the American Brachytherapy Society suggested guidelines for the use of HDR brachytherapy for carcinoma of the cervix. The group emphasized the importance of factors that were already considered critical to successful LDR treatment—optimized applicator position, balanced use of external beam therapy and brachytherapy, compact overall treatment duration, and delivery of an adequate dose to tumor while respecting normal tissue tolerance limits.
Image-based brachytherapy is becoming increasingly common, particularly in Europe. Institutions are using MRI-based treatment planning to plan their doses to tumor as well as doses to avoid structures such as the bladder and rectum; however, large prospective studies and careful analysis will be needed before this becoming standard of care.
Interstitial brachytherapy
Several groups have advocated the use of interstitial perineal template brachytherapy in patients with poor anatomy or parametrial or pelvic sidewall disease. These implants are usually placed transperineally, with placement guided by a Lucite template that encourages parallel placement of hollow needles that penetrate the cervix and paracervical spaces and are usually loaded with iridium 192. Advocates state that the advantages of this method are the ease of inserting implants in patients whose uterus is difficult to probe, the ability to place sources directly into the parametrium, and the relatively homogeneous dose. Recent updates report poor survival rates and higher rates of major complications for patients with stage IIB and IIIB disease.181, 182 Outside of an investigational setting, interstitial treatment of primary cervical cancers should probably be limited to patients who cannot accommodate intrauterine brachytherapy and patients with distal vaginal disease that requires a boost with interstitial brachytherapy.
Patient selection
For patients with IB1 tumors, the choice between surgery and radiation therapy is based primarily on patient preference, anesthetic and surgical risks, physician preference, and understanding of the nature and incidence of complications of radiation therapy and hysterectomy. In general, surgery is often chosen for younger patients in the hope of preserving ovarian function and hopefully reducing vaginal shortening, while radiation therapy is often selected for older postmenopausal women to avoid the morbidity of a major surgical procedure. Patients with stage IB2 disease can be treated with either surgery followed by radiation therapy or definitive radiation therapy. The biases are so large that the Gynecologic Oncology Group (GOG) could not complete trial randomizing patients with stage IB2 disease between surgery followed by chemoradiation therapy and definitive radiation therapy. Radiation therapy is the primary local therapy for most patients with stage IIB-IVA disease.
Outcomes
Stage IB-IIA disease
Radical radiation therapy achieves excellent survival and pelvic disease control rates in patients with stage IB-IIA cervical cancer. Eifel et al.164 reported 5-year DSS rates of 90%, 86%, and 67% in patients with stage IB tumors with cervical diameters of <4, 4–4.9, and >5 cm, respectively. The 5-year survival rates of patients with stage IIA disease range from 70% to 85% and, like survival rates in patients with stage IB disease, are strongly correlated with tumor size.112, 183, 184 Studies suggest that results for patients with bulky tumors may be improved further by concurrent administration of chemotherapy.185, 186
Stage IIB-IVA disease
Five-year survival rates of 65–75%, 35–50%, and 15–20% have been reported in patients who received radiation therapy alone for stage IIB, IIIB, and IV tumors, respectively (Table 5).111, 112, 184, 187 The addition of cisplatin-containing regimens may further improve local control and survival.186, 188, 189
Table 5 Pelvic disease control and survival ratesa
| FIGO stage | Patients (n) | Control rate (%) | Survival rate (%) |
| I | 229 | 93 | 89 |
| IIA | 315 | 88 | 85 |
| IIB | 314 | 80 | 62 |
| IIIA | 266 | 63 | 62 |
| IIIB | 216 | 57 | 50 |
| IV | 43 | 18 | 20 |
a Pelvic disease control rates and survival rates of 1383 patients with carcinoma of the intact uterine cervix treated with irradiation alone, according to the Fletcher guidelines: a French cooperative study.
Source: Barillot et al. 1997.112 Reproduced with permission of Elsevier.
Complications
Acute side effects
Acute side effects of pelvic irradiation include symptoms related to the bowel, bladder, rectum, and vagina. Most patients experience mild fatigue and mild to moderate diarrhea that usually is controllable with antidiarrheal medication and dietary modifications. Less frequently, patients may complain of bladder or urethral irritation. These symptoms may be treated with Pyridium or antispasmodics after urinalysis and urine culture have ruled out a urinary tract infection. Patients treated with extended-field radiation may have nausea, gastric irritation, and mild depression of peripheral blood cell counts. Acute symptoms may be increased in patients receiving concurrent chemotherapy. Unless the ovaries have been transposed, all premenopausal patients who receive pelvic radiotherapy experience ovarian failure by the completion of treatment.
Fatal or life-threatening complications of ICRT are rare. Thromboembolic events, uterine perforation, fever, vaginal laceration, and the usual risk of anesthesia are other less serious complications associated with ICRT.190
Problems after therapy
For patients with cervical cancer, overall estimates of the risk of major complications of radiation therapy usually range between 5% and 15%.191, 192 The risk of experiencing a late complication is greatest within the first 3 years after treatment; however, major complications have been reported as late as 30 years or more after treatment. Most complications occurring after radiation involve the rectum, bladder, or small bowel. During the first 3 years after treatment, rectal complications are most common and include bleeding, stricture, ulceration, and fistula. The average onset of major urinary tract complications tends to be somewhat later than that of intestinal complication, with an actuarial risk of hematuria requiring transfusion of 2.6% at 5 years.191 The overall risk of developing a gastrointestinal or urinary tract fistula was 1.7% at 5 years with an increased risk of patients who underwent adjuvant hysterectomy or pretreatment transperitoneal lymphadenectomy. The risk of small bowel obstruction is strongly correlated with a number of patient characteristics and treatment factors including type of surgery, history of pelvic infection, history of smoking, and body habitus.150, 191, 192
Patients who are treated with radiation for cervical cancer tend to have varying degrees of atrophy, telangiectasia, or scarring of the upper third of the vagina. However, more severe shortening can occur. Changes may be greater in patients with very extensive tumors and in patients who are elderly, sexually inactive, or hypoestrogenic.192, 193 Regular intercourse and use of vaginal dilators may help prevent vaginal shortening.
Current practice by disease stage
CIS and stage IA1 disease
Treatment of HGSILs is rapidly changing. LEEP is one effective therapy for HGSILs. Other techniques such as cryosurgery and laser ablation have also proven effective. Regardless of the technique used, HGSILs should be entirely visible by colposcopy, the entire transformation zone should be visualized, and the ECC specimen should be negative. Conization with a knife is preferred when the ECC specimen is positive. All margins must be clear of intraepithelial lesions.
Stage IA1 (microinvasive) squamous cell carcinomas that invade the stroma <3 mm and have no LVSI are usually not visible to the unaided eye, and cytology is abnormal. The diagnosis requires cone biopsy, which yields a specimen adequate for diagnostic purposes. Conization alone is also potentially a low-risk therapy for patients who wish to retain fertility and who have invasion <3 mm in depth with no LVSI. It is important to remember that the margins of a cone specimen must be negative. When the conization specimen has a positive margin, a second tissue specimen must be obtained because foci of frankly invasive carcinoma may lie adjacent to the positive margin. Patients should be informed that the conservative approach carries a small risk. There is very little current information on which to base a recommendation regarding the use of conization as therapy for women with nonsquamous cervical cancers.155 Patients who elect conization therapy must be willing to be monitored closely for the development of residual or recurrent cervical neoplasia.
The most conventional treatment of stage IA1 disease is a type 1 (simple extrafascial) hysterectomy. If the patient is young, the ovaries are left in place. Patients can also be treated with radiation therapy alone, usually consisting of intracavitary therapy alone. The 10-year disease control rate with radiation therapy alone is 95–100%.194
Stage IA2 disease
Small lesions that invade 3–5 mm have an average risk of lymph node metastasis of about 5%. The standard treatment of patients with this stage of disease is type II (modified radical) hysterectomy and pelvic node dissection.
Recently, a number of studies have explored less radical surgical options for early-stage cervical cancer, including simple hysterectomy, simple hysterectomy, simple trachelectomy, and cervical conization with or without sentinel lymph node biopsy and pelvic lymph node dissections especially in patients who wish to preserve fertility. Such options may be available for patients with low-risk early-stage cervical cancer including patients with any histology except neuroendocrine tumor, tumor size <2 cm, stromal invasion <10 mm, and no LVSI. Presently, three international trials are underway looking at this option for patients with early-stage disease.
If surgery is contraindicated, these patients can be treated with radiation alone, usually with pelvic radiation therapy plus brachytherapy. However, in special situations, brachytherapy alone may be sufficient in controlling disease.
Stage IB1 and small-stage IIA disease
Treatment of invasive carcinoma or carcinoma of FIGO stage IB or greater is determined on the basis of tumor size and the presence or absence of lymph node metastases (Figure 5).
Stage IB1 tumors, which are <4 cm in size, are considered small; however, they are associated with a significant risk of microscopic paracervical extension or lymph node metastasis. In a prospective study in patients treated with radical hysterectomy who had clinically estimated maximum tumor diameter <3 cm, the GOG reported that 16% of the patients (42 of 261) had lymph node metastases.195 Landoni et al.163 found a 25% incidence of positive nodes (28 of 114 patients) in patients treated with radical hysterectomy for stage IB-IIA tumors that measured 4 cm or less on initial clinical examination. Therefore, for patients with stage IB1 or small IIA (<4 cm diameter) disease, the treatment is either type III (radical) hysterectomy plus bilateral pelvic lymphadenectomy or radical radiation therapy. The goal of both of these treatment options is to destroy malignant cells in the cervix, paracervical tissues, and regional lymph nodes.
OS rates for patients with stage IB1 or small IIA disease treated with either surgery or radiation therapy are usually in the range 80–90%, suggesting that the two treatments are equally effective. However, only one prospective randomized trial has compared the two treatments directly.163 There were no significant differences in the rates of relapse or survival between the two arms, but the overall rate of grade 2 and 3 complications was greater for patients treated with hysterectomy. Therefore, for patients with stage IB1, the choice of treatment is based on patient preference, anesthetic and surgical risks, physician preference, and an understanding of the nature and incidence of complication with radiation therapy and hysterectomy. The role of pelvic radiation therapy after radical hysterectomy is still being defined. The GOG, in a prospective trial, randomized patients with intermediate risk of recurrence after radical hysterectomy for stage IB carcinoma to pelvic irradiation or no further treatment.196 The preliminary analysis showed a 47% reduction in the risk of recurrence when postoperative irradiation was given (15% vs 28%, p = 0.008). An update of this study published in 2006 showed a statistically reduction in the risk of recurrence and PFS however no statistical difference in OS with the addition of postoperative radiation therapy. The conclusion by the authors was that pelvic radiotherapy after radical surgery significantly reduced the risk of recurrence and prolongs PFS in women with stage IB cervical cancer, particularly in patients with adenocarcinoma or adenosquamous histologies.197 The Southwest Oncology Group reported the results of a randomized trial of patients treated with radical hysterectomy followed by radiation therapy versus radiation therapy plus cisplatin-based chemotherapy in patients with high risk factors including pelvic lymph node metastases, parametrial involvement, and or positive margins. The authors found that patients who received radiation therapy plus chemotherapy had a significant improvement in survival compared with the survival of patients treated with radiation alone.117
Stage IB2 disease
Patients with tumors >4 cm in diameter whose para-aortic lymph nodes are found to be free of disease have excellent survival rates when treated with whole-pelvis EBRT plus brachytherapy. However, these tumors appear on clinical examination to be technically resectable, and the ideal management of these tumors is a subject of considerable controversy. The approach to these tumors differs widely between centers, and the biases are so large that the GOG closed a trial early owing to a lack of accrual that compared surgery plus chemoradiation versus chemoradiation alone.
Although radiation therapy is effective for many patients with stage IB2 disease, central disease recurrences occur in at least 8–10% of patients with bulky endocervical cancers.167, 183, 184 The literature contains numerous reports on the use of an adjunctive hysterectomy for patients with bulky endocervical carcinomas treated with primary radiation therapy. However, in 1999, the GOG concluded from a study that evaluated adjunctive hysterectomy, that adjunctive hysterectomy did not improve local control or survival but did increase toxicity when added to chemoradiation therapy in patients with stage IB2 disease.185
Several investigators recommend treating these tumors with initial surgery followed by postoperative radiation therapy or chemoradiation depending on the pathology findings. In a randomized trial by Landoni et al.,163 patients with stage IB2 tumors were randomized to initial surgery versus definitive radiation therapy. In this trial, even though the survival was similar in both arms, 84% of the patients treated with initial surgery required postoperative radiation therapy, which contributed to a higher complication rate in that arm.
Several trials have been initiated to study the role of induction chemotherapy before surgical exploration in patients with bulky lesions who would otherwise be poor candidates for radical hysterectomy.198, 199 A meta-analysis200 collected individual patient data (IPD) from 21 randomized trials of neoadjuvant chemotherapy in locally advanced cervical cancer. This analysis included data from comparison of the benefits of neoadjuvant chemotherapy followed by radical radiotherapy versus radical radiotherapy alone (2074) and for comparison of neoadjuvant chemotherapy followed by surgery (±radiotherapy) versus radical radiotherapy alone (872 patients). They found that the group of trials using cycles lasting longer than 14 days showed a significant 25% increase in the relative risk of death with neoadjuvant chemotherapy, representing an absolute 8% reduction in 5-year survival compared to shorter chemotherapy cycle lengths, where there was a significant 17% decrease in the relative risk of death, representing an absolute improvement in 5-year survival of 7%. They also found a significant 35% increase in the risk of death for trials that used <25 mg/m2 cisplatin per week, reducing absolute 5-year survival by 11%; however, the results for the higher dose-intensity group were less clear, with only a trend toward increased survival. The second comparison of patients treated with neoadjuvant chemotherapy and surgery versus radiation therapy only showed that patients treated with neoadjuvant chemotherapy had a highly significant 35% reduction in the risk of death (p = 0.0004) that translated into a 14% absolute increase in 5-year OS.201 Unfortunately, this meta-analysis only included trials before 1999, when radiotherapy alone was still the standard treatment. Ultimately, however, this approach may need to be compared with optimized chemoradiation to determine the most effective, least toxic therapy for these patients. The EORTC (European Organisation for Research and Treatment of Cancer) completed a trial addressing this specific question. Patients with IB2 cancers are being randomized to neoadjuvant chemotherapy followed by radiation or current chemotherapy and radiation.
Two prospective randomized trials185, 186 indicate that patients who are treated with radiation for bulky central disease benefit from concurrent administration of cisplatin-containing chemotherapy. A third study suggests that patients who require postoperative radiotherapy because of findings of lymph node metastasis or involved surgical margins also benefit from concurrent chemoradiation.117 These studies are discussed in more detail in the section titled “Concurrent Chemoradiation,” later in this chapter.
Stage IIB-IVA disease
Radiation therapy is the primary local treatment of most patients with locoregionally advanced (stages IIIB-IVA) cervical carcinoma. The success of treatment depends on a careful balance between EBRT and brachytherapy that optimizes the dose to tumor and normal tissues and on the overall duration of treatment. In the French Cooperative Group study of 1875 patients treated with radiation therapy according to Fletcher guidelines, Barillot et al.112 reported 5-year survival rates of 70%, 45%, and 10% for patients with stage IIB, IIIB, and IVA tumors, respectively.
Local and distant disease recurrences continue to be a problem for patients with locally advanced disease. Neoadjuvant chemotherapy has produced excellent tumor responses; however, randomized trials have failed to demonstrate improvements in survival. Other approaches that have been used to try to improve outcome in these patients, including neutrons, hyperbaric oxygen, and hypoxic cell sensitizers, have also produced disappointing results.
Concurrent chemoradiation
In 1999, five prospective randomized trials, involving patients with locoregionally advanced cervical cancer,117, 185, 186, 188, 189 provided compelling evidence that the addition of concurrent cisplatin-containing chemotherapy to standard radiotherapy reduces the risk of disease recurrence by as much as 50% and thereby improves the rates of pelvic disease control and survival (Table 6).
Table 6 Prospective randomized trials: role of concurrent radiotherapy and cisplatin-containing chemotherapya
| First author (references) | Eligibility | Patients (numbers) | CT: investigational arm | CT: control arm | Relative risk of recurrence (90% CI) | p Values |
| Rose188 | FIGO IIB-IVA | 526 | Cisplatin 40 mg/m2/week (up to 6 cycles) | HU 3 g/m2 (2×/week) | 0.57 (0.42–0.78) | <0.001 |
| Cisplatin 50 mg/m2; 5-FU 4 g/m2/96 h; HU 2 g/m2 (2×/week) (2 cycles) | HU 3 g/m2 (2×/week) | 0.55 (0.40–0.75) | <0.001 | |||
| Morris186 | FIGO IB-IIA (≥5 cm); IIB-IVA or pelvic nodes involved | 403 | Cisplatin 75 mg/m2; 5-FU 4 g/m2/96 h (3 cycles) | Nonea | 0.48 (0.35–0.66) | <0.001 |
| Keys185 | FIGO IB (≥4 cm) | 369 | Cisplatin 40 mg/m2/week (up to 6 cycles) | Noneb | 0.51 (0.34–0.75) | 0.001 |
| Whitney189 | FIGO IIB-IVA | 368 | Cisplatin 50 mg/m2; 5-FU 4 g/m2/96 h (2 cycles) | HU 3 g/m2 (2×/week) | 0.79 (0.62–0.99) | 0.03 |
| Peters117 | FIGO I-IIA after radical hysterectomy with nodes, margins, or parametrium positive | 268 | Cisplatin 50 mg/m2; 5-FU 4 g/m2/96 h (2 cycles) | None | 0.50 (0.29–0.84) | 0.01 |
| Pearcey202 | FIGO IB-IIA (≥5 cm), IIB-IVA or pelvic nodes involved | 259 | Cisplatin 40 mg/m2/week (up to 6 cycles) | None | 0.91 (0.62–1.35)c | 0.43 |
| Wong203 | FIGO IB-IIA (>4 cm), IIB-III | 220 | Epirubicin 60 mg/m2, then 90 mg/m2 q4 weeks for 5 more cyclesd | None | ∼0.65 | 0.02 |
| Lorvidhaya204 | FIGO IB-IVA | 926 | Mitomycin-C 10 mg/m2 day 1 and 29 Oral 5-FU 300 mg/day days 1–14 and 29–42 | None | 0.001 | — |
Abbreviations: CI, confidence interval; FU, follow-up; HU, hydroxyurea; PA, para-aortic; RT, radiotherapy.
Results from prospective randomized trials that investigate the role of concurrent radiotherapy and cisplatin-containing chemotherapy for patients with locoregionally advanced cervical cancer.
a Patients in control arm had prophylactic para-aortic irradiation.
b All patients had extrafascial hysterectomy after radiotherapy.
c Survival.
d Chemotherapy was begun on day 1 and continued every 4 weeks throughout and after radiation therapy.
e This study had four arms: arm 1, conventional radiation therapy (RT); arm 2, conventional RT with adjuvant chemotherapy consisting of 5-FU orally at 200 mg/day given for 3 courses of 4 weeks, with a 2-week rest every 6 weeks; arm 3, conventional RT with concurrent chemotherapy; and arm 4, conventional RT with concurrent and adjuvant chemotherapy. The addition of adjuvant therapy did not affect recurrence, but there was a significant difference in the recurrence rate between the conventional RT and the conventional RT plus concurrent chemotherapy arms.
In two studies,188, 189 the GOG randomly assigned patients with stages IIB-IVA disease to receive either hydroxyurea or cisplatin-containing chemotherapy during external beam irradiation. All three of the cisplatin-containing regimens in these trials produced local control and survival rates superior to those for the control (hydroxyurea and radiation). In a third study,185 patients with stage IB tumors measuring at least 4 cm in diameter were randomly assigned to receive radiation alone or radiation plus weekly cisplatin before an extrafascial hysterectomy. Patients who received cisplatin were more likely to have a complete histologic response and were more likely to be disease free at the time of preliminary analysis. A fourth study,117 sponsored by the Southwest Oncology Group and the GOG, randomized patients after a radical hysterectomy pelvic radiation therapy or pelvic radiation therapy plus chemotherapy if they had high risk factors. The trial found improvement of survival of chemotherapy and radiation therapy compared to radiation therapy alone at 5 years; however, the benefit of the chemotherapy to the radiation therapy in a univariate analysis was less significant in tumors size <2 cm and patients with only one node positive.205 The absolute improvement in 5-year survival for adjuvant chemotherapy in patients with tumors ≤2 cm was only 5% (77% vs 82%), while for those with tumors >2 cm, it was 19% (58% vs 77%). Similarly, the absolute 5-year survival benefit was less evident among patients with one nodal metastasis (79% vs 83%) than when at least two nodes were positive (55% vs 75%).205
During the same interval, the Radiation Therapy Oncology Group (RTOG)186 conducted a trial in which radiotherapy alone was compared with pelvic irradiation (including prophylactic para-aortic irradiation) plus concurrent cisplatin and 5-FU. This study demonstrated a significant improvement in outcome for patients with stages III or IV disease as well as for patients with stages IB2 or II cervical cancer with concurrent chemotherapy.194 An update of this trial, with a median follow-up period of 6.6 years for the surviving patients,206 reported that the OS rate for patients treated with chemotherapy and radiation therapy compared to radiation therapy was still significantly better (67% vs 41% at 8 years, p = < 0.0001) and the conclusion by the authors was that chemotherapy with radiation therapy significantly improved the survival rate of women with locally advanced cervical cancer without increasing the rate of late treatment-related side effects.206 Only one large randomized trial has failed to demonstrate a significant advantage from concurrent cisplatin-based chemotherapy in cervical cancer patients. Pearcey et al.202 published this trial in 2002. The authors suggest that difference in technique could explain the difference between their results and the results of the five earlier trials, although the survival rate in their control arm indicated that the margin for improvement was smaller than that in the earlier trials. This trial was also the smallest of the six trials.
Taken together, the randomized trials provide strong evidence that the addition of concurrent cisplatin-containing chemotherapy to pelvic radiotherapy benefits selected patients with locally advanced cervical cancer. It remains to be resolved whether or not 5-FU plays a significant role. The combination of cisplatin plus FU was tested in four of the trials [RTOG 90-01, GOG 85, GOG 120, and Southwest Oncology Group (SWOG) 8797]. In GOG 120, the frequency of grade 3 and 4 leukopenia was significantly higher in the arm with cisplatin/5-FU and hydroxyurea compared to the arm with cisplatin and hydroxyurea alone, with equal effectiveness. Therefore, it was felt that cisplatin alone was equally effective as cisplatin-5-FU and less toxic, although there has been no adequately powered direct randomized comparison of these two regimens without hydroxyurea.
A meta-analysis of 18 trials207 with concurrent chemotherapy and radiation (4580 patients) in patients with cervical cancer concluded that concomitant chemotherapy and radiotherapy improved overall and PFS and reduced local and distant recurrence in selected patients with cervical cancer. Concomitant chemotherapy and radiation therapy produced a 12% absolute increase in survival with greater evidence of benefit in the trials using platinum-based chemotherapy than in those with nonplatinum-based chemotherapy.207 More recently, a systematic review and meta-analysis based on IPD208has documented a 7% absolute improvement in 5-year survival. The benefits were similar in the trials using platinum and nonplatinum chemoradiotherapy.208 In this analysis, there is a suggestion that the magnitude of benefit with chemoradiotherapy varies according to stage, but not to other patients characteristics, suggesting a greater benefit in patients with early-stage disease compared to stage III and IV disease.208
In 2012, Duenas-Gonzales published a large phase III study that randomized patients with locally advanced cervical cancer to cisplatin plus radiation versus cisplatin plus gemcitabine and radiation therapy.209 Women assigned to treatment with the combination regimen also received additional systemic therapy after chemoradiation was complete. With a median follow-up of 3 years, women who received cisplatin plus gemcitabine had a 32% reduction in disease progression and a higher rate of PFS at 3 years compared with those treated with cisplatin alone (74% vs 65%, respectively). In addition, cisplatin plus gemcitabine resulted in a 32% survival advantage. However, there were methodological problems with follow-up in this study and a second publication by the authors purported no benefit in various subgroups.210 Despite these results, there are reports from other investigators that the combination of cisplatin, gemcitabine, and pelvic irradiation results in unacceptable toxicity211 and presently is not commonly used in the United States.
These studies raise other interesting questions that will undoubtedly be the subjects of future studies. Although North American studies have emphasized cisplatin-containing regimens, investigators in Southeast Asia have reported improved outcome when radiation was combined with epirubicin203 or mitomycin and 5-FU.204 Other drugs that are being studied for their radiosensitizing effects in patients with advanced disease are paclitaxel,212 carboplatin,213 nedaplatin,214 topotecan,215 and multiple biologic response modifiers. RTOG just published results of a phase II study evaluating the combination of bevacizumab with cisplatin in combination with radiation therapy and found results that were promising with 3-years OS, DFS, and LRF of 81.3%, 68.7%, and 23.3%, respectively, in 49 patients.216 Another potential pathway is the inhibition of ribonucleotide reductase (RNR). Elevated RNR levels are associated with an increased risk of an incomplete response to chemoradiation and an increased risk of disease recurrence.217 Therefore, targets that inhibit RNR may be of potential value in combination with RT. One such agent, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, has been evaluated in a single-institution phase 2 trial and with a median follow-up of 20 months; clinical responses were observed in 24 of 25 patients with suggested metabolic complete responses noted in 23 of 24 patients evaluated by PET/CT at 3 months.218 These encouraging results have prompted a larger, multi-institutional, randomized phase II study as well as a possible phase III through NRG. Other potential targets/pathways that are being investigated include EGFR expression and inhibition of poly (adenosine diphosphate-ribose) polymerase (PARP).
Para-aortic metastasis
Metastatic cancer in the para-aortic lymph nodes can be confirmed with fine-needle aspiration, laparoscopy, or extra-peritoneal laparotomy. Laparoscopy and laparotomy, using the extra-peritoneal approach, allows the removal of positive nodes and sampling of other para-aortic nodes, which may enhance control with radiation therapy and help design the treatment field.
The superior boundary of the extended EBRT portal provides a margin of 3 cm above the most cephalad of the positive nodes. The superior boundary limit is the T12 vertebra. Currently, patients with positive pelvic nodes or low common iliac nodes may have the field extended to the L1–L2 interspace.
Patients with para-aortic lymph node involvement can be treated effectively with extended-field irradiation. Five-year survival rates range from 25% to 50%.219, 220 The value of prophylactic extended-field irradiation was tested in two randomized trials and both trials found no advantage in the use of prophylactic para-aortic radiation.221, 222
The role of concurrent chemotherapy with extended-field irradiation has been evaluated in several phase II studies.223 Although, side effects are greater when treatment fields are enlarged, combined therapy may be tolerable if careful consideration is given to the chemotherapy regimen, volume of tissue irradiated, and other factors that might increase the risk of serious toxicity. In conclusion, it is tempting to extrapolate from the results achieved with combinations of pelvic radiation and chemotherapy; however, patients need to be informed that the cost-benefit ratio of concurrent chemotherapy and extended-field irradiation has not been formally tested but this is where IMRT may be a benefit in reducing acute toxicity.
Unsuspected invasive cancer discovered after simple hysterectomy
Sometimes the pathologist discovers unsuspected invasive cervical carcinoma in the tissue specimen in patients who undergo hysterectomy for what is presumed to be a benign pelvic condition. Many factors can lead to such an event.224
Patients with unsuspected invasive cervical carcinoma detected after simple hysterectomy have been classified in five groups according to the amount of disease and presentation: (1) microinvasive cancer, (2) tumor confined to the cervix with negative surgical margins, (3) positive surgical margins but no gross residual tumor, (4) gross residual tumor by clinical examination documented by biopsy, and (5) patients referred for treatment more than 6 months after hysterectomy (usually for recurrent disease).225 The therapy plan is based on the amount of residual disease. Patients with minimal invasion and no residual disease require at most brachytherapy to the vaginal apex; patients with gross disease at the specimen margin require full-intensity therapy. Patients with minimal or no known gross residual disease (groups 1–3) have excellent 5-year survival rates (59–79%), whereas rates for patients with gross residual disease (groups 4 and 5) are poorer (in the range of only 41%).226
Recurrent disease
Prognostic factors
Various clinicopathologic features have been associated with adverse outcomes in patients with recurrent cervical carcinoma in the pelvis; however, the relatively small numbers of patients and the heterogeneity of clinical and treatment parameters in most series preclude detailed statistical analysis of these factors. Two clinical factors commonly correlated with the probability of the success of salvage therapy are the location (central vs side wall involvement) and the size of the recurrent pelvic tumor.227 The presence of nodal disease in conjunction with pelvic relapse portends a dismal outcome; other unfavorable clinical variables include nonsquamous histologies (particularly adenocarcinomas) and a higher FIGO stage at the time of diagnosis of the primary tumor.228 Controversial factors include the interval between primary therapy and relapse and symptomatic versus asymptomatic pelvic failures.172, 228, 229
Radical hysterectomy
In rare, carefully selected patients initially treated with primary radiation therapy, radical hysterectomy for salvage may be a feasible alternative to exenterative surgery. Coleman et al.230 reported 50 patients who underwent radical hysterectomy for persistent or recurrent disease after definitive radiation therapy. The 5- and 10-year survival rates were 72% and 60%, respectively. Severe complications were noted in 64% of patients, and 42% of patients had permanent complications. The authors concluded that radical hysterectomy was an alternative to exenteration in patients with small, centrally recurrent cervical cancer, but that it should be used only in carefully selected patients.230
Pelvic exenteration
Pelvic exenteration is a potentially curative procedure for patients who, following radiation therapy, have a central pelvic recurrence or a new primary tumor in the irradiated field.231–233 Advances in surgical technique, anesthesia, and postoperative care have decreased intraoperative and postoperative complications, and thus have greatly reduced operative mortality.224, 232, 233 Advances in ostomy appliances and care have given patients the opportunity to live a nearly normal life and to be able to meet their personal needs and responsibilities after surgery. The type of exenteration is determined by the anatomical site of the cancer (Figure 19).
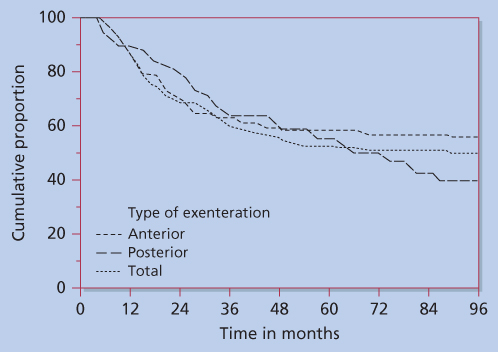
Figure 19 Survival curves are shown to compare the three types of exenterations performed in an MD Anderson series (1955–1984). Although the curves are similar, it should be noted that posterior exenteration is performed more frequently than the other procedures for vulvar and anorectal cancer and is associated with more local cancer recurrences.
Source: Rutledge 1987.231
Anterior exenteration
Anterior exenteration encompasses the removal of the uterus, adnexa, bladder, urethra, and vagina. Patients selected for this operation have cancers that are sufficiently anterior to allow clearance of the rectum and do not extend to involve the vaginal apex or the posterior vaginal wall. Vaginal reconstruction is performed as indicated.
Posterior exenteration
In posterior exenteration, the uterus, adnexa, anus, rectosigmoid colon, levator muscles, and vagina are removed. Many gynecologic oncologists leave a portion of the anterior vaginal wall to support the urethra, and this can lessen postoperative urinary incontinence. This procedure is performed in patients with lesions confined to the posterior vaginal wall and rectovaginal septum.
Total pelvic exenteration
In total pelvic exenteration, the uterus, adnexa, bladder and urethra, vagina, rectosigmoid colon, levatores, and anus are all removed. Patients treated with this procedure usually have lesions that are central or involve the upper half of the vagina. Contiguous extension to the base of the bladder and rectovaginal septum leaves no opportunity to perform a less extensive operation. Vaginal reconstruction is performed for functional purposes and to aid in the reconstruction of the pelvic floor. An omental pedicled graft is required to aid in reconstruction of the pelvic floor, and this technique for bringing in a new blood supply has been a major factor in reducing postoperative complications.234 In both anterior and total pelvic exenteration, a continent urinary conduit can be constructed.
Total pelvic exenteration is widely used, and 5-year survival rates of 40–50% can be expected. At MD Anderson, a total of 448 exenterations were done from 1955 to 1984 and reported by Rutledge.231 The 5-year survival rates for patients treated with exenteration are shown in Figure 19.
Radiation therapy
Patients who have an isolated pelvic recurrence after initially being treated with a radical hysterectomy should be treated with radical radiation therapy. A literature review by Lanciano found disease-free survival rates ranging from 20% to 50% following radiation therapy for locoregional failures. More favorable outcomes were reported in patients with small-volume disease and a central pelvic relapse location.227 In most patients, treatment consisted of EBRT with or without brachytherapy. Patients who have isolated central recurrences without pelvic wall fixation or regional metastasis can be cured in up to 60–70% of cases.229 The prognosis is much poorer when the pelvic wall is involved (usually 10–20% of patients survive 5 years after radiation therapy).
Treatment of stage IVB or recurrent disease
Chemotherapy
Single-agent chemotherapy
Using chemotherapy to treat metastatic or recurrent carcinoma of the cervix is relatively ineffective. Median survival ranges between 4 and 8 months. The most active single agents include cisplatin, paclitaxel, topotecan, vinorelbine, and ifosfamide. Cisplatin is regarded as the most active agent in cancer of the cervix. The response rate (RR) is 17–21%.235, 236 Doses used range from 50 to 100 mg/m2. Bonomi and colleagues compared 50–100 mg/m2 and noted that the higher dose was associated with a better partial RR (31% vs 21%) and a slightly better complete RR (13% vs 10%). Response duration, PFS, and survival measures failed to improve with the higher dose.236 Carboplatin given at 340–400 mg/m2 every 28 days to 175 patients induced a complete response in 10 (5.7%).237 The total RR for carboplatin from a number of studies is 19%.
Paclitaxel has consistently demonstrated clinical activity, even in patients who received prior platin therapy with RRs of 17–31% and median survival of about 7 months, including those patients with nonsquamous histology. The other three—topotecan, vinorelbine, and ifosfamide238–240—have also shown substantial responses and are being used in combination therapy in phases II and III trials.
Combination chemotherapy
Nineteen single agents have activity against cervical cancer, defined as the ability to induce a 15% RR when used as a single-agent (Table 7), but survival rates have not been improved by adding other drugs to cisplatin. Numerous reports describe a higher RR with combination therapies accompanied by an increase in toxicity. Ifosfamide has received the most interest until recently. Several small phase II studies evaluated treatment with combinations of ifosfamide and either cisplatin or carboplatin in patients who had not received radiotherapy. RRs for these combinations ranged between 50% and 62%.241–244
Table 7 Single-agent chemotherapy for cervical cancer
| Agent | Response (%) |
| Alkylating agents | |
| Cyclophosphamide | 38/251 (15) |
| Chlorambucil | 11/44 (25) |
| Melphalan | 4/20 (20) |
| Ifosfamide | 35/157 (22) |
| Mitolactol | 16/55 (29) |
| Heavy metal complexes | |
| Cisplatin | 190/815 (23) |
| Carboplatin | 27/175 (15) |
| Antitumor antibiotics | |
| Doxorubicin | 45/266 (17) |
| Bleomycin | 19/176 (11) |
| Mitomycin | 5/23 (22) |
| Antimetabolites | |
| Fluorouracil | 29/142 (20) |
| Methotrexate | 17/96 (18) |
| Hydroxyurea | 0/14 (0) |
| Plant alkaloids | |
| Vincristine | 10/55 (18) |
| Vinblastine | 2/20 (10) |
| Etoposide | 0/31 (0) |
| Miscellaneous agents | |
| Altretamine | 12/64 (19) |
| Irinotecan (CPT-11) | 36/192 (19) |
| Paclitaxel | 14/74 (19) |
| Docetaxel | 1/13 (8) |
| Topotecan | 8/43 (19) |
| Hexamethylmelamine | 12/64 (19) |
| Razoxane | 5/28 (18) |
Source: Data from Refs 235–240.
Combinations of cisplatin and continuous infusion 5-FU,245–247 cisplatin and paclitaxel,248, 249 cisplatin and vinorelbine,250 and cisplatin and gemcitabine251, 252 also produce high response in previously untreated patients. The combination of carboplatin and liposomal doxorubicin has shown modest activity in this clinical setting.253 Again, RRs decrease significantly if patients have had previous irradiation.246, 252
A GOG-randomized phase III trial reported that combining topotecan with cisplatin had a better RR than cisplatin alone.254 The RR was 13% in the cisplatin arm and 27% in the combination arm. There was also an improvement in median survival by 3 months as well as a slight improvement in quality of life as determined by the functional assessment of cancer therapy-cervical (FACT-CX). They found the previous radiosensitizing chemotherapy and interval from diagnosis predicted for response. Response was more frequent in nonirradiated sites (70% vs 23%). This has led the GOG to their next prospective trial for primary stage IVB or recurrent/persistent carcinoma of the cervix. In this trial, patients were randomized between four arms: arm 1, paclitaxel and cisplatin (PC); arm 2, vinorelbine and cisplatin (VC); arm 3, gemcitabine and cisplatin (GC); and arm 4, topotecan and cisplatin (TC). This study was closed early due to futility. However, in 513 patients, VC, GC, or TC was not superior to PC in terms of OS, but the trend in RR, PFS, and OS favored PC.255 In a separate report, health-related quality of life outcomes for this study showed no difference between the four arms except that PC had a higher rate of neuropathy, but the final conclusion was that PC should be the standard of care for recurrent or metastatic cervical cancer.256
Recently, however, a major step forward in the treatment of patients with recurrent or metastatic cervical cancer was achieved with the result of GOG 240, which randomly assigned patients to treatment with 1 of 2 chemotherapy regimens (cisplatin plus paclitaxel vs paclitaxel plus topotecan) and to bevacizumab versus no bevacizumab.257 There was no difference in outcomes noted between the chemotherapy regimens. However, when compared with chemotherapy alone, the addition of bevacizumab significantly improved OS (17 months vs 13.3 months), PFS (8.2 months vs 5.9 months), and RR (48% vs 36%) without a significant deterioration in health-related quality of life.258 Major treatment-related toxicities included fistula (3%), thromboembolism (8%), and easily managed hypertension (25%).258 Since this publication, cisplatin-paclitaxel-bevacizumab triplet has been listed as Category 2A in the National comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Cervical Cancer.259
Despite the result of GOG 240, the prognosis of patients with recurrent or metastatic cervical cancer remains poor and represents an urgent unmet need worldwide. Therefore, better alternatives need to be explored including determining prognostic factors, administration of novel agents that may improve the therapeutic index of definitive chemoradiation and various immunotherapeutic approaches. Moore et al.260 evaluated 428 patients enrolled in three GOG trials and determined 5 prognostic factors including race, performance status, pelvic disease, prior radiosensitizer, and time interval from diagnosis to the first recurrence <1 years, that may have utility in clinical practice to identify women who are not likely to respond to standard cisplatin-based regimens and that may benefit from investigational trials.
The rationale for immunotherapy in cervical cancer is based on the causative role of HPV infection in the disease. HPV infection invokes cellular immune response, and regulatory T cells appear to play a role in local immune suppression in HPV-associated tumors. New agents that interrupt the mechanisms involved in cancer immune evasion have soon promising results. Basu presented final results of a randomized phase II trial involving 110 women from India with recurrent/refractory cervical cancer who were randomized to receive live attenuated Listeria monocytogenes vaccine with or without cisplatin at the 2014 Annual Meeting of the American Society of Clinical Oncology.261 The final 12-month OS was 36% and 18 month OS was 28%.261 The overall RR was 11% and included 6 complete responders and 6 partial responders. 321The addition of cisplatin did not significantly improve OS or RR.261 These promising results have led to a trial in the United States using the same live-attenuated vaccine under the auspices of the NRG (GOG 265/NCT01266460).
Other pathways including regulatory pathways that limit the immune response to cancer including the upregulation of cytotoxic T lymphocyte-associated molecule-4 (CTLA-4) and PD-1 are increasing being well characterized. In addition, the role of adoptive immunotherapy using tumor-infiltrating lymphocytes represents another potential useful approach for patients with advanced or metastatic disease. In this technology, the tumor is excised and enriched for tumor-infiltrating lymphocytes, which are expanded after selection and reinfused to patients after lymphodepletion. On the basis of the responses noted in tumors such as malignant melanoma, this approach is being evaluated in an ongoing trial at the NCI for patients with recurrent or refractory metastatic cervical cancer (NCT01266460).
Signaling pathways affected by HPV are also an area where novel targeted drugs may be a benefit in the fight against cervical cancer. Genomic data have documented KRAS and P13KCA mutations and are now awaiting proof of concept by demonstration that these pathways are both turned on and necessary for the cervical cancer success. Endpoints including showing purposeful activations of MEK/ERK and AKT proliferation, invasion and survival pathways would provide justification for testing active agents targeting these pathways. Potential new agents include cetuximab, gefitinib, erlotinib, and selumetinib. Irradiation is a major cause of DNA damage. Therefore, inhibition of PARP provides another potential target for the treatment of cervical cancer. Two trials currently are being conducted among patients with cervical cancer through NRG (GOG 127W/NCT0101266447and GOG76HH/NCT01282852).
Summary
In patients with recurrent cervical cancer, it is important first to determine if the patient is a candidate for definitive surgery or radiation therapy. Five-year survival rates range from 20% to 50% if one of these therapies can be administered. Systemic chemotherapy can be used for treatment of both recurrent and metastatic disease, but careful attention should be paid to balancing benefit and toxicity. Further research is needed to determine the impact of chemotherapy on quality of life and the sensitivity to chemotherapy of patients who received prior chemotherapy as part of chemoradiation therapy as well as the importance of biological and immunotherapy agents. The key to success will be the transportation of success seen in the developing countries to success in areas of the world where advanced-stage invasive cervical cancer is most common.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree