Neoplasms of the breast
Hope S. Rugo, MD  Melanie Majure, MD
Melanie Majure, MD  Anthony Dragun, MD
Anthony Dragun, MD  Meredith Buxton, PhD
Meredith Buxton, PhD  Laura Esserman, MD, MBA
Laura Esserman, MD, MBA
Overview
Breast cancer in women remains a major medical problem with significant public health and societal ramifications, including issues related to screening, risk factors, prevention, diagnosis, treatment, and survival following diagnosis. Major advances have markedly improved the understanding of clinical phenotypes, as well as the biologic pathways that drive tumor growth and resistance. This research has led to dramatic changes in treatment that have contributed to a significant reduction in breast cancer mortality over the last two decades, and is the basis of ongoing clinical research. Molecular profiling has provided insights into the heterogeneity of breast cancer subtypes; combining biology and tumor burden has allowed stratification of both risk and treatment to begin the process of individualizing screening, prevention, and treatment. As new information accumulates, new paradigms of management become the standard of care reflected in international guidelines. Our challenge is to apply new formation and treatment appropriately and effectively, and to understand both response and resistance. Information obtained from molecular, biologic, and pathologic investigations and clinical trials provides the major focus of this chapter.
Epidemiology
Breast cancer is the most common malignancy in North American women and in women throughout the industrialized world. In the United States, breast cancer accounts for 29% of all cancers in women. The American Cancer Society (ACS) estimated 231,840 women to be diagnosed with breast cancer in 2015.1
The lifetime risk for a woman being diagnosed with breast cancer is 1 in 8 or 12%.2 Age-specific probabilities of developing breast cancer are provided in Table 1. This risk is even higher for women with certain risk factors, such as a strong family history or known genetic mutations. These figures exclude the 64,640 expected cases of in situ breast cancer. In addition, 2350 men are expected to be diagnosed with breast cancer.
Table 1 Age-specific probabilities of developing invasive breast cancer (cancers diagnosed in females of all races between 2008 and 2010)
| Current age | Probability of developing breast cancer in the next 10 years (%) | Corresponding to a risk of |
| 20 | 0.06 | 1 in 1732 |
| 30 | 0.44 | 1 in 228 |
| 40 | 1.45 | 1 in 69 |
| 50 | 2.31 | 1 in 43 |
| 60 | 3.49 | 1 in 29 |
| 70 | 3.84 | 1 in 26 |
| Lifetime risk | 12.29 | 1 in 8 |
Source: DeSantis 2014.3 Reproduced with permission of Wiley.
The incidence of breast cancer increased about 30% between 1980 and the late 1990s in Western countries, with a marked decline between 2002 and 2003. This increase in diagnosis was attributed to increased screening, as well as to increased use of postmenopausal hormone replacement therapy (HRT) and changes in reproductive factors. In 2002, the first results of the Women’s Health Initiative Trial were published, revealing a significant increase in the risk of breast cancer in postmenopausal women undergoing HRT. These results led to a major decrease in the use of HRT, and correspondingly between 2002 and 2003, breast cancer rates dropped 7%, primarily affecting Caucasian women aged 55 and higher.4, 5 Since 2004, the incidence of breast cancer in the United States has been relatively stable.2, 3 It varies significantly by race and ethnicity, as described in Table 2. However, rates between white and African–American women in the United States are now converging (see below).3
Table 2 Rates by race or ethnicity: United States, 2007–2011
| Non-Hispanic White | African-American | Hispanic-Latino | American Indian/Alaskan Native | Asian/Pacific Islander | |
| Incidence rates (per 100,000) | 127.6 | 123.0 | 86 | 91.7 | 86.0 |
| Mortality rates (per 100,000) | 22.2 | 31.4 | 14.5 | 15.2 | 11.3 |
Source: Siegel 2015.1 Reproduced with permission of Wiley.
Breast cancer is the most frequently diagnosed cancer in women worldwide. In 2012, it was estimated that 1.7 million cases would be diagnosed, accounting for 25% of all cancer cases in women.6 The rates vary by geographic region, with about one-half of the incidences occurring in more developed countries, with generally higher rates found in Northern America, Australia/New Zealand, and Northern and Western Europe. By contrast, the rates are intermediate in Central and Eastern Europe, Latin American, the Caribbean, and Western Asia, and low in most of Africa and Southern and Eastern Asia. The cumulative risk of developing breast cancer in less developed areas is a 3.3% (until age 74) compared to an 8% risk in more developed areas. The variation in international incidence rates is likely due to differences in risk factors as well as the availability of early detection methods.7 The incidence of breast cancer has been increasing in many countries in Asia, South America, and Africa, possibly because of changes in lifestyle, including reproductive patterns, diet, obesity, and physical activity.6, 8
The mortality rate from breast cancer in the United States slowly increased by 0.4% from 1975 to 1990, then decreased by 34% from 1990 to 2010.3 This decrease has been attributed to improvements in treatment as well as early detection,9 with the largest decrease in women below 50 years of age (an annual decrease of 3.1% vs 1.9% in those aged 50 years and higher). Breast cancer is the second leading cause of cancer-related deaths in women in the United States (after lung cancer) and the leading cause of cancer death in those between the ages of 20 and 59. The ACS estimated 40,290 deaths of women from breast cancer in 2015, representing 6.8% of all cancer deaths in the United States. Mortality rates vary by race and ethnicity with African-American women experiencing the highest annual breast cancer death rate, despite having a lower incidence rate than white women. This difference has been attributed to variations in biologic subtype, later stage of disease at diagnosis, and poorer survival by stage, driven in large part by differences in socioeconomic status. Death rates declined in almost all racial and ethnic groups from 2001 to 2010, with a much higher reduction in white than African-American women.
A majority of breast cancers are diagnosed at an early stage, with 61% diagnosed when the disease is localized to the breast; another 32% are diagnosed after the cancer has spread to the regional lymph nodes and only 6% have metastasized at the time of initial diagnosis. Five year survival rates depend on the stage of tumor detection, with 98.6% alive when diagnosed at a localized stage, 84.9% alive when the tumor has spread to regional lymph nodes, and only 26% when diagnosed with distant spread of disease.
Risk factors
Although breast cancer is common, the risk of developing the disease varies depending on a number of factors, with female gender and increasing age being the most important ones. Germline mutations in DNA repair genes markedly increase the lifetime risk of developing breast and other cancers and increase the risk at younger ages. Other lifestyle-related factors have a more modest impact on risk, and the effect of altering modifiable factors on an individual’s risk of developing breast cancer is largely unknown.10 The risk factors associated with the development of breast cancer are clearly defined in Table 3.
Table 3 Risk factors associated with the development of breast cancer
| Major increase |
| Mutations in BRCA1, BRCA2, tp53 (Li–Fraumeni syndrome) |
| Increasing age |
| Developed countries |
| Family history of breast or ovarian cancer in first-degree relatives |
| Atypical hyperplasia, LCIS before the age of 45 |
| Exposure to ionizing radiation |
| Moderate increase |
| Prior diagnosis of breast cancer |
| Early menarche |
| Late menopause |
| Nulliparity or delayed first full-term pregnancy (above age 30) |
| High socioeconomic status |
| Alcohol intake |
| Atypical hyperplasia, LCIS over the age of 45 |
| Obesity (postmenopausal women only) |
| High breast density |
| Diagnosis of soft-tissue sarcoma in son or daughter |
| Prior diagnosis of uterine, ovarian, or colon cancer |
| Modest increase |
| Benign breast disease with hyperplasia (no atypia) |
| Oral contraceptives (for longer than 10 years) |
| Postmenopausal estrogen replacement therapy |
| Questionable increase (no evidence to support) |
| Interrupted first pregnancy |
| High-fat diet |
| Complex fibroadenoma |
| Decrease |
| Full-term pregnancy before age 20 |
| Multiple pregnancies |
| Ovariectomy before age 45 |
| Regular exercise, particularly during adolescence and early adulthood |
| Breast-feeding |
| No effect |
| Breast reduction |
Gender
The incidence rate of age-adjusted breast cancer is more than 100-fold higher in women than in men in the United States, a ratio that is similar worldwide. Male breast cancer represents less than 1% of all cancer in men and about 1% of all breast cancer. Germline mutations in BRCA1 and BRCA2 are the best understood risk factors for breast cancer in men, with a range of lifetime risk of just over 1% (BRCA1) to almost 7% (BRCA2). Worldwide, mortality has decreased less in men than women, and clinical trials have been unsuccessful to date because of low accrual. An international consortium has recently started collaborative clinical trials focusing on male breast cancer.
Age
The median age of breast cancer diagnosis in women in the United States is 61, with the majority of cases diagnosed in women between the ages of 55 and 64. In other parts of the world, where life expectancy is shorter, the median age of development of breast cancer is 10–15 years younger. Age-related mortality rates parallel this pattern.
Socioeconomic class
Breast cancer is diagnosed more frequently in women of higher economic class and educational status.10, 11 This finding is likely related to lifestyle factors such as diet, age at first childbirth, exogenous hormonal use, and alcohol consumption. However, mortality is higher in women of lower socioeconomic classes, correlating with observed differences including higher stage at diagnosis, more aggressive tumor biology, and reduced access to care.
Ethnicity
The incidence and mortality rates of breast cancer vary considerably by ethnicity and race, as outlined in Table 2. In the United States, from 2010 to 2012, the risk of being diagnosed with and dying from breast cancer was 12.64% and 2.66% for whites, 11.14% and 3.26% for blacks, 10.25% and 1.74% for Asian/Pacific Islanders, 9.81% and 2.08% for Hispanics, and 8.15% and 1.66% for American Indians/Alaskans.1 Studies of migrant populations showed that when people living in low-risk geographic areas move to high-risk areas (e.g., a move from Asia to the United States), their incidence of breast cancer increases, approaching the rates of the host population within one to two generations, suggesting an important role of lifestyle in determination of risk even within ethnic and racial groups.12
Family history and genetic mutations
Family history is a significant risk factor for breast cancer, but existing data are complicated by associations established before routine genetic testing (see below).13 In general, compared with a woman with no affected relatives, a single affected first-degree relative approximately doubles the risk. Two first-degree relatives triple the risk, and three or more quadruples the risk. A first-degree relative affected at an early age increases risk further to about threefold, twofold, and 1.5-fold if diagnosed below 40 years, from 40 to 59 years, and from 50 to 60 years of age, respectively. There is little impact from breast cancer diagnosed at older ages unless multiple family members are diagnosed.
Individuals inheriting a germline mutation in either BRCA1 or BRCA2 have a markedly higher lifetime risk of breast and ovarian cancers than the general population. The risk of developing breast cancer is estimated to be 50–85% in women and is often at a younger age of onset.14 There is also an increased risk of second primary breast cancers, estimated to be about 40–60%. These mutations are inherited by autosomal dominant transmission, and more than 2000 different mutations, polymorphisms, and variants have been reported in BRCA1 on chromosome 17 and BRCA2 on chromosome 13.15, 16 There is clearly a higher risk of carrying a mutation in populations with homogeneous ethnicity, such as those with Ashkenazi Jewish heritage, with associated characteristic BRCA1 (185delAG, 5382insC) and BRCA2 (6174delT) mutations; the combined frequency of these genes in the general population exceeds 2%.17–19 Similar “founder” mutations have been identified in Belgium, Denmark, Finland, France, Holland, Hungary, Iceland, Norway, Russia, and West Africa, among other ethnic communities.20 The BRCA genes encode DNA repair enzymes that play a major role in double-stranded DNA repair through the homologous recombination pathway. The resulting defect in DNA repair in cells with dysfunctional BRCA activity has been currently used to design specific therapies such as PARP inhibitors that further damage DNA, leading to tumor cell death (see treatment sections).
Familial breast cancer accounts for less than 10% of all breast cancers, and BRCA1– and BRCA2-related familial breast cancers appear to be responsible for only about two-thirds of these cases. A number of other genes have been identified, which increase the risk of developing breast and other cancers, including TP53 (Li–Fraumeni syndrome), PTEN (Cowden syndrome), ATM, CHECK2 and PALB2, as well as others.21–25 A large number (>75) of more common risk variants (>5%) have been identified by genome-wide association studies (GWAS) over the last decade and validated by large consortia26, 27 that have a moderate impact on risk [relative risk (RR) < 1.5].28 While being individually associated with a modest effect on breast cancer, in combination they can contribute substantially to overall risk.
Interestingly, certain breast cancer phenotypes have been associated with specific mutations, although all phenotypes have been reported. Triple-negative [estrogen receptor (ER) and progesterone receptor (PR) and HER2/neu nonamplified] tumors occur more frequently in women with BRCA1 mutations, hormone receptor-positive (HR+) tumors in those with mutations in BRCA2 or CHEK2, and HER2/neu and HR+ in those with the Li–Fraumeni syndrome.29, 30 Importantly, many of the common risk alleles are more strongly associated with the development of HR+ breast cancer.28, 31 A total of seven variants are associated with the more aggressive hormone receptor-negative (HR−) disease,32 with four new loci recently identified.33 Multigene testing panels are now widely available and have the potential to detect an additional 4% of individuals with potentially deleterious mutations, for whom counseling and testing may be of value.34
The National Comprehensive Cancer Network® (NCCN®) recommends that patients with a personal history of breast cancer and one or more of the following should be tested for germline mutations: A family history of a deleterious mutation in BRCA1 or BRCA2, diagnosis at age ≤45, diagnosis at age ≤50 with an additional primary cancer, ≥1 close blood relative with breast cancer at any age, an unknown or limited family history, or diagnosis at age ≤ 60 with triple-negative breast cancer (TNBC). Additional criteria include patients diagnosed at any age with breast cancer and ≥1 close blood relative with breast cancer diagnosed ≤50 or ovarian cancer, ≥2 close blood relatives with breast cancer or with pancreatic cancer and/or prostate cancer at any age. Patients with a personal history of ovarian cancer, male breast cancer, as well as several other criteria are also included in this recommendation. In general, testing should first be performed on the affected family member with testing for those without a cancer diagnosis reserved for situations when the affected family member is not available for testing.378 One recent series of more than 200 patients with TNBC found BRCA mutations in 15.4%; this rate increased to 18.3% in those meeting NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) for screening; rates vary based on age, ethnicity, and race.35
Additional criteria for genetic screening include diagnosis at any age with breast cancer; a close blood relative with ovarian cancer, pancreatic cancer, or prostate cancer; or any male relative with breast cancer. Those with ethnicities associated with higher mutation frequency should always be considered for testing. Indeed, one trial found that approximately 12% of breast cancers in Ashkenazic women could be attributed to mutations in BRCA1 or BRCA2.17
Identification of germline mutations in women with breast cancer, and in women at risk for mutations, is extremely important. Screening and risk-reducing surgery have been found to reduce mortality from cancer.36–39 Educating people about these strategies as well as the mechanism of identifying tumors is critical.
Endocrine and reproductive risk factors
Duration and extent of exposure to estrogen and progesterone clearly affects the risk of developing breast cancer, regardless of subtype. Longer duration of ovulation, as indicated by earlier age of menarche, and later age at menopause are associated with an annual increase of 3–4% in the risk of breast cancer.11 Younger age at first childbirth decreases the risk of breast cancer about 3% per year from age 30 to 20, with multiparity having a lesser protective effect. Women having their first child at age >30 years have a higher risk of breast cancer than nulliparous women, particularly within the first 5 years after delivery. The possible mechanism for this apparent enhancement in risk might be the stimulatory effect of pregnancy (and its altered hormonal environment) on an otherwise involuting epithelium. Prolonged breast-feeding appears to reduce risk, with short durations providing little impact and with higher protection when lactation is at younger ages.40 In a recent meta-analysis, each birth decreased the RR of breast cancer by 7%, and each year of breast-feeding decreased the RR by an additional 4.3%.41
Exogenous hormones
Although controversial for many years, results from the large Women’s Health Initiative randomized controlled trial provided definitive evidence of the risks associated with postmenopausal HRT with combined estrogen and progesterone. In this study, 16,608 postmenopausal, otherwise healthy, women were randomly assigned to conjugated estrogen plus medroxy progesterone acetate or placebo.42 The combined estrogen and progesterone arm was stopped in July 2002 after a median 5.6 years of follow-up, because health risks exceeded health benefits. There were a 29% increase in coronary heart disease, 26% increase in breast cancer, 41% increase in stroke, and 13% increase in pulmonary embolism associated with HRT. Simultaneously, there were a 47% reduction in colorectal cancer and 34% reduction in hip fractures among women on hormonal replacement. No protective effect was found for memory loss or other measures of intellectual function. Although risk decreased right after stopping HRT, the overall increased risk persisted during long-term follow-up. The Million Women Study and the HERS II study reached similar conclusions.43–45
The United States Preventive Services Task Force (USPSTF) concluded that the harmful effects of combined estrogen and progestin exceed the prevention of chronic disease effects in most women. Although short-term use of HRT might be beneficial for control of vasomotor symptoms related to menopause, long-term use is not indicated.
The use of oral contraceptives has long been associated with a slight increased risk of breast cancer. A recent large study of a US health care delivery system in women between the ages of 20 and 49 years has found increased risk in recent users taking high-dose estrogen preparations, but not in those taking low-dose estrogen contraceptives.46 Risk does not appear to be sustained after cessation of use.
Exercise and obesity
There is convincing evidence that lack of physical activity is a risk factor for postmenopausal breast cancer and that active women have a relative reduction in breast cancer risk.47, 48 This benefit is clearly modified by weight, with little to no benefit from exercise seen in obese women. In the Women’s Health Initiative, women engaging in regular strenuous exercise at age 35 had a 14% relative decrease of risk of breast cancer. Even 1.25–2.5 h per week of brisk walking was associated with an 18% relative decrease in breast cancer risk compared with sedentary women, with a slightly higher risk reduction in those who reported an additional 10 h of brisk walking or equivalent exercise per week.49
A high body mass index (>25 kg/m2), particularly when associated with weight gain after menopause and abdominal obesity, has been clearly associated with an increased risk of postmenopausal breast cancer.10, 50–52 The RR for postmenopausal women is about 1.5 for those with a body mass index > 25 and 2 for those who are obese (body mass index > 30). In the Women’s Health Initiative trials, obesity was also associated with an increased risk of HR+ breast cancer and more advanced disease at diagnosis.53 The data in premenopausal women are more complicated, without a clear relationship between weight and risk of breast cancer.54
Higher physical activity and reduced body mass index have been associated with lower relative levels of estradiol and estrone as well as serum insulin levels, which may in part explain the impact of exercise on breast cancer risk.55–57 The ACS has published guidelines on exercise and nutrition to reduce cancer risk.58, 59 Adherence to these guidelines has been associated with lower risk of breast cancer.54
Metformin and diabetes mellitus
In the Women’s Health Initiative, use of metformin in diabetic women reduced the risk of breast cancer.60 Metformin is known to increase insulin sensitivity and reduce hyperinsulinemia, and hyperinsulinemia has been associated with carcinogenesis and proliferation in preclinical models.61 In addition, signaling through expression of the insulin-like growth factor receptor is related to both proliferation and therapeutic resistance in breast cancer, and metformin may inhibit downstream signaling through the mammalian target of rapamycin (mTOR).62–65 Diabetes mellitus has been associated with a higher risk of breast cancer in some studies, but not in others; however, it is more clearly shown to worse outcome after diagnosis of breast cancer.52, 66–69 Metformin is currently being tested in women with early-stage breast cancer (ESBC) in a multicenter clinical trial.70, 71
Breast density
Breast density is a common and significant risk factor for breast cancer across ethnicities and is inversely associated with age and body mass index.72–76 Numerous state laws require reporting of breast density information to women at the time of screening mammography.75 The highest quartile of breast density appears to have significantly elevated risk, with a 4.5- to 5-fold higher risk than those with the least dense breast tissue. Using the density reported on standard mammography (1, 2, 3, and 4 relating to fatty, scattered fibroglandular, heterogeneous, and homogeneous densities, respectively), studies reproducibly show strong correlation with breast cancer risk. The impact of breast density is modified by individual risk factors, as measured by the Breast Cancer Surveillance Consortium (BCSC) 5-year risk model, where women at moderate or high risk for breast cancer using this model and extremely high density had the highest risk for interval cancer.76 The hazard ratio for interval cancer cases was 1.62 for those with high or very high BCSC 5-year risk and extremely dense breasts, rising to 3.45 in those aged 70–74 years. A risk model has been developed for this purpose combining the BCSC risk model and breast density and benign breast disease (BBD), which can assist in accurately identifying high-risk women who might be eligible for primary prevention.70 The Gail Risk model for estimating 5-year and lifetime risks for breast cancer has also been modified by using a multiplier of breast density and Gail model risk, with a modest impact on breast cancer risk assessment.77, 78 In addition, these patients could possibly be referred for additional imaging, although to date there have been no definitive data that alternate imaging modalities will provide better information and improve cancer detection, stage at diagnosis or breast cancer specific survival.
Alcohol
Numerous studies suggest that alcohol increases the risk of breast cancer. This is thought to be due to increased serum and tissue concentrations of estradiol mediated by the impact of ethanol on hepatic clearance. Alcohol was associated with a modest increase in risk of breast cancer in the Women’s Health Initiative observational study, with the maximum increase observed with the highest daily consumption. This impact appeared to be subtype specific.79 When compared with women who never drink, those who were reported to consume more than seven drinks per week had almost a twofold increased risk of invasive lobular cancer (hazard ratio 1.82 with a 95% confidence interval (CI) of 1.18–2.81), but there was no difference in the risk of invasive ductal cancer, even when both subtypes were HR+.
Radiation exposure
Exposure to ionizing radiation is a known risk factor for breast cancer. Atomic bomb survivors and patients treated in the past with irradiation for postpartum mastitis, acne, hirsutism, or arthritic conditions and repeated fluoroscopic chest radiography used to monitor tuberculosis have an increased incidence of breast cancer, even after low or moderate radiation doses.80, 81 Survivors of Hodgkin’s disease who received radiation therapy to the chest in adolescence or at a young age, particularly when the radiation was combined with chemotherapy, have a marked increase in breast cancer risk.82 The latency period between radiation exposure and development of breast cancer is long, with a median of 30 years although this time is shorter in those treated with both radiation and chemotherapy. The risk of developing breast cancer as a result of common diagnostic radiologic procedures is minimal, and radiology technicians do not have an increased incidence of breast cancer.83 Recent evidence has suggested that therapeutic radiation administered to treat primary breast cancer modestly increases (∼30%) the risk of developing contralateral breast cancer more than 5 years after treatment.84
Benign breast disease
Most forms of BBD appear to be unrelated to an increased breast cancer risk, and the majority of women with lumpy breasts and most of those with BBD do not have a significantly increased risk of breast cancer.
However, a number of studies, including a recent meta-analysis, suggest that the presence (or history) of BBD, particularly in those with a previous biopsy for benign disease, is associated with an increase in breast cancer risk.85 This association is generally limited to biopsy-proven lesions with histologic atypia or proliferation (atypical ductal or lobular hyperplasia) (Table 4).87, 88 When compared with women who never had a breast biopsy, women with BBD without hyperplasia had an odds ratio of developing breast cancer of 1.5, women with hyperplasia without atypia had an odds ratio of 1.9, and women with hyperplasia and atypia had an odds ratio of 2.6–5.3, and this increased to 11 in women with both atypia and family history.86, 89 A study from Mayo Clinic on 9087 women with BBD suggested that the combination of young age (<45 years) and atypia had a hazard ratio of 6.99 for developing breast cancer.88 The importance of identifying this risk factor is the ability to significantly reduce risk using chemoprevention with hormone therapy and to use a risk-based screening approach.87, 88
Table 4 Relative riska of invasive breast carcinoma based on histologic examination of breast tissue without carcinoma
| No increased risk (no proliferative disease) |
| Adenosis |
| Apocrine change |
| Duct ectasia |
| Mild epithelial hyperplasia of usual type |
| Slightly increased risk (1.5–2 times) (proliferative disease without atypia) |
| Hyperplasia of usual type, moderate, or florid |
| Papilloma (probably) |
| Sclerosing adenosis |
| Moderately increased risk (4–5 times) (atypical hyperplasia or borderline lesion) |
| Atypical ductal hyperplasia |
| Atypical lobular hyperplasia |
| High risk (8–10 times) (carcinoma in situ)b |
| Lobular carcinoma in situ—both breasts |
| Ductal carcinoma in situ (noncomedo)—unilateral, local |
a Women in each category are compared with women matched for age who have had no breast biopsies for the risk of invasive breast cancer during the ensuing 10–20 years. These risks are not lifetime risks.
b Only smaller examples of noncomedo ductal carcinoma have consistently been assessed as risk indicators after biopsy only.
Source: Dupont 1985.86
Other
Bisphosphonates
The use of oral bisphosphonates has been associated with a reduced risk of invasive breast cancer, particularly the incidence of HR+ disease.90–92 Osteoporosis may be a marker for lower postmenopausal exposure to estrogen and has been associated with lower breast cancer risk, and hence the possibility of interaction between this variable and the cancer-inhibiting effects of bisphosphonates exists.93, 94 A recent meta-analysis suggested a 15% RR reduction in any breast cancer and 32% RR reduction in invasive disease, with the benefit seen in patients who used bisphosphonates for more than 1 year compared with nonusers.95
Calcium and vitamin D
Although previous studies supported a possible role of vitamin D and calcium in reducing breast cancer incidence, subsequent studies have failed to find an association between risk of breast cancer and dietary intake.96 A large study showed a lower breast density in younger women taking supplemental vitamin D, and another demonstrated a possible impact in women with the highest mammographic breast density.93, 94
Other dietary factors
A diet high in animal fat and low in fruits and vegetables has been associated with a higher risk of breast cancer. However, this is closely related to additional factors such as exercise and body fat. A primary prevention trial was conducted in more than 48,000 postmenopausal women without a history of breast cancer.97 Women in the intervention group were assigned to a diet with low total fat intake (20% of energy) and consumption of five to six servings of vegetables, fruits, and grains daily. At 8 years of follow-up, there was no reduction in invasive breast cancer risk, but a trend toward decreased risk in the most adherent group was shown. Given that this diet is also associated with reduction in a number of other comorbid conditions and that a longer intervention might have more impact, this seems a reasonable and easily applied lifestyle modification.
Breast cancer risk assessment models
Statistical models can be used to estimate a woman’s risk of breast cancer. Some models predict the risk of developing breast cancer framing both short-term and lifetime risk. The models are highly dependent on the age of the person; thus, a very low short-term risk for a young woman may be accompanied by a high lifetime risk. The Gail Risk Assessment model98–100 and the Claus model101 are the most frequently used models to predict a woman’s risk of breast cancer.102 Other models such as BRCAPRO,103 Frank,104 and Couch105 predict the risk or probability of carrying a genetic mutation. These models should be used to guide decisions to perform genetic testing for the presence of cancer-causing mutations in BRCA1 or BRCA2. They do not identify the risk of developing cancer; rather, prediction will depend on the results of the test aiming at determining inherited predisposition for breast cancer. The interpretation of the results of genetic testing depends on knowing whether the proband (person with a cancer) is the one being tested and whether a known cancer-causing mutation is present in the family. Over the past 5 years, new models have emerged that include breast density, exposures, family history, and single nucleotide polymorphisms such as the Breast Cancer Surveillance Consortium model has been validated in over 1 million women.106
Gail model
The Gail Risk Assessment model is the most commonly used statistical model for estimating the risk of developing breast cancer in women undergoing annual screening. Gail and colleagues used data from 284,780 predominantly white women in 28 participating centers of the Breast Cancer Detection Demonstration Project to develop the model. This is an unconditional logistic regression model based on the relationship between risk in a woman with specified risk factors and the risk in a woman with no risk factors. Risk factors used in this model include age, age at menarche, age at first live birth, number of first-degree relatives with breast cancer (mother, sisters, or daughters only), number of breast biopsies, and breast pathology exhibiting atypical hyperplasia.
The Gail model is applicable to the largest number of women. A score of 1.67 (which is the average 5-year Gail score for a 60-year-old woman) was used as the minimum risk criterion to join the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-01 prevention trial in the United States. The Gail Risk model has been currently used widely for clinical decision making for individual patients, with many forms of access (National Cancer Institute’s Web site, handheld, and computer applications). It is good at predicting the risk for a population, but a cutoff of 1.67 does not show high discriminatory power.107 Ozanne and colleagues developed improved methods for incorporating Gail Risk by comparing a woman’s Gail Risk in context with other women. Being able to show women that they are in the highest quartile or decile of risk is much more discriminatory and much more helpful in identifying truly high-risk women.108 A limitation of the model is the treatment of family history: neither paternal family history of breast cancer nor age at onset in affected relatives is accounted for in the model. As described earlier, a multiplier can be used to incorporate breast density with subsequent improvement in predictive performance of the model.74, 109
The BCSC model, a similar tool that incorporates BI-RADS breast density, exhibited a better risk discrimination and calibration than the Gail model in a multiethnic cohort of more than one million American women.74 Neither Gail nor BCSC incorporates modern knowledge of genetic risk factors. The addition of polygenic risk (76 single nucleotide polymorphisms) improved the performance of the BCSC model, including density and polygenic risk. Other models have also been developed to include multiple risk factors, including family history, more comprehensive genetic factors, and exposures.100, 103, 184–186
Hereditary models
Models such as BRCAPRO,101 Frank,102 and Couch103 are designed to predict the chance of carrying a BRCA1 or BRCA2 mutation based on an autosomal dominant pattern of inheritance and the incidence of mutations within high-risk populations, and the BRCAPRO model also predicts the risk for developing breast and ovarian cancer as a separate output. The models are based on multiple variables in both maternal and paternal cancer history, including age of diagnosis, multiple primary cancers, bilateral breast cancer, Ashkenazi Jewish ancestry, and the number of first-, second-, and third-degree relatives with breast cancer. The BRCAPRO model also includes ovarian and pancreatic cancers. This high-risk category rarely occurs.
The hereditary models are used clinically for women with a positive family history of breast cancer and are discriminatory and well calibrated for predicting the presence of a BRCA mutation. It is predicted that at most, 10–15% of diagnosed breast cancers are in women with either BRCA1 or BRCA2 GENE mutations. BRCAPRO performs well in predicting mutations in families of African-American descent similarly to Caucasian families. It has been suspected that approximately 20% of breast cancers are caused by unidentified hereditary factors. Indeed, the use of clinical genetic testing with panels of multiple genes known to be associated with hereditary breast or ovarian cancer is identifying patients who carry a mutation in one of these less common genes. This, in turn, increases the percentage of breast cancers that are related to hereditary factors. The remaining 70% are thought to be sporadic or nonhereditary cancers.
Biomarkers
Germline mutations such as BRCA1 and BRCA2 (BRCA1/2) predict lifetime risk and risk at a younger age. However, there are no tests that are predictive for a specific age of onset, or the short-term risk of developing breast cancer. Understanding individual risk at specific time points will help design effective screening and prevention strategies and will also provide markers for the effectiveness of preventive strategies. These markers would need to be modifiable like risk factors. Potential modifiable risk factors include atypia and breast density. The presence of atypia has also been shown to predict a higher benefit from the use of tamoxifen, with a risk reduction of up to 89% in the NSABP P-1 study.110 Multiple investigators are conducting biomarker studies to determine whether these markers are reliable surrogate markers that change in response to interventions.111 How would these biomarkers then be used to test prevention interventions? Agents such as selective estrogen modifiers or aromatase inhibitors (AIs) that are known to target ER-positive disease can be used to treat women with cytologically documented atypia and the impact of the intervention measured by reduction in atypia. Change in breast density can also be measured over the course of the treatment (6–12 months) and used as a surrogate to better define those populations most likely to benefit from preventive interventions. Other modifiable risks include exercise, diet, and weight. The use of frameworks for communicating risk and shared decision making for risk reduction is critical.112–114
Regulation of breast cancer growth
Understanding the basis for the development and regulation of breast cancer growth provides us with a foundation for developing strategies for breast cancer prevention and treatment. The adult female breast is composed of epithelial lactiferous ducts terminating in secretory alveoli embedded in a fibrous tissue framework and fat. Normal breast growth and development are regulated by the complex interaction of many hormones and growth factors, some of which are secreted by the mammary cells themselves and may have autocrine functions. Estradiol regulates the expression of several genes corresponding to peptides and proteins involved in mammary cell growth control mechanisms. Binding to specific receptors triggers effects of these growth factors and hormones. Polypeptide hormone receptors are typically located on the cell membrane, whereas receptors of the steroid hormone family are found mostly in the nuclear compartment of the cell.115, 116 However, steroid hormone receptors can also be found localized to the cell membrane. The interaction of growth factors, cytokines, and hormones with specific membrane receptors triggers a cascade of intracellular biochemical signals, resulting in the activation and repression of various subsets of genes. Several of these hormones have been shown to play an active role in breast epithelial cell growth and development and in lactation. Because these hormones and their receptors regulate normal breast tissue, it is not surprising that malignant cells arising from breast tissue might also express receptors for many of these hormones and might retain some degree of hormonal dependence.
Genetic aberrations in growth factor signaling pathways, for the most part acquired, are inextricably linked to developmental abnormalities and to a variety of chronic diseases, including cancer. Malignant cells arise as a result of a stepwise progression of genetic events that include the unregulated expression of growth factors or components of their signaling pathways.
Growth regulation of breast cancer cells by hormones and growth factors is shown schematically in Figure 1. The biological role of estrogens is mediated through high-affinity binding to ER by molecules belonging to a family of ligand-inducible nuclear receptors that have steroid and thyroid hormones and vitamins as known ligands.114
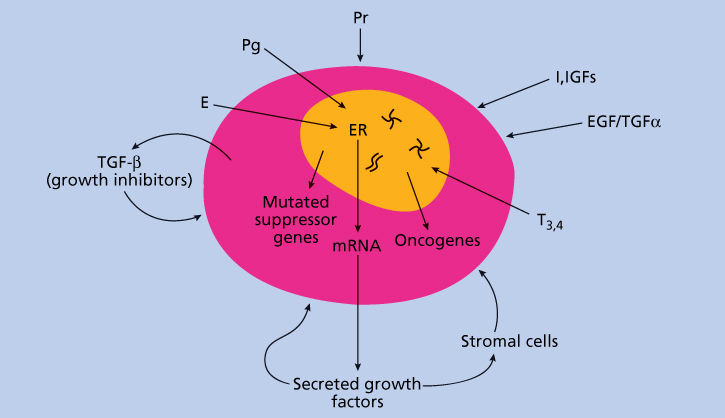
Figure 1 Growth regulation of breast cancer by hormones and growth factors. Abbreviations: E, estrogen; EGF, epidermal growth factors; I, insulin; IGFs, insulin-like growth factors; Pg, progesterone; Pr, prolactin; T3,4, thyroid hormones; TGF-α, transforming growth factor alpha; TGF-β, transforming growth factor beta.
Breast cancer cells under estrogen control can synthesize and secrete their own growth factors that could auto stimulate breast cancer cells or adjacent stromal tissues through autocrine or paracrine mechanisms.117 Aromatase is abundantly expressed in many breast cancers, providing the malignant cell with the ability to synthesize its own major growth factor, estrogen. Stromal tissues may also secrete IGF-1 and IGF-2 that can stimulate breast cancer cells. The identified potential autocrine/paracrine growth factors include epidermal growth factor (EGF), TGF-α, IGF-2, platelet-derived growth factor, and fibroblast growth factor (FGF). EGF, TGF-α, IGF-1, and IGF-2 have been found to be expressed and secreted by cultured breast cancer cells and human breast cancer tissue specimens.118 They are potential mitogens for the epithelial (malignant) component of the tumor. Platelet-derived growth factor and FGF are secreted by breast cancer cells and may be responsible for the proliferation of the mesenchymal stromal component evident in many breast cancers.
Human breast cancer cells also secrete several peptides that may have autocrine inhibitory activity. TGF-β is a family of growth factors that inhibit the proliferation of epithelial tissues and stimulate the proliferation of stromal tissues.50 Studies suggest that ER-negative breast cancer cells are more sensitive to TGF-β than cells expressing ER. The malignant potential of breast cancer is likely to depend, in part, on the balance between growth stimulators and growth inhibitors produced by the tumors. The epithelial and/or stromal cells within the tumor also secrete proteases, such as the cathepsins, stromelysins, gelatinases, or urokinase plasminogen activator, which may participate in tumor invasiveness and metastatic potential.
In ER-positive breast cancer cells, expression and secretion of certain autocrine growth factors, such as TGF-α and IGF-2, are stimulated by estrogen and inhibited by antiestrogens. In ER-negative breast cancer cells, secretion of these factors is not estrogen regulated. Investigators have hypothesized that changes in the expression of these secreted factors may mediate to some extent the growth effects of estrogens and antiestrogens. Estrogens and antiestrogens have a variety of other effects on breast cancer cells. Estrogen stimulates RNA, DNA, protein synthesis, and the activity of key regulatory enzymes. Antiestrogens have the opposite effects in most tissues. Estrogens ultimately regulate movement of the cells through the cell cycle and mitosis.
Disturbance of normal growth control mechanisms within a cell can result in uncontrolled cell division and the development of cancer. Such cellular transformation occurs through the activation of oncogenes, loss or mutation of tumor suppressor genes, or both. The normal counterparts of oncogenes, termed proto-oncogenes, function as growth regulators in normal cells. Alterations of proto-oncogenes are associated with the initiation, promotion, and/or maintenance of tumors in animals and humans. The products of oncogenes are frequently growth factors, growth factor receptors, molecular switching, or transcription factors. Oncogenes often found overexpressed in human breast cancer tissue include members of the myc and ras family (c-myc, Ha-ras-1), int-2, which is involved in mouse (and, presumably, human) mammary gland carcinogenesis, and the members of the epidermal growth factor receptor (EGFR, erbB) family, including erbB-2 (also known as HER2 or neu), HER3, and HER4. Overexpression and mutation of growth factor receptors often lead to constitutive activation of these receptors (i.e., signaling in the absence of their cognate ligands). Growth-promoting signals may be continuously transmitted into the cells, resulting in activation of multiple intracellular signal transduction pathways and unregulated cell growth. Genes normally involved in cell cycle control, particularly members of the cyclin D and E families, may also function as oncogenes. Overexpression of these oncogenes may contribute to the initiation and maintenance of the malignant phenotype. Tissue-specific expression of myc, ras, and HER2 in mammary glands of transgenic mice has been shown to result in an increased incidence of both benign and malignant breast pathology. Altered expression of these otherwise normal genes can have profound effects on growth homeostasis of breast epithelium. Recent studies have shown that blockade of these growth factor receptors or pathways has therapeutic implications.68 Monoclonal antibodies to HER2 have dramatic antitumor effects, and they downregulate the phosphatidyl inositol 3-kinase (PI3K) signaling pathway. Furthermore, these antibodies have synergistic interactions with cytotoxic agents, such as the anthracyclines, the platinum analogs, vinorelbine, and the taxanes. The EGFR, when overexpressed, confers an adverse prognosis to patients with EGFR-overexpressing tumors. However, EGFR does not seem to be a critical driver of malignant behavior in breast cancer, and monoclonal antibodies against this target have had only marginal success in clinical trials.
Quantification of the expression of these oncogenes in human breast cancer specimens has been shown to provide valuable information on tumor aggressiveness, prognosis, and sensitivity to therapy.69 Signaling molecules downstream from the cell surface receptors are often activated or otherwise altered in malignant cells. The PI3K pathway and the MAP kinase pathway are frequently activated in breast cancer, even in the absence of EGFR or HER2 overexpression.
Tumor suppressor genes also play a role in breast carcinogenesis. Loss of the normal “suppressor” function of these genes through mutations or deletion may cause cancer. Alterations in known suppressor genes, such as the retinoblastoma gene (RB1) and the human TP53 gene, have been identified in human breast cancer cells, as well as in other solid tumors. Mutations in the TP53 gene have been found in families with the Li–Fraumeni syndrome, who have a markedly increased incidence of breast cancer and other neoplasms. In addition, up to 50% of breast cancers have been shown to have mutations in the TP53 gene. The two mutated genes associated with familial breast cancer, BRCA1/2, are also considered tumor suppressor genes. The normal function of the protein products of these genes is to control cell proliferation (RB1 and TP53) or facilitate/mediate DNA repair (TP53, BRCA1/2). Mutations lead to mutated proteins and thus to dysregulated transit of cells through the cell cycle. Recognition that mutational inactivation of suppressor genes is associated with breast cancer could lead to early recognition of high-risk families, as well as to new treatment strategies to reverse the malignant phenotype by introducing normal gene copies through gene therapy or by treatment with the normal suppressor protein itself. Such strategies are under active investigation, both in the laboratory and early clinical trials.
Estrogen and progesterone receptors
ERs are members of the nuclear hormone receptor superfamily and have several functional domains. There are two subtypes, with each subtype having several isoforms and splice variants. The ERα (alpha) gene has been mapped to the long arm of chromosome 6 (6q24-q27), whereas the ERβ (beta) gene is located on band q22-24 of chromosome 14. There are at least three PR subtypes. Other estrogen-induced proteins regulate events leading to cell proliferation. When receptors are bound to antiestrogens, such as tamoxifen, transcription of growth-promoting genes is blocked, although other genes might be activated by tamoxifen.
Nuclear localization of ER leads to its genomic effects. Upon binding its ligand, the ER–ligand complex binds to the estrogenresponsive element and initiates transcription of estrogen-driven genes. In its cell membrane localization, ER mediates the nongenomic effects of ligand binding, mostly through cross talk with peptide growth factor receptors (EGFR and HER2). Upon development of antiestrogen resistance, there is marked increase in the nongenomic effects of ER.
The most important application of the ER assay is the selection of appropriate patients for endocrine therapy. Approximately 50–60% of patients with ER-positive tumors benefit from endocrine therapy. This percentage includes patients with metastatic disease who achieve a major objective remission (partial or complete) and those who derive long-term (>6 months) stability of the disease with endocrine therapy; both groups have equivalent survival expectations. The ER status predicts equally well for all modalities of endocrine therapy. Patients with no detectable ER or PR in their tumors do not benefit from endocrine therapy; however, breast cancers with very low but detectable ER and/or PR respond, albeit infrequently, to endocrine therapy (see systemic therapy sections).
Tumor ER and PR status can change over time or with intervening therapy; thus, repeat biopsies of accessible tissue may be helpful in selecting sequential therapies. However, ER status on the primary tumor still predicts reasonably well for endocrine response at the time of relapse. It is not known why 40–50% of ER-positive tumors fail to respond to hormonal therapy despite the presence of receptor. Clearly, an assay that would identify truly hormone-sensitive tumors would be more clinically useful. At least one multigene assay, the Oncotype DX, is being used increasingly in the United States to characterize the risk of developing distant metastases after 5 years of tamoxifen for women with ER-positive disease.119
Variant and/or mutated ERs have been identified in breast cancer tissue.120 Current data suggest that the frequency of mutations in ESR1 increases under pressure, so that it is increasingly common with progression of metastatic diseases. Some of these altered receptors are constitutively active (activate transcription in the absence of estrogen), some are inactive, and some have predominant negative activity. The presence of these mutations has been associated with resistance to some types of endocrine therapy, but response to others.
Pathology
Histologic types
Pathologic classifications of mammary carcinoma are frequently confusing to the individual who is not a specialist in breast disease. Table 5 lists the distribution of various histologic types of invasive breast cancer.
Table 5 Distribution of histologic types of invasive breast cancers 2008–2012, selected
| Histologic type | No. | % |
| Adenocarcinoma | 287,384 | 97.4 |
| Infiltrating duct carcinoma | 216,104 | 73.2 |
| Lobular carcinoma | 26,726 | 9.1 |
| Mixed ductal and lobular carcinoma | 27,371 | 9.3 |
| Inflammatory adenocarcinoma | 1003 | 0.3 |
| Mucinous adenocarcinoma | 5737 | 1.9 |
| Tubular adenocarcinoma | 1850 | 0.6 |
| Papillary adenocarcinoma | 1754 | 0.6 |
| Paget disease | 1259 | 0.4 |
| Other adenocarcinomas | 3336 | 1.1 |
| Adenocarcinoma, NOS | 2176 | 0.7 |
| Epidermoid carcinoma | 145 | 0 |
| Other specific carcinomas | 2415 | 0.8 |
| Medullary adenocarcinoma | 815 | 0.3 |
| Other | 1600 | 0.5 |
| Unspecified, carcinoma, NOS | 3356 | 1.1 |
Abbreviation: NOS, not otherwise specified.
Source: Howlader 2015.2
Epithelial neoplasms of the breast
Tumors arising from ductal epithelium may be found only within the lumen of the ducts of origin; that is, the carcinomas are intraductal and do not penetrate the basement membrane or invade surrounding stroma. Most frequently, such tumors arise from large ducts and may present as several types. If they grow into the ducts with a papillary configuration, they are recognized as papillary carcinomas (Figure 2). Such lesions are rare, accounting for only 1% of breast cancers. Histologically, pleomorphic duct epithelial cells with disturbed polarity can be demonstrated, as can their “heaping up” into papillae. Difficulty may be encountered in differentiating a papillary carcinoma from a benign atypical papilloma. Papillary carcinomas rarely invade the surrounding stroma. A survival rate approaching 100% may be anticipated upon complete excision of such tumors. When these tumors do invade surrounding tissue, they grow rather slowly and attain considerable bulk. Skin and fascial attachments are unusual, and axillary node involvement is a late feature. Clinically, noninvasive tumors are found to be movable, circumscribed lesions that have a soft consistency not unlike that of fibroadenomas.
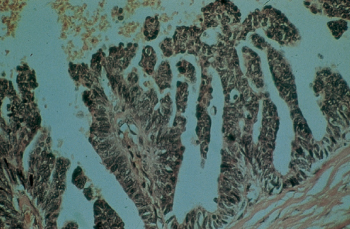
Figure 2 Papillary carcinoma of the breast. This uncommon tumor, <1%, rarely infiltrates and has a favorable prognosis.
The noninvasive variety of ductal carcinoma, referred to as intraductal carcinoma or ductal carcinoma in situ (DCIS), is a proliferation of a subgroup of epithelial cells confined to the mammary ducts without light microscopic evidence of invasion through the basement membrane into the stroma. The histologic diagnosis of DCIS poses certain problems. It is often difficult to distinguish between benign but highly atypical hyperplasia and DCIS, and it is sometimes difficult to identify small foci of stromal invasion. Occasionally, it is difficult to distinguish between DCIS and lobular carcinoma in situ (LCIS), as the former may extend into breast lobules and the latter may involve extralobular ducts. Some lesions may be intermediate between the two. A variety of histologic patterns of DCIS has been recognized. The most frequently encountered are comedo, cribriform, solid, papillary, and micropapillary (Figure 3). The different histologic patterns have been associated with differences in biologic behavior. The proliferative rate has been found to vary according to the histologic characteristics of DCIS. A high proliferative rate has been observed with comedo DCIS, and a low proliferative rate with cribriform, papillary, and solid DCIS. A type of carcinoma known as comedocarcinoma is characterized by ducts that are dilated and filled with carcinoma cells. These are necrotic and can be expressed as semisolid necrotic plugs. Such cancers are not usually regarded as a separate cell type, but they rather represent a descriptive variant of intraductal carcinoma. Patients whose DCIS exhibits comedo features have been shown to have increased rates of local recurrence and may progress more rapidly to invasive breast cancer than other types (Figure 4). Human EGF-receptor 2 (HER2/neu or HER2) protein overexpression has been observed in solid and comedo types of DCIS, but not in papillary or cribriform types.
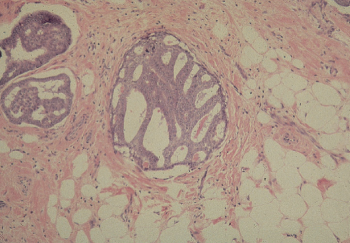
Figure 3 Ductal carcinoma in situ (DCIS), cribriform type. Duct spaces are completely involved by a proliferation of ductal cells with relatively uniform nuclei, arranged in back-to-back (cribriform) glands. The glands are almost uniform in size and shape and exhibit rigid inner borders (so-called cookie-cutter appearance).
Source: Courtesy of Dr. Ira Bleiweiss, Mount Sinai School of Medicine.
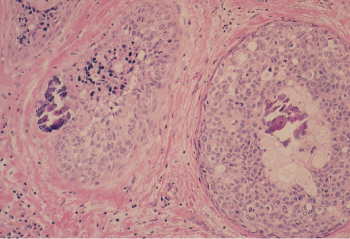
Figure 4 Ductal carcinoma in situ (DCIS), comedo type. Two duct spaces contain tumor cells with high nuclear grade, focal necrosis, and calcifications. The combination of high-grade nuclei and central necrosis is diagnostic of comedocarcinoma.
Source: Courtesy of Dr. Ira Bleiweiss, Mount Sinai School of Medicine.
Lobular carcinoma arises from the small end ducts of the breast. The noninvasive variety—the so-called LCIS—is characterized by small cells of low nuclear grade that fill and expand lobules without penetration of the basement membrane (Figure 5). When this lesion extends beyond the boundary of the lobule or terminal duct from which it arises, it is known as invasive lobular carcinoma. Often the small cells interdigitate between collagen bundles in a single line, the so-called “Indian file.” At other times, lobular carcinoma may be almost indistinguishable from the conventional invasive ductal carcinoma (Figure 6). Noninvasive mammary carcinomas comprise almost 22% of all neoplastic lesions of the female breast, and LCIS accounts for about 60% of these, or 12% of all tumors. Whereas DCIS often accompanies invasive ductal carcinoma, and may well be its usual precursor, LCIS may be followed by invasive ductal or invasive lobular carcinomas in either breast. Thus, LCIS is more a systemic marker than a local precursor. With the increased use of mammography, a much higher proportion of noninvasive cancers is being detected.
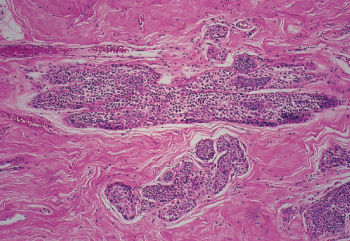
Figure 5 Lobular carcinoma in situ (LCIS). Terminal ducts and acini are completely filled and dilated by a uniform small cell proliferation.
Source: Courtesy of Dr. Ira Bleiweiss, Mount Sinai School of Medicine.
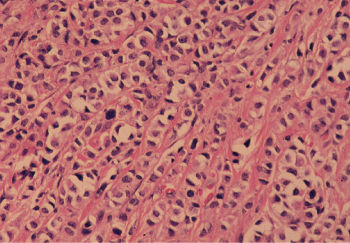
Figure 6 Infiltrating lobular carcinoma. Tumor cells with relatively uniform nuclei invade in a single file or linear pattern (so-called Indian file).
Source: Courtesy of Dr. Ira Bleiweiss, Mount Sinai School of Medicine.
Invasive ductal carcinomas in which no special type of histologic structure is recognized are designated “not otherwise specified” (NOS) and are the most common duct tumors, accounting for almost 80% of breast cancers (Figure 7). They are characterized clinically by their stony hardness to palpation. When they are transected, a gritty resistance is encountered, and the tumor retracts below the cut surface. Yellowish chalky streaks that represent necrotic foci are observed. Histologically, varying degrees of fibrotic response are present. They frequently metastasize to axillary lymph nodes, and their prognosis is the poorest of the various tumor types. More than half (52.6%) of breast cancers are pure invasive ductal lesions (NOS).
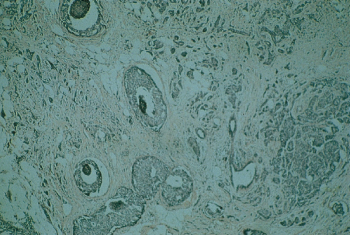
Figure 7 Infiltrating ductal carcinoma of the breast, not otherwise specified (NOS). Approximately 80% of breast cancers exhibit this histology, about one-third of the time with additional types of differentiation.
Several other types of invasive carcinomas arise from large ducts, and each has its own distinct histopathologic image. Medullary carcinoma, comprising 3–6% of all mammary carcinomas, often attains large dimensions (Figure 8). This tumor is formed by cells of relatively high nuclear grade, and it usually exhibits an extensive infiltration of the tumor by small lymphocytes. Medullary carcinomas have a relatively well-circumscribed border, sometimes described as a “pushing” border, in contrast to the NOS tumors in which small nests of cells tend to infiltrate the adjacent stroma more extensively. A study of medullary cancer using 336 typical and 273 atypical medullary breast cancers from 6404 patients enrolled in various stage I and stage II NSABP trials indicated that the survival of patients with typical medullary cancers was higher than those with NOS invasive ductal carcinomas. Survival was comparable for those with atypical medullary and NOS types.121
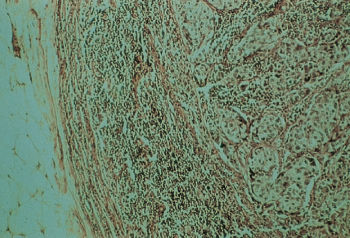
Figure 8 Medullary carcinoma of the breast accounts for approximately 5–7% of breast cancers. Despite its relatively poor differentiation, this tumor has a better prognosis than does infiltrating ductal carcinoma.
Tubular carcinoma is an invasive carcinoma in which tubule formation is highly prominent. This tumor represents 1–2% of breast cancers and has a low nuclear grade with some cell polarity (Figure 9). Its prognosis is favorable, and, when combined with small size, it is a curable tumor.
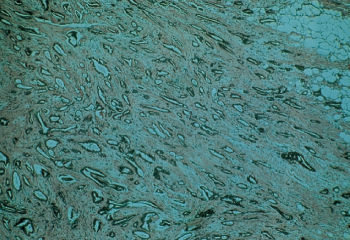
Figure 9 Tubular carcinoma of the breast. This tumor is rare in its pure form, <1%, but has a better prognosis than infiltrating duct carcinoma not otherwise specified (NOS). Partial tubular differentiation is seen in 20% of infiltrating duct carcinomas NOS.
Mucinous or colloid carcinoma, which comprises about 1–2% of all mammary carcinomas, is characterized on microscopy by nests and strands of epithelial cells floating in a mucinous matrix. It usually grows slowly and can reach bulky proportions. When the tumor is predominantly mucinous, the prognosis tends to be good (Figure 10).
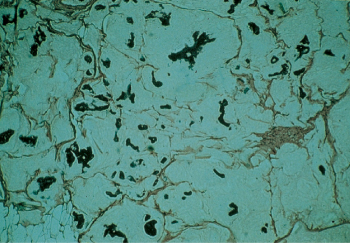
Figure 10 Mucinous or colloid carcinoma of the breast. This tumor is uncommon (∼2%), but has a rather favorable prognosis.
Two entities represent special manifestations of mammary carcinoma. Paget disease of breast occurs in 1–4% of all patients with breast cancer. Clinically, the patient presents with a relatively long history of eczematoid changes in the nipple, with itching, burning, oozing, and/or bleeding. The nipple changes are associated with an underlying carcinoma in the breast that can be palpated in about two-thirds of the patients. The subjacent tumor may be either intraductal or of the invasive duct type. Prognosis is related to the invasiveness and histologic type of the associated tumor. Histologically, the nipple epithelium contains nests of carcinoma cells.
Inflammatory breast cancer (IBC), or “dermal lymphatic carcinomatosis” of the breast, is characterized clinically by skin redness, warmth, edema (peau d’orange), visible erysipeloid margin, induration of the underlying breast, and rapid evolution, usually less than 3 months from first sign to diagnosis. These features must be present at the time of primary diagnosis. Biopsies of the erythematous areas and adjacent normal-appearing skin often but not always reveal poorly differentiated cancer cells filling and obstructing the subdermal lymphatics. Inflammatory cells are rarely present. Patients typically have signs of advanced cancer, including palpable axillary nodes, supraclavicular nodes, and/or distant metastases. IBC represents about 1–2% of breast cancers in the United States and Western Europe, although its incidence is reportedly higher in North Africa and the Middle East.
Several other histologic types of mammary carcinomas have been described but are rarely (<1%) encountered. Adenocystic carcinoma, carcinosarcomas, pure squamous cell carcinoma, basal cell carcinomas, and the so-called lipid-rich carcinomas have been observed. Because of their rarity, clinical correlates are practically nonexistent. Metaplastic cancers, previously rare, are increasingly recognized as a distinct and poor prognosis subset of TNBCs.
Nonepithelial neoplasms of the breast
A variety of nonepithelial neoplasms of the breast have been described. Fibrosarcomas, leiomyosarcomas, rhabdomyosarcomas, and angiosarcomas are all infrequent.122 Non-Hodgkin lymphomas can have their initial onset in the breast and can also occur as a focus of generalized disease. These usually have a B-cell phenotype. Some cases resemble carcinoma on routine histology; immunohistochemistry (IHC) is often helpful in resolving this issue. Only a few cases of Hodgkin disease and leukemia have been reported with initial manifestation in the breast.
Phyllodes tumor (cystosarcoma phyllodes) is a rare biphasic neoplasm that is partially epithelial and partially mesenchymal (Figure 11). Phyllodes tumors may achieve a large size and, not infrequently, demonstrate some invasion of adjacent breast tissue. They are best managed by local excision with a rim of normal breast tissue. Phyllodes tumors are classified as benign, borderline or indeterminate, and malignant. Malignant phyllodes tumors can metastasize and are highly resistant to therapy, with no known effective therapy. High mitotic rate, cellular atypia, stromal overgrowth, infiltrative margins, hemorrhage, and necrosis are considered features of malignancy; however, these findings are extremely rare. Although it is difficult to determine from clinical or histologic appearance the tumors that behave malignantly and metastasize, malignant histology and stromal overgrowth are predictive of metastasis.123
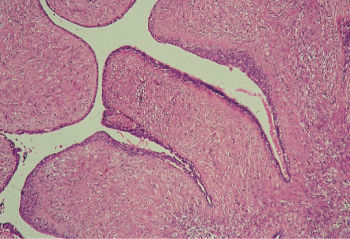
Figure 11 Phyllodes tumor (cystosarcoma phyllodes). Leaf like projections, lined by benign epithelium, contain a hypercellular stroma.
Prognostic factors
Tumor size
In addition to being a determinant for optimal local therapy, tumor size has prognostic significance in the determination of additional therapy. As the size of the tumor increases, the risk of recurrence (ROR) or metastasis also increases, for both lymph node-negative and node-positive tumors. Because the risk of treatment failure is already high for patients with node-positive breast cancer, increasing tumor size adds relatively little prognostic value. However, tumor size is often the main prognostic indicator in node-negative breast cancer. This variable is particularly important to decide whether to use or not adjuvant systemic therapy in patients with node-negative breast cancer. Tumor size refers only to the invasive component and hence should be determined in all three dimensions by the pathologist. Approximately 25–30% of patients with negative lymph nodes and a primary tumor less than 2 cm in diameter will experience a recurrence within 20 years of follow-up.124 Patients with tumors ≤1 cm in diameter have an excellent prognosis, with <15% recurring at 10 years without effective adjuvant therapy. The largest database demonstrating the relationship among tumor size, lymph node status, and breast cancer survival comes from the Surveillance, Epidemiology, and End Results (SEER) program.1 Less than 2% of patients with tumors under 1 cm and negative nodes died of breast cancer within 5 years, and only 4% at 10 years.125 Considering the excellent prognosis for this group of patients with very small tumors, as well as the expense and toxicity of treatment, routine use of chemotherapy is not indicated. The combination of poor nuclear grade and lymphatic vessel invasion identifies a small subset (∼10%) of patients with T1a, b N0 M0 breast cancer with a significant risk of relapse, up to 30%, that warrants systemic adjuvant therapy.126
Axillary lymph node involvement
Involvement of the ipsilateral axillary lymph nodes is still the most reliable and reproducible prognostic indicator for primary breast cancer. In general, 50–70% of patients with positive lymph nodes have a relapse, whereas only 15–45% of patients with all lymph nodes negative for metastatic disease have a relapse after locoregional treatments only. The risk of tumor recurrence in a patient with primary breast cancer is a continuum related to the number of positive axillary lymph nodes.127 With each additional positive lymph node found, the ROR and metastasis increase by a few percentage points. Thus, patients with 4–10 positive lymph nodes have a higher risk than those with one to three positive nodes, and those with 10 or more positive nodes have a higher risk than 80% probability of recurrence and metastasis.
Both macro- and micrometastases within the lymph nodes have prognostic significance.128 Recent data suggest that micrometastases (N1mic) result in an intermediate risk compared with macrometastases, although this remains controversial.129, 130 By contrast, isolated tumor cells (ITC, <0.2 mm) appear to be without prognostic significance.131
In recent years, primary breast cancer has been diagnosed in earlier and mostly localized stages. A classic axillary lymph node dissection (ALND) has no therapeutic benefit for patients with a node-negative axilla and is associated with considerable short- and long-term morbidity. An alternative (diagnostic) staging procedure for these patients is the sentinel lymph node biopsy (SLNB)132 that markedly limits the extent of the surgical procedure in the axilla and, for the high majority of patients with negative axillary lymph nodes, precludes the need for formal axillary dissection while providing similar (and in some cases superior) diagnostic and prognostic information. The identification of a single (or only a few) sentinel node also permits the pathologist to perform a more detailed assessment to detect micrometastases by combining light microscopy, IHC, and even more sensitive molecular techniques. The finding of ITCs does not convey prognostic impact and should not be used to direct either local or systemic therapy. Randomized trials comparing axillary dissection with SLNB have demonstrated that the latter is associated with significantly reduced morbidity. Because of these results, SLNB has replaced classic axillary dissection for early localized breast cancer in a substantial percentage of patients with clinically negative axillae.
Although axillary lymph node status is still the most powerful prognostic indicator, 15–45% of patients whose lymph nodes do not contain metastases still experience a recurrence and die. Because of this limitation, other prognostic markers have been developed to improve prognostic accuracy, particularly in the group of patients with node-negative tumors. Molecular tests based on gene expression suggest that biology of the tumor may be more important than its stage. Women with node-positive breast cancer who were determined to be of low risk based on the MP gene assay had excellent recurrence-free survival regardless of whether chemotherapy was administered or not. This contrasted with the recurrence-free survival of those with a high-risk score, whose outcome was significantly poorer, but appeared to be moderated by chemotherapy.133 A similar finding has been seen with the Oncotype DX gene assay.134 This will continue to be an important area of research, and molecular analysis of tumors is already a major tool for determining therapy.
Histologic type
Several histologic variables have been reported to have prognostic significance.120 The prognoses of ductal and lobular carcinomas are sufficiently similar to prompt the same treatment modalities. Several less common cancers, including pure tubular carcinoma, mucinous or colloid carcinoma, papillary carcinoma, and all noninvasive breast cancers, have substantially better prognoses, particularly when found in a node-negative stage.135 The more favorable prognosis of these histologic types often justifies omission of adjuvant systemic treatment, particularly for small tumors (<3 cm). Because most of these special types have small dimensions when diagnosed, and are node negative, regional treatment is usually all that is required.
Histologic grade or differentiation
Tumor grade has been shown to be an important prognostic indicator. In general, tumors expressing features that indicate a high degree of tumor differentiation are associated with the most favorable prognosis. Multiple studies have shown that higher grade is associated with higher rates of recurrence and metastases and poorer survival. These correlations are independent of tumor size and lymph node involvement. High tumor grade is also associated with HR− and increased response to cytotoxic therapy. Conversely, low grade is associated with hormonal sensitivity and lower response to chemotherapy.
The clear definition of various histologic differentiation grades led to the recognition that those grades had reproducible prognostic significance. A similar finding can be observed for nuclear grade, although some find that histologic grade is a more reliable prognostic indicator as it includes cellular and tissue-related criteria. Nuclear grade can be determined in cytologic specimens.
The most frequently used grading system is the Elston–Ellis modification of the Scarff–Bloom–Richardson system.136 In this system, invasive ductal breast cancers are categorized into three histologic grades, depending on their degree of tubular and/or gland formation, cellular pleomorphism, and the number of mitoses per high-power microscopic field. Within each of these categories, a score of 1–3 is assigned, with 1 representing the most favorable findings (e.g., prominent gland formation, little cellular pleomorphism, and low mitotic rate) and 3 indicating the least favorable ones. The scores for each of these categories are added together. Grade 1 carcinoma (well differentiated or low grade) is defined as having a total of 3–5 points, grade II (moderately differentiated or intermediate grade) 6–7 points, and grade 3 (poorly differentiated or high grade) 8–9 points.
Tumor necrosis
Tumor necrosis of varying degrees was encountered in 60% of 1539 patients with invasive breast cancer in NSABP protocol B-04. Necrosis, particularly when observed to be of marked degree, was positively correlated with increased rates of treatment failure. Although necrosis was observed to be significantly associated with a number of clinical and histopathologic features purportedly related to worse prognosis in this disease, it was not correlated with pathologic nodal status, and multivariate analysis revealed it to influence treatment failure independently of tumor size in lesions less than 5 cm in their highest diameter. It is likely that tumor necrosis is a marker of proliferation and not a unique prognostic factor.
Lymphatic and blood vessel invasion
Lymphatic and blood vessel invasion has been associated with poor prognosis in numerous clinical reports. One-third of NSABP patients exhibited extension into lymphatics within the predominant mass, and the remaining 23% were considered questionable (Figure 12). Such a finding was associated with other unfavorable characteristics. Blood vessel invasion was observed in only 5% of patients and was associated with the finding of four or more positive axillary nodes, lymphatic invasion, and certain other unfavorable findings (Figure 13).
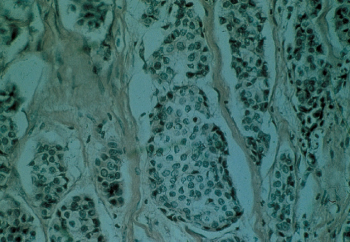
Figure 12 Lymphatic invasion by breast cancer. The vessel walls are thin and lined with endothelial cells.
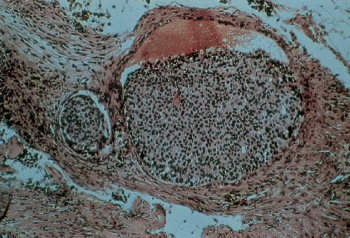
Figure 13 Blood vessel invasion by breast cancer. The vessel wall structure is recognizable, together with erythrocytes in the vessel.
Multicentricity
Many breast cancers are multicentric in origin. In an examination of 904 NSABP cases, either invasive or noninvasive cancers regarded as independent were found in 13.4% patients. The frequency of invasive and noninvasive multicentric cancers was 4.1% and 9.3%, respectively. Increased utilization of magnetic resonance imaging (MRI) of the breast for preoperative assessment of the extent of disease indicated that multicentricity is more common than that it was previously determined by mammography.
Despite the significant incidence of multifocal lesions in both breasts in a woman with a primary breast cancer, two or more clinically overt primary cancers in the primary breast are uncommon. Similarly, synchronous bilateral tumors are uncommon, and the incidence of a second asynchronous primary tumor in the uninvolved or opposite breast (∼4–6% in 10 years) fails to approach the incidence predicted by the number of occult lesions detected by random biopsy, autopsy, or MRI.
Markers of proliferative capacity
Measurement of the proliferation rates of malignant tissues found high prognostic values for several types of cancer, including breast cancer. Several techniques are used to evaluate the proliferative capacity of the malignant cell, including mitotic indices, thymidine-labeling indices (TLIs) and S-phase fraction (SPF). The mitotic index is determined by counting mitotic figures using light microscopy on a tumor specimen stained with hematoxylin and eosin. It has been validated by both univariate and multivariate analyses. Many proteins play a role in the control of the cell cycle or are expressed at higher levels during certain phases of the cell cycle. Ki-67 and proliferating cell nuclear antigen (PCNA) are additional markers for the proliferation rate of malignant tumors.92, 93 Of these, Ki-67 has been more extensively studied, and it correlates strongly with the results of SPF determination and, therefore, long-term prognosis. This technique can be performed on fresh or frozen tissues and archival paraffin-embedded material. A low value indicates a more slowly proliferating tumor and is associated with a lower rate of recurrence, regardless of axillary nodal status. A high Ki-67 fraction is strongly correlated with other adverse prognostic factors, such as high histologic and cytologic grades, aneuploidy, and a negative steroid receptor status. Not surprisingly, the predictive molecular assays that have emerged are driven in part by genes that regulate proliferation.
Immunologic factors
Tumor-infiltrating lymphocytes137 have been associated with improved outcome in aggressive breast cancer subtypes, such as TNBC. Preliminary data demonstrating efficacy of immune checkpoint inhibitors in breast cancer have highlighted the need to better understand the individual immune environment in individual tumor cases.
Diagnosis and screening
Historically, the primary presenting symptom of breast cancer was a palpable mass, often first detected by the patient. At present, the increasing use of mammography, particularly in screening programs, has resulted in many cancers being found at a preclinical stage. A simple discussion of the signs and symptoms of breast cancer without consideration of these preclinical manifestations would be incomplete. To some extent, this indicates a higher complexity in selecting for biopsy patients who are suspected of having carcinoma. The clinical and mammographic signs and symptoms are best understood against the background knowledge of the anatomy and biology of breast cancer—how it grows and extends locally.
Patient history
The patient’s history should include standard epidemiologic and reproductive information to assess the RR factors. Information about lumps, pain, or any changes in the breast should be obtained and correlated with physical findings. Although pain is probably the most frequent breast complaint, it is uncommonly the presenting factor in cancer. Breast cancer, particularly in its early stages, is usually painless. Most breast pain is related to hormone stimulation and swelling of breast tissue (although these symptoms may draw attention to a mass that proves to be cancer). Careful questioning of the patient usually reveals that the pain is cyclic, beginning any time between ovulation and the onset of menstruation, and that commonly it is most intense a few days before menstruation. Pain usually disappears in the first or second day of the menstrual period, only to return in the next cycle. Cyclic pain is present at a mild level in more than 50% of women of childbearing age. Less frequently, the pain can reach intense proportions. Some patients report that, during the worst days, it is too painful even to take a shower.
The most effective treatment is explanation and reassurance, although some patients who are extremely symptomatic and incapacitated by the pain may require treatments with hormones or hormone-blocking drugs. There are occasional reports that caffeine limitation or low-fat diets help, but relief seems to be individual, and these reports are not supported by persuasive clinical trials.
A patient who reports a lump or any other physical change in her breast needs careful attention. The history should describe any change in the character or size of the lump and whether or not it has been tender. Pain should be described with respect to its timing in the menstrual cycle. Lumpy changes associated with a fibrocystic process may wax and wane, but it is distinctly unusual for a carcinoma to do anything but increase in size. If the physician is unsure, the patient should be reexamined after the menstrual period.
Other descriptive changes, such as skin thickening or discoloration, the presence of axillary masses, or nipple discharge, should be elicited. Nipple discharge may be serous, watery, or milk-like. It may be clear or have a yellow or greenish hue, or it may be sero sanguineous or bloody. Although the latter may indicate a neoplasm, this is most commonly an intraductal papilloma, which is benign. It is possible, but rare, for such a discharge to signal an intraductal papillary carcinoma; all bloody discharges require further investigation.
Clear or serous discharge, particularly if it involves more than one major duct opening on a nipple, is likely to be benign. Nonbloody discharge that is not spontaneous but requires manual compression to elicit is also likely to be benign. In an apocrine system such as the breast, there are always some cell desquamation and liquefaction and, therefore, some fluid present in the duct system. If this is not well absorbed, it can make its way through the collecting ducts to the nipple and present as a discharge. Similarly, if the duct is blocked by fibrosis or inspissated material, the pressure of secretion can cause dilation and cyst formation. Cytologic examination of the discharge is less accurate and not very useful.
Physical examination
The patient should be examined, first in a sitting and then in a supine position. When the patient is sitting erect, more useful information is obtained visually than by palpation. When the arms are raised and stretched upward, the contour of the skin is pulled tight, allowing for easier detection of contour abnormalities in the upper half of the breast. This position also emphasizes dimpling, particularly in the lower half of the breast. Because much of the breast tissue coalesces in the sitting position, it is very difficult when palpating to appreciate true masses and often easy to be confused by confluent tissue. The axilla is palpated by relaxing and adducting the patient’s arm, but this is best done with the patient in the sitting position.
With the patient supine and the arm raised such that the hand is behind the head and the elbow lies flat on the pillow, the breast tissue can be spread across the chest wall, allowing for proper palpation. The patient should be slightly turned to the contralateral side to aid this process. It is important to proceed in a pattern, but whether it be performed by quadrants or strips is up to the examiner. Skin changes such as dimpling, peau d’orange (edema), erythema, or areas of fixation and ulceration suggest advanced cancer that has invaded the skin or the immediate subcutaneous tissue. Skin retraction is often more easily detected when the patient is sitting with the arms raised or when the patient is leaning forward. Retraction or asymmetry of the nipple is another worrisome sign unless the patient reports that this has been present all her life. A subtle reddish thickening of the nipple and areola or flaking of the superficial epithelium may suggest Paget disease.
The examination is concluded with a search for axillary, infraclavicular, and supraclavicular nodes and palpation of the liver to detect enlargement. Although palpably enlarged axillary nodes increase the probability of metastases, careful studies have shown that clinical judgment is highly inaccurate. In a study conducted by the NSABP, a group of cancer patients were judged by their clinicians to have normal axillary nodes, where 38% showed histologic evidence of metastatic tumor when the specimens were examined pathologically.120 Conversely, in 25% of such cases, nodes that appeared enlarged and were judged to contain cancer were found to be normal. Evaluation should include axillary ultrasound, with a fine-needle aspiration (FNA) of abnormal-appearing nodes.
The most difficult clinical decision is differentiating between a pathologic mass and physiologic density associated with fibroglandular (or fibrocystic) changes. A true lump has definite margins. Whether these are smooth, as in a gross cyst or fibroadenoma, or somewhat irregular, as in carcinoma, they delineate a discrete mass that requires further investigation. Invasive ductal carcinomas are usually hard, and predominant in the breast, in contrast to multiple firm masses that may exist in BBD. Lobular carcinomas are not usually so hard and thus are more difficult to recognize.
It is important to measure the size of a tumor so that subsequent examinations can more accurately establish any change in size. Advanced cancers may be fixed, but early palpable lesions will certainly be mobile with respect to skin or fascia and muscle of the chest wall. There is, however, a subtle difference in mobility (better called “movability”) characteristic of cysts or fibroadenomas, which have capsules and move much more easily within the surrounding breast tissue. Carcinoma, on the contrary, which has no capsule and is surrounded by an infiltrating desmoplastic process, tends to move with the neighboring breast tissue rather than within it, because the process “locks” it into the stroma and surrounding glandular tissues, even when it is not fixed to surrounding structures, such as the skin or muscle. Even an experienced examiner could fail to distinguish correctly between benign and malignant lesions.
Imaging
For almost 30 years, annual mammograms for women over 40 years of age have been a cornerstone of the strategy to reduce mortality from breast cancer. Advances in our understanding of breast cancer biology, and screening in general, have led to revise and improve our screening strategy.138 In 2009, the USPSTF introduced changes to screening guidelines, recommending that annual mammograms for all women of age 40–75 years be replaced by biennial screening for women of age 50–75 and that screening between 40 and 50 years of age should be individualized by considering patient context, including the patient’s values regarding specific benefits and harms. The 2015 update further supported these recommendations (http://www.uspreventiveservicestaskforce.org/). The ACS revised its mammography screening guidelines in October 2015.139 Screening was still strongly recommended, but the age at its start was modified. Annual screening was recommended for women aged 45 between 54 years, and women of age ≥ 55 years could transition to screen every other year. However, the ACS also noted that women should have the opportunity of annual screening and should continue to screen as long as they have a life expectancy of at least 10 years or more. It also recommended that women between the 40 and 44 years of age should have the opportunity of annual screening, if advised (Table 6).
Table 6 International screening strategies
| Country | Start age | Stop age | Frequency |
| USA | 40 | NA | Annually |
| Sweden | 40 | 74 | Biennially |
| UK | 50 | 70 | Triennially |
| The Netherlands | 50 | 70 | Biennially |
| France | 50 | 74 | Biennially |
| Italy | 50 | 70 | Biennially |
| Germany | 50 | 70 | Biennially |
Screening recommendations continue to spark debate, and scientific opinion on the effectiveness of annual screening is greatly divided. One side argues that annual mammograms starting at 40 reduce interval cancers, but others believe that annual screening results in more false positives with unnecessary treatment and that a more targeted approach could result in fewer false negatives and less overdiagnosis without increasing interval cancers. For most women, the discomfort and inconvenience of the annual mammogram is offset by the belief that it is one of the best ways to find cancers at an earlier, more curable stage. Unfortunately, cancers that are most likely to present at a more advanced stage or have a worse prognosis are those that are most likely to develop in between screening or are less likely to be visualized by standard radiographic tests. The reduced screening frequency recommended by the 2009 USPSTF was meant to balance benefits and harms and is more in line with policies of most other Western nations.
An increasingly vocal group questions the effectiveness of screening altogether. The 25-year follow-up results of the Canadian National Breast Screening Study (CNBSS), one of the early pivotal trials of screening, were published in February 2014 when they concluded (confirming their previous findings from 10- to 15-year follow-ups) that annual mammograms do not reduce deaths versus simple clinical breast exams.140 Switzerland has recommended ending mammography screening altogether because of lack of evidence that the benefits outweigh the harms.141 Editorials and perspectives regularly feature opposing viewpoints,142–144 highlighting the broad disagreement over screening guidelines.
The annual screening approach to mammography, which is still the current standard, has its roots in the large randomized screening trials of the 1980s.145 The overview of the Swedish trials showed a relative reduction in breast cancer mortality of 21%, with the maximum benefit for women in their 60s. The screening interval in the Swedish trials ranged from 18 to 33 months. Much has changed since these initial studies. Most of the effective systemic therapies used today were not available at the time when these screening studies were initiated, but currently have a clear role in reducing breast cancer mortality.146 At present, systemic therapy has been thought to constitute up to two-thirds of the reduction in mortality and mammography about one-third.9, 147 Importantly, the extensive use and effectiveness of endocrine therapy148, 149 may mitigate the impact of finding some cancers later.
The potential harm caused by mammographic screening that is most familiar to women is the “false-positive.” With each mammogram, a woman’s risk of receiving a false-positive increases, and with it, the risk of a biopsy that turns out to be benign. After 10 years of annual screening, over half of all women will receive a false-positive recall, and 7–9% will have a false-positive biopsy.150 In general, the specificity of mammography is approximately 90%, indicating a 1/10 probability of occurrence of a false-positive result. However, only approximately 5 in 1000 women actually have breast cancer when they are screened, so the majority of abnormal mammograms are false-positives.151 These affect women in several ways. A recent systematic review has shown that psychological distress can endure for up to 3 years, reducing adherence to subsequent screening.152 Similarly, a study from Holland showed 93% of women return after negative screens, but after one false-positive, only 56% return, and after two false-positive recalls, only 44% return.153 Biennial screening, relative to annual screening, was shown in the BCSC to reduce the false-positive rate by 50%, with only a small but statistically insignificant increase of late-stage cancer diagnoses.154
Another potential harm of breast screening comes from “overdiagnosis.”155 Two decades of research has shown us that breast cancer is a spectrum of disease, spanning indolent lesions of epithelial origin (IDLE)156 to aggressive disease. Screening, by nature, is more likely to identify slow growing and IDLE tumors136 as highlighted by the 25-year follow-up results of the CNBSS. Half of the mammography-detected cancers that would not otherwise have been found by regular breast examination were deemed to be clinically insignificant.138 A recent meta-analysis estimated that 20% of all cancers (up to 50% of screen-detected cancers) fall into the category of overdiagnosis.157 Data suggest that a woman has a greater chance of being overdiagnosed than of having her life saved by screening.158 Similar results were obtained when molecular markers were used to identify ultralow-risk disease.159, 160
Most precancerous breast lesions and DCIS likely qualify as overdiagnosis. DCIS was rarely diagnosed before screening was adopted but increased 500-fold afterward.161 The tumor cells comprising DCIS are morphologically similar to those of invasive cancer, and hence it has been assumed that DCIS ultimately progresses to invasive cancer. Because routine care for DCIS is mastectomy or lumpectomy and radiation, the natural history of DCIS is unclear.162, 163 Epidemiologic evidence suggests that only a subset progresses to invasive disease over a lifetime.164 Furthermore, unlike cervical cancer, where the removal of precancerous lesions caused a sharp decline in invasive cervical cancer, after a decade of removing DCIS lesions from more than 50,000 women per year, there has been no decline in incidence of invasive cancers162 (there was a short period of decline, but it was due to the sharp reduction in HRT after results of the Women’s Health Initiative study were released).4, 165 Molecular markers are now available that identify a low risk form of DCIS whose 5-year risk for developing breast cancer is about the same as that of an average 65-year-old woman (2.5%)166, 167 Yet DCIS diagnoses result in >20,000 mastectomies per year, many of which are bilateral.168, 169
Research to improve screening is ongoing, focusing on identifying the populations of patients who are most likely to benefit and developing molecular signatures to identify cancers with an extremely low risk of progressing.156, 170, 171 Criteria and threshold for recall after an abnormal scan and biopsy are being actively evaluated and improved. The BI-RADS172 recommends biopsy with a score of 4, which has a >2–95% chance of being either invasive cancer or DCIS. BI-RADS 4A (low suspicion <10%), 4B (intermediate 10–50%), and 4C (moderate >50%, but <95% risk of cancer) categories could help better refine biopsy thresholds.173
However, reducing the burden of overdiagnosis and false-positive recall could perhaps be best accomplished by modernizing our screening approach by incorporating our improved understanding of individual breast cancer risk.
The idea of risk-stratifying screening recommendations has been attracting attention recently.174 The Institute of Medicine has advocated for technology integration and biology and risk stratification in the development of breast-screening models. They noted that personalization—more frequent screening of those at highest risk—could improve the positive predictive value of the screening test and potentially lead to fewer unnecessary interventions.175, 176 Because most people have a relatively low risk, personalized screening will result in the majority of the population getting less-frequent examinations, reducing false-positive recalls and biopsy rates.154 This was also the conclusion of the USPSTF following their comprehensive review of mammography in 2009155 that the balance of benefits and harms of screening depends on individual risk factors for women in their 40s and comorbidities for women over 74 years of age.177 Women in their 40s with twice the average risk were thought to obtain the same benefits from screening compared with women in their 50s.178 As such, the decision whether to screen women in their 40s should be based on individual risk factors. The USPSTF urged clinicians to discuss the pros and cons of screening with their patients in the context of their individual risk. For women of ages 50–74, USPSTF found that screening every other year would preserve benefits and minimize risks compared with annual screening.
The USPSTF guidelines have met resistance179–181 and the medical community has failed to reach consensus on the issue. Women have also been reluctant to believe that less screening could somehow be beneficial, which, on the surface, is understandable.177 Finally, USPSTF guidelines did not state how to integrate risk assessment into practice. Thus, while we have witnessed major advances in the diagnosis, prognosis, and treatment of breast cancer, our approach to screening has not fundamentally changed since the 1980s. It is imperative to move beyond the current debate and test new approaches to breast cancer screening to maximize the benefits and minimize the harms to women. Clearly, this means new studies to help us understand screening in the context of modern adjuvant therapies. However, it also indicates using three decades of knowledge on the natural history of breast cancer and the factors that contribute to an individual’s personal risk of developing and/or dying from the disease. Just as our approach to treatment has evolved from one-size-fits-all to more individualized, patient-centered, evidence-based treatment, so too must our approach toward screening.147
In the last decade, concomitant advances in our understanding of disease mechanisms and analytical capabilities have given us the ability to treat disease with a higher degree of precision than ever before. In breast cancer, this is reflected in how we select specific therapies based on measured characteristics of an individual’s disease.117, 168, 182, 183 On the contrary, we largely approach breast cancer screening as if everyone is the same. A more risk-adapted approach has the potential to benefit patients, providers, and payors. The Patient-Centered Outcomes Research Institute (PCORI), established as part of the Affordable Care Act, has recently funded a randomized trial to test personalized screening versus annual screening, in an effort to focus forward on how to make screening better for women and their providers (wisdomstudy.org). The proposed pragmatic trial seeks to resolve this controversy by comparing an updated individualized approach to breast screening with annual screening. Despite our vastly improved understanding of breast cancer risk, the only criterion used to establish a woman’s screening recommendations is her age (and BRCA status if known). However, currently, there have been models that incorporate family history and breast density, endocrine exposures, gene mutations, and atypia to assess breast cancer risk,.100, 103, 184–186Most recently, certain common gene variants have been confirmed predictors as well.187 Advances in breast cancer biology, risk assessment (genomics), and imaging (density) have provided us with all the tools and knowledge required to implement a personalized model; one that provides recommendations on when to start, when to stop, and how often to screen, depending upon well-characterized measures of their personal risk. The study is being conducted by the University of California-wide Athena Breast Health Network. The potential benefits of risk-based screening to health care payers are numerous and enormous. Our current breast cancer screening costs $8–10 billion and results in 600,000 benign biopsies annually. If we compare the costs of the most widely adopted annual screening practice of women of age 40–80, with the USPSTF recommendations, the difference in cost would be approximately $6.3 billion—a figure larger than the NCI’s annual budget.188, 189
MRI screening
MRI is increasingly being used in the management of breast cancer. MRI has the advantages of providing a three-dimensional (3D) view of the breast, performing with high sensitivity in dense breast tissue, and using nonionizing radiation. MRI has the following drawbacks as well: high cost, variability in performance, and moderate specificity that in combination with high sensitivity often leads to unnecessary workup.190
MRI is not appropriate as a general screening tool. It is at least 10 times costlier than mammography. MRI should be reserved for those situations where there is a high prior probability of identifying a cancer (high risk) and very high sensitivity is preferred and where other less expensive, robust screening tools (e.g., mammography) are known to be less sensitive,156, 191 such as BRCA1 or BRCA2 mutation carriers, which have an 85% lifetime risk of developing breast cancer. Although an average 35-year-old woman would have a 1/10,000 chance of having a cancer, a mutation carrier would have a risk in the range of 1 to 5/100, and MRI would be much more sensitive in this population than mammography. Women with a very high 5-year Gail risk and very dense breast tissue may also fall into this category. Use of a density-modified Gail Risk score,52 which combines both risk and breast density as recorded on a mammogram (BI-RADS density), enables the identification of women both at high risk and at risk for false-negatives with mammography. The density-modified Gail risk is calculated by multiplying the lifetime Gail risk by 0.59, 1.00, 1.41, or 1.94 for a BIRADS of 1, 2, 3, or 4, respectively. Women with a lifetime risk of >50%, as calculated by the density-modified Gail model, are recommended for MRI screening. Consideration can be given to women with a 35–49% lifetime risk using this tool, although there has been no current evidence to support the addition of annual MRI screening.
The true measure of a screening test is not whether it finds more cancers, but whether finding the cancers decreases mortality and morbidity from breast cancer. No study has yet shown that cancers found by MRI decrease mortality from breast cancer. However, two large studies have shown that screening using MRI is more sensitive in high-risk women, with remarkably similar results.192, 193 If tumor size and lymph node involvement are used as surrogates for outcome, MRI does improve the stage at which tumors are identified in women in the highest risk cohort. In the Netherlands study, the cancers found in the 1909 women screened with mammography and MRI were compared with two appropriate control groups of mutation carriers and high-risk patients, none of whom had access to MRI screening. In the highest risk group (50–85% estimated lifetime risk), 63% of mutation carriers with screened cancers had negative nodes when screened with MRI compared with 47% with negative nodes in the controls. In the moderate-risk group (15–30% estimated lifetime risk), only 12% had lymph node involvement and 87% had negative nodes (compared with 52–56% positive nodes and 44–48% negative nodes in the control groups). Therefore, MRI appears to be capable of finding cancers at an earlier stage. However, the rate of detection of cancer is also important, and MRI should be used where that rate is high and significantly higher than that in the usual screened group. Of note, the cancer detection rates were 26.5, 5.4, and 7.8 per 1000 woman years for the mutation carriers, high-risk group, and moderate-risk group, respectively. This should be compared with the detection rate of 5 to 7/1000 women where mammography is most cost-effective, in women aged 50–70 years. The only group that had a higher rate of cancer development was the highest risk group, the mutation carriers, and we should be careful to restrict the use of MRI for those women.
Screening comes at a price, both financial and psychological. In the Netherlands study of 1909 women screened for 10 years, 1200 extra procedures were performed. In the process of finding the 45 cancers, MRI led to twice as many extra procedures (420) compared with mammography (207) and three times as many unnecessary biopsies, 24 versus 7 for MRI and mammography, respectively.190 It is common to recommend 3- or 6-month follow-up studies after an abnormal imaging test. However, MRI examinations cost US $1000–2000, so it is inappropriate to order these tests unless there truly is a situation where the likelihood of finding an abnormality is much higher than in the general population and where mammography would be unlikely to be effective. The two key messages are that we need to find ways of stratifying risk to appropriately tailor the use of technology and MRI screening should be undertaken only in facilities that have the capability of investigating MRI abnormalities, both with ultrasound and MRI-guided biopsy, if necessary. The Blue Cross/Blue Shield technology assessment concluded in 2003, which showed that MRI screening was justified in women who carry an inherited predisposition to breast cancer. The studies conducted in the Netherlands and Canada190, 194 strengthened this conclusion. The moderate- and high-risk women probably gain less overall because the risk is not as high, suggesting that MR must become more specific and follow-up of abnormalities easier before we implement widespread screening of intermediate risk women.
Not all MRI exams are alike. There is a great deal of variability in technique, sequences, interpretation, and capability for follow-up and biopsy. Although the use of breast MRI has proliferated rapidly, standards have not. Clinicians ordering breast MRIs need to know that technique, time of menses (midcycle is optimal 4–14 days after starting menses190), and the skill in interpretation affect results. Interpreting images performed in different institutions is also a challenge because of the relative inability to transfer and view images electronically. Each of these areas is under active investigation, and further research and technological improvements will substantially improve our ability to appropriately integrate MRI into breast cancer management.
MRI has also been used after a diagnostic test reveals an abnormality. The most definitive study on the performance characteristics of MRI for the evaluation of mammographic abnormalities and palpable masses is the multi-institutional International Breast MRI Consortium (IBMC) study. High sensitivity and moderate specificity were confirmed in this study. Diagnostic characterization of lesions by MRI is improving, but it is not sufficiently specific to substitute a biopsy. MRI after an abnormal diagnostic mammogram significantly increases false-positives. Currently, there has been no role for MRI in the diagnosis of breast cancer unless suspicious mammographic findings cannot be evaluated or localized or unless there is another compelling reason to order MRI (e.g., if a patient is a mutation carrier or has a very high risk of cancer and very dense breast tissue).
Perhaps, the most important role for MRI is the staging of known cancer in the breast and monitoring the response to therapy.190 MRI reveals that tumors form distinct patterns in the breast, and different types of tumors, such as lobular and inflammatory cancers, are more commonly associated with distinct patterns. Initial imaging characteristics identify women likely to have a particularly poor response, such as diffuse tumors with large volumes.195
Risk and prevention
If we can reduce the frequency of screening at those with lower risk, in accordance with the recommendations of the USPSTF, those at lower risk to develop breast cancer will be screened less, thus reducing false-positive results. The process of callbacks and biopsies from false-positives is extremely stressful, characterized by increased anxiety and changes in screening choices that have been shown to persist long after the issue is resolved.152, 193 Personal risk-assessment may improve the general understanding of their personal breast cancer risk. At present, only one in 10 women has accurate perceptions of her personal risk, while four in 10 have never discussed their personal breast cancer risk with a doctor.196 They are not aware that some tumors grow rapidly and present between screens (interval cancers) and need attention regardless of a prior normal screen.197 There are effective, viable prevention options available to the estimated 2 million American women who are at high risk, yet many are completely unaware of their predisposition198 and so cannot benefit from them. There are three level I studies that have demonstrated the ability of endocrine risk-reducing agents. The NSABP P-01 study of over 13,000 women with a Gail Risk of at least 1.67, randomized to tamoxifen versus placebo, showed that risk of developing invasive cancer or DCIS could be reduced by 50% and more (85%) in the setting of atypia.199 This has been confirmed by the IBIS trial,200 and furthermore, younger women (below 50 years of age) had more to gain, and benefits continued for 10 years, even when therapy was stopped after 5 years. The STAR trial compared tamoxifen with raloxifene, and a selective estrogen receptor modulator (SERM) was developed to improve breast density, but noted to reduce hormone-positive breast cancer in postmenopausal women and reduce fracture risk in the MORE study.201 Raloxifene does not stimulate the endometrial lining and has fewer endometrial side effects including bleeding and endometrial cancer, and although the risk-reducing impact was not quite as high as tamoxifen, it is considered the better choice for postmenopausal women.202 This is supported by the long-term follow-up of the IBIS 2 trial,203 which showed an increased risk of death from endometrial cancer in postmenopausal women on tamoxifen. Finally, a prevention study (MAP.3) using the AI exemestane showed a 60% reduction in the risk of hormone-positive invasive breast cancer in postmenopausal women compared with those on tamoxifen.204 There has been a poor uptake of endocrine risk reduction in the United States and worldwide. This is likely from a combination of a failure to automate or integrate risk assessment into primary care, a failure to appreciate the benefits of risk reduction, a failure to assess whether a woman receives specific benefit. Cholesterol, for example, is a measure that is used to demonstrate the lowering of cardiac risk. Breast density has been proposed as a possible dynamic measure of risk, where women on tamoxifen who demonstrate a reduction in breast cancer density are the ones who derive the risk reduction benefit.205, 206 This is an important point of study and is likely incorporated into future prevention studies. Furthermore, we know that communicating individual risk can motivate increased screening uptake207 and that women are more motivated to use preventive interventions when they understand they are high risk112 and specifically stand to benefit.
Biopsy
When a woman presents with diagnostic abnormality, a decision to intervene should be made in the context of the likelihood of the lesion being invasive cancer or in situ cancer, as well as the age, underlying health condition, and life expectancy of the patient. A decision about which type of biopsy to perform is made by thinking about the need for future procedures. The goal should be to minimize the total number of procedures (including definitive cancer surgery), discomfort and scarring, and diagnostic wait time and anxiety and to enable the optimal timing of procedures.
A number of options are available for the diagnosis of masses and mammographic abnormalities. For palpable lesions, the options include FNA, core biopsy, or excisional biopsy. Minimally invasive techniques, core biopsy, and FNA are quite accurate in experienced hands and when the “triple assessment” is used. Triple assessment is the consideration of the imaging, clinical, and pathologic findings. If there is significant discordance, further evaluation should be pursued. In general, excisional biopsy is not the optimal diagnostic procedure. Minimally invasive biopsy techniques can be performed immediately in the office and facilitate a rapid diagnosis and discussion of options and full evaluation of the extent of disease in the breast before definitive surgery. FNA or core biopsies can also be used to confirm the suspicion of multicentric disease and thereby avoid multiple trips to the operating room and in general allow the optimal sequencing of interventions.
The type of biopsy performed should depend on the expertise at a given institution. FNA is highly accurate, with sensitivity and specificity of 98% and 99%, respectively. FNA both for palpable and mammographic lesions has been used extensively and successfully in Sweden and the United Kingdom, where it is the standard diagnostic tool. This technique requires practitioners who have training and experience in sampling the lesion (the aspect prone to the highest error), preparing the slides, and interpreting cytology.208 The advantage is that it can be performed right away and yields results within a day. Both FNA and core biopsy are preferred when there is a high suspicion of invasive cancer and the anticipation that a sentinel lymph node dissection (SLND) will be performed. When an SLND is performed, the type of biopsy performed affects the accuracy of the SLND. Core biopsy and FNA have a lower false-negative rate than excisional biopsy when a subsequent sentinel node dissection is performed: 8% compared with 14% for incisional biopsy and 15% for excisional biopsy. In the setting of a patient with a large obvious tumor, FNA can facilitate the rapid confirmation of a diagnosis and a discussion of options, including neoadjuvant therapy and clinical trials. For patients who opt for surgical management, no further test is needed. For those who opt for neoadjuvant therapy, a core biopsy should be obtained for histology to confirm invasive disease, to save for future studies in the event of complete pathologic response and potentially for clinical trials. In the event that cytology expertise does not exist in a given institution, a core biopsy can also be rapidly performed in the office setting.
Recall for mammographic abnormalities is common, and the likelihood of cancer being diagnosed from a mammographic biopsy (cancer to biopsy rate) is highly variable, from 10% to 40%. A low cancer to biopsy rate is not necessary for high sensitivity, and in fact, the most experienced and highly trained mammographers find more cancers and order fewer biopsies for what turns out to be benign.116 Cancer to biopsy rates decline over time in settings where quality improvement and feedback on performance are the rule. Several ways exist to avoid biopsies of noncancerous tissue. The first is to take the extra time to get old mammograms for comparison. Circumscribed mass lesions that have been stable for over 2 years will be converted to probably benign and not require a biopsy. If an experienced mammographer has not read the films, a second opinion can always be obtained.
In the event that a biopsy is recommended, it is important to make sure that the lesion is not palpable. If it is, and particularly if the lesion is suspicious for cancer, an FNA not only establishes the diagnosis but also confirms that the palpable mass is indeed the cancer, avoiding the need for wire localization at the time of lumpectomy. An attempt to locate nonpalpable suspicious mammographic masses with ultrasound will enable diagnostic and definitive procedures to be ultrasound-guided, which is more comfortable for patients. In the event that a mammographic lesion can only be seen on mammogram, a stereotactic biopsy can be performed using digital images to locate the lesion and direct the core biopsy. The sensitivity of this procedure is as high as 98% by experienced practioners.206 A specimen radiograph is obtained to confirm that the target has been obtained (usually calcifications). If there is a risk that all calcifications will be removed, it is then critical to leave a clip to localize the area later.
It is important to perform procedures in the context of their value to the overall management of the patient. If cancer is not present, an adequate sampling of the calcifications or lesions should be performed. If cancer is present, the minimal amount to establish the diagnosis is sufficient. At this point, the diagnostic procedure is not definitive and wide excision is needed. Some radiologists take over 30 core biopsy samples. This is not necessary and can create hematomas, distortion of tissue, and difficulty assessing the true extent of the lesion. It is also unpleasant for the patient.
Some mammographic lesions cannot be biopsied using stereotactic techniques because either the lesion is too close to the chest wall or the breast compresses to less than 3 cm in the direction the biopsy needle would be placed. In this case, an excisional biopsy must be performed. A wire is placed by the radiologist to guide the surgery, with the tip of the wire just under or at the level of the calcifications or mass. The surgeon should use the radiologist’s estimate for the likelihood of malignancy to determine the extent of resection. For lesions more likely to be cancer, the lesion and a 1-cm rim of tissue should be taken. For less-suspicion lesions, a smaller volume of tissue can be taken. In order to avoid taking unnecessary tissue, an incision should be made near the tip of the wire or the expected location of the abnormality. Starting the excision at the insertion site of the wire only leads to excessive tissue being removed and usually results in a close margin at the end of the wire. Making the incision over the lesion is helpful in locating the biopsy cavity in the event that an additional resection is necessary. All specimens need to be sent to mammography or evaluated using a Faxitron to assure the surgeon that the target lesion has been removed. Mammographers routinely recommend a biopsy of any lesion that is a BI-RADS 4 or higher. However, a BI-RADS classification of 4 includes lesions that have a risk of as low as 3% or as high as 75% for being malignant. The lesion may be suspicious for either in situ or invasive cancer—no distinction is implied by the categorization of a BI-RADS 4. The surgeon should understand both the type of lesion suspected and a more specific estimate of risk, because they may make a difference in how the patient is evaluated. An older woman with several comorbidities and a mammogram with a BI-RADS 4, if she has a lesion that is approximately 90% likely to be benign and 10% likely to be DCIS, may not need a biopsy. This is the type of lesion that could be followed on mammography. In general, we recommend that a minimally invasive biopsy be used to establish a diagnosis, but excisional biopsy may be the procedure of choice for a woman who has a confined cluster of linear calcifications that are highly suspicious for high-grade DCIS. In this situation, a core biopsy would likely reveal DCIS, and a negative biopsy would be discordant and require wire localization and excision. If the likelihood of associated invasive cancer is low, a sentinel node dissection or axillary sampling will not be necessary, and therefore, there is little value in starting with a stereotactic biopsy.
Ductal carcinoma in situ
DCIS is defined by cytologically malignant epithelial cells within the ductal system of the breast that have not invaded through the basement membrane into the breast parenchyma. Figure 3 shows a histological example of DCIS.
Before the advent of screening, DCIS comprised about 3% of breast cancers detected. As the interest in finding smaller cancers increased, and targeted calcifications rather than only masses, we began to identify DCIS more frequently. DCIS now accounts for approximately 20–25% of screen-detected breast cancers. The cells that comprise DCIS appear similar to invasive cancer both pathologically and molecularly, and therefore the presumption was made that these lesions were the precursors of cancer and that early removal and treatment would reduce cancer incidence and mortality. However, long-term epidemiology studies have amply demonstrated that the removal of 50,000 to 60,000 DCIS lesions annually has not been accompanied by a reduction in the rate of occurrence of invasive breast cancers.209 This is in contrast to the experience with the removal of colonic polyps and cervical intraepithelial neoplasia (CIN) lesions of the cervix, where the removal of precursor lesions has led to a decrease in the incidence of colon and cervical cancer, respectively.164 For low-grade DCIS, there is no evidence from SEER that women have the same survival whether they have any intervention or not. Of the 57,222 women diagnosed with DCIS, 2% (1169) had observation only. In women with low-grade DCIS, survival was identical at 10 years with and without surgery (98.6% vs 98.8%, respectively).210
From the results of a large observational study of >100,000 women diagnosed with DCIS, Narod and colleagues showed that the risk of dying from breast cancer is extremely low.211 Less than 1% of patients in this 20-year study died of breast cancer (compared with 5% of patients who died of other causes). Using the Kaplan–Meier method, the breast cancer-specific mortality rate is 3.3% at 20 years, almost similar to the statistic listed by ACS as the chance that breast cancer will be responsible for a woman’s death (ACS.org).
Two ongoing therapeutic questions related to the treatment of DCIS concern the use of radiation and hormonal therapy. While radiation decreases the chance of an invasive breast cancer occurrence, and a recurrence of DCIS, radiation after lumpectomy for DCIS does not result in a reduction in breast cancer mortality. This has been shown consistently.212 While some reports suggest that low-grade DCIS with wide margins (>10 mm) recur in as few as 3% of cases with surgery only without radiotherapy. Two large prospective studies by the Dana-Farber Cancer Institute and the Eastern Cooperative Oncology Group (ECOG) encountered local recurrence rates of 12.5% and 7% in low- and intermediate-grade DCIS, respectively.
One of the leading proponents of treating favorable DCIS with lumpectomy only has been Silverstein and colleagues, who, in 1995, developed the Van Nuys Prognostic Index (VNPI) to help define selection criteria for cases with low recurrence risk.136 The original VNPI classes were determined from the retrospective analysis of 333 patients treated at two institutions between 1979 and 1995. Patients received 1–3 points for three factors: tumor grade and the presence or absence of necrosis (combined into one category), tumor size, and margin status. Over two-thirds of the patients had an intermediate-risk VNPI (cumulative score of 5–7), and, similar to the randomized data, radiation reduced the probability of breast cancer recurrence in this cohort (32% vs 15%; p = 0.17).160 Less than one-third of their patients had small, low-grade disease with widely negative surgical margins, and the breast cancer recurrence rate was low after breast conservation surgery (BCS) only (3% breast cancer recurrence rate among 76 patients treated without radiation). Subsequently, this group has also incorporated patient age into their prognostic index. Similar data were subsequently reported from a population-based cohort study of 1036 women ≥40 years of age in San Francisco treated with lumpectomy only for DCIS. This study found overall a relatively high recurrence rate (20% and 10% for all recurrence and invasive recurrence, respectively, at 5 years) but reported that the subset of patients with mammographically detected low-grade disease treated with surgery that achieved widely negative margins had a lower ROR after surgery only.137
Using an expansion of this original cohort, Silverstein and colleagues reanalyzed their data, focusing on the importance of margin status for patients treated with DCIS. In this analysis,138 they reported that 93 patients with a margin width of 10 mm or more had an 8-year local recurrence rate of only 3%. This rate was found to be much higher after treatment with lumpectomy without radiation when the margin width was 1–10 mm (recurrence rate 20%) and margins were under 1 mm (58%). When this study was updated and expanded to include 212 patients with margin widths of ≥10 mm, the breast recurrence rate in this cohort increased to 14%.139 In addition, the low recurrence rate noted in the setting of 10-mm margins could not be confirmed in a single-arm prospective trial conducted at the Dana-Farber/Harvard Cancer Center. In this study, 157 patients with grade 1 or 2 DCIS, ≤2.5 cm, underwent wide local excision only with achievement of negative margins of ≥1 cm.142 The trial was closed early because the recurrence rate met predefined stopping rules, with an estimated 5-year local recurrence rate of 12.5% (invasive cancer occurrence of 6%). The authors concluded that even in this highly selected group of patients, there was a substantial local recurrence rate. The ECOG has also conducted a prospective study of 711 patients with DCIS treated with surgery plus/minus tamoxifen without radiation. Two different patient strata were enrolled: (1) low- or intermediate-grade DCIS, size < 2.5 cm, and (2) high-grade DCIS, size < 1 cm. All patients were required to have a negative post lumpectomy mammogram and negative margins of 3 mm or more. The 5-year risk of in-breast tumor recurrence (IBTR) was 7% in the low- and intermediate-grade stratum and 14% in the high-grade stratum.141 Given the success of salvage therapies and the low risk of cancer-associated death with surgical-only treatment, it is reasonable to inform patients about the available data and have them participate in their locoregional treatment decisions.
At present, a debate has been ongoing regarding the overdiagnosis and subsequent overtreatment of pure DCIS in the modern era, given the technologic improvements in breast screening over the past decade. At the same time, long-term combined follow-up of the NSABP B-17 and B-24 trials shows that an invasive recurrence after surgery only for DCIS increases the risk of breast cancer-related death and that radiation reduces this risk of invasive recurrence by more than half (19.4% vs 8.9%).211 Although the VNPI and ECOG studies cited above provide guidance in terms of identification of a low-risk subgroup of DCIS, they do not hold the same weight as a prospective randomized trial.
In 1998, the RTOG initiated such a trial for selected women with low-/intermediate-grade DCIS ≤ 2.5 cm in the highest extent with ≥3-mm margins, originally designed for 1800 patients.213 Unfortunately, the study was ended in 2006 because of poor accrual; however, in the 636 patients enrolled, radiation reduced the local failure rate from 6.7% to 0.9% at 7 years (p < 0.001). The clinical impact of this statistical benefit is up for discussion, and patients must be counseled regarding the trade-offs of toxicity and inconvenience of adjuvant radiation versus the higher risk of a (possibly invasive) recurrence and need for subsequent salvage therapy. An emerging clinical tool to counsel patients and tailor adjuvant recommendations for DCIS may be genomic sequencing. In a tissue analysis of over 300 patients treated on the abovementioned phase II ECOG trial, the 21-gene Oncotype DX assay (see section on adjuvant therapy) was used to score patients according to the risk of invasive recurrence and was found to be predictive, independent of clinical factors.165 It remains to be seen whether such a strategy provides cost-effective utility over the currently used clinical selection factors.
Radiation has small but real risks.214 Given the lack of mortality benefit, it is appropriate to consider reserving external beam radiation for breast conservation when invasive cancer occurs in the setting of DCIS with many high-risk features. Many invasive lesions do not require radiation in the postmenopausal setting,215–217 and hence certainly it should not be a surprise that there is no benefit of radiation in the noninvasive setting.
The second major area of research and controversy regarding the management of DCIS concerns the use of hormonal therapy. After completion of the B-17 trial, the NSABP conducted the B-24 trial, which randomized 1802 patients treated with lumpectomy and radiation to receive either tamoxifen or placebo for 5 years.218 The eligibility of this trial differed from B-17 in that it included patients with positive surgical margins, which ended up being present in 25% of the study population. In addition, the primary end point of the study was not ipsilateral breast recurrence, but rather the probability of having an ipsilateral recurrence or developing a contralateral breast cancer. Therefore, this trial was designed to investigate the combined therapeutic benefit of tamoxifen against the index DCIS and the chemopreventive benefit of tamoxifen in reducing subsequent independent breast cancers.
The results of the B-24 trial indicated that patients treated with tamoxifen, surgery, and radiation had a 5-year rate of noninvasive or invasive breast cancers of 8.2% compared with 13.4% for those treated only with surgery and radiation.142 Clearly, a component of this benefit was the chemoprevention effects of tamoxifen. In B-24, the use of tamoxifen resulted in a 41% reduction in the 5-year incidence of contralateral breast cancers. These data are consistent with the reduction in second primary breast cancers seen in the adjuvant tamoxifen trials and the NSABP P-1 trial, which found that tamoxifen decreased breast cancer development in women at increased risk for breast cancer by 49%.110 In a subsequent retrospective analysis of ER expression from stored tumor material from 628 patients (327 placebo, 301 tamoxifen), it was found that the benefit of tamoxifen was limited to 482 patients (77%) with ER-positive disease. In this cohort, tamoxifen was associated with a significant reduction in both ipsilateral breast recurrence and the development of contralateral breast cancer. There was no apparent benefit of tamoxifen in those with ER-negative tumors, but the sample size precluded, detecting a small benefit.
The UK/ANZ trial provides additional information concerning the interaction of radiation and tamoxifen after lumpectomy in patients with DCIS.219 In this study, use of tamoxifen decreased the rate of DCIS recurrence, but not invasive recurrence for patients who did not receive radiation. However, in patients treated with radiation after lumpectomy, tamoxifen provided no benefit in ipsilateral breast events. Unlike the B-24 trial, this study required all patients to have negative surgical margins after lumpectomy and did not include the development of a contralateral breast cancer as an event in their primary outcome measure.
These data suggest that, for patients treated with an adequate lumpectomy that achieves negative surgical margins and subsequently receive radiation, the therapeutic benefits of tamoxifen are likely to be very low in reducing ROR in the ipsilateral breast, but there will be a protective benefit in the contralateral breast. Interestingly, a survey found that 56% of US practitioners routinely recommend tamoxifen for women with DCIS compared with only 22% of European practitioners.146 Despite the lack of clarity concerning the therapeutic benefits of tamoxifen in DCIS, for patients with ER-positive disease, the addition of tamoxifen is likely to have chemopreventive effects and minimize the risks of subsequent new breast cancers. NSABP B = 35 is a phase III trial that compared the effects of 5-year tamoxifen use to the AI anastrozole in 3104 postmenopausal women diagnosed with DCIS.220 With a median follow-up of 9 years, there was an absolute difference of 4.3% in breast cancer-free interval favoring anastrozole. This difference was magnified in women under the age of 60, where the absolute difference was 6.7%. Consistent with prior studies, no difference in overall survival (OS) was observed; less uterine cancers and more osteoporotic fractures were observed in patients treated with anastrozole.
Among DCIS patients, breast cancer-specific mortality is associated with age at diagnosis, ethnicity, and DCIS characteristics such as ER status, grade, size (>5 cm), and comedonecrosis. Despite their significance in a multivariable analysis, high-risk characteristics, such as ER-negativity and high grade, often overlap. Only a small minority of patients will have one or more of these high-risk characteristics. For young women (<40 years of age) who present with symptomatic DCIS, about 5% of the population, we should be cognizant that this is a different disease than the average DCIS. In addition, African-American women who are more likely to be at risk for hormone-negative breast cancer and women with hormone-negative or human epidermal growth factor receptor-2 positive (HER2+) breast cancer may be those that should continue to be treated according to the current aggressive standards. In total, these groups probably constitute about 20% of the population of DCIS.
There are uncommon cases where DCIS is associated with a higher risk than has been appreciated. When DCIS is diagnosed before the age of 35 or even 40, some of these lesions do pose an increased risk of breast cancer-specific mortality. DCIS diagnosed before the age of 40 is likely different, as it would present as a symptomatic event (e.g., a mass or bloody nipple discharge), as screening before the age of 40 is rare.
It has always been assumed that DCIS indicates a higher local risk—but the similarity of the ipsilateral and contralateral invasive breast cancer risk (5.9% and 6.2%) with long-term follow-up211 suggests that DCIS may behave more as a risk factor like atypia. Oncotype DCIS, a gene expression score obtained on formalin-fixed tissues, demonstrated that low-risk lesions simply excised appear to carry the risk equivalent to a Gail Risk of 2.5167 A unilateral recurrence of DCIS or contralateral DCIS event has no effect on mortality. However, an invasive cancer does: 18-fold for unilateral and 13-fold for contralateral, suggesting that all risk depends on the probability of the occurrence of an invasive cancer. DCIS may best represent an opportunity to alter the environment of the breast and approach using standard risk reduction or endocrine risk reduction. For premenopausal women, tamoxifen is a good choice for HR+ DCIS. For postmenopausal women, AIs have been shown to have a higher impact on risk reduction. If AIs produce adverse side effects, raloxifene is a good alternative. Tamoxifen should probably not be used for DCIS in postmenopausal women given the risks reported with longer duration hormone therapy in IBIS 2.203
High-risk lesions (e.g., HER2+, <40, ER−, large size) should be aggressively treated, but the Narod analysis suggests that our current approach of surgical removal and radiation may not suffice for the rare cases that lead to breast cancer mortality and new approaches need to be investigated. In a study characterizing the immune environment of high-risk lesions that are most likely to recur, we found that the tumor microenvironment associated with recurrence was replete with activated macrophages and a paucity of activated T cells.221
Treatment of early-stage breast cancer
Staging and classification
The purposes of staging are to (1) plan a therapeutic strategy that is most appropriate for the patient, (2) allow for more intelligent projection of outcome based on the disease status of the patient, and (3) permit comparison of therapeutic results obtained from different sources by different means. The common staging methods are clinical and pathologic, but newer methods involving biologic assessments are under development and validation. Regardless of the staging method used, it is important to remember that the stage represents the state of disease or biologic potential of a patient’s tumor. The benefits of a particular staging method must be judged against its accuracy in performing this task.
The tumor node metastasis (TNM) classification devised by the Union for International Against Cancer (UICC) and accepted by the American Joint Committee on Cancer Staging is of world standard.222 The TNM is based on the clinical features of tumor (T), the regional lymph nodes (N), and the presence or absence of distant metastases (M) (Table 7).
Table 7 TNM stage definitions.
| Primary tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Tis (DCIS) | Ductal carcinoma in situ |
| Tis (LCIS) | Lobular carcinoma in situ |
| Tis (Paget’s) | Paget disease of the nipple with no tumor |
| T1 | Tumor 2.0 cm or less in highest dimension |
| T1mic | Microinvasion 0.1 cm or less in highest dimension |
| T1a | Tumor more than 0.1 but not more than 0.5 cm in highest dimension |
| T1b | Tumor more than 0.5 cm but not more than 1.0 cm in highest dimension |
| T1c | Tumor more than 1.0 cm but not more than 2.0 cm in highest dimension |
| T2 | Tumor more than 2.0 cm but not more than 5.0 cm in highest dimension |
| T3 | Tumor more than 5.0 cm in highest dimension |
| T4 | Tumor of any size with direct extension to (1) chest wall or (2) skin, only as described below |
| T4a | Extension to chest wall, not including only pectoralis muscle adherence/invasion |
| T4b | Edema (including peau d’orange) and/or ulceration of the skin of the breast and/or satellite skin nodules confined to the same breast |
| T4c | Both T4a and T4b |
| T4d | Inflammatory carcinoma |
| Regional lymph nodes (N) | |
| NX | Regional lymph nodes cannot be assessed (e.g., previously removed) |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to movable ipsilateral axillary lymph node(s) |
| N2 | Metastasis in ipsilateral axillary lymph node(s) fixed or matted, or in clinically apparent ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastasis |
| N3 | Metastasis in ipsilateral infraclavicular lymph node(s) with or without axillary lymph node involvement, or in clinically apparent ipsilateral internal mammary lymph node(s) and in the presence of clinically evident axillary lymph node metastasis, or metastasis in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement |
| Pathologic classification (pN) | |
| pNX | Regional lymph nodes cannot be assessed (previously removed or not removed for pathologic study) |
| pN0 | No regional lymph node metastasis identified histologically; isolated tumor cell (ITC) clusters are no higher than 0.2 mm, single tumor cells, or a cluster of fewer than 200 cells in a single histologic cross section, detected by histology or immunohistochemistry (IHC) |
| pN0(i-) | No regional lymph node metastases histologically, negative IHC |
| pN0(i+) | Malignant cells in regional lymph node(s) no higher than 0.2 mm |
| pN0(mol-) | No regional lymph node metastases histologically, negative molecular findings by RT-PCR |
| pN0(mol+) | Positive molecular findings by RT-PCR, but no regional LN metastases detected by histology or IHC |
| pN1 | Micrometastases, or metastases in 1–3 axillary lymph nodes, and/or in internal mammary nodes with metastases detected by sentinel lymph node biopsy, but not clinically detected |
| pN1mi | Micrometastases (>0.2 mm and/or more than 200 cells, but none higher than 2.0 mm) |
| pN1a | Metastases in 1–3 axillary lymph nodes, at least one metastasis higher than 2.0 mm |
| pN1b | Metastases in internal mammary nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy, but not clinically detected |
| pN1c | Metastases in 1–3 axillary lymph nodes and in internal mammary nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy, but not clinically detected |
| pN2 | Metastases in 4–9 axillary lymph nodes, or in clinically apparent internal mammary lymph nodes in the absence of axillary lymph node metastasis |
| pN2a | Metastases in 4–9 axillary lymph nodes; or in clinically detected lymph nodes in the absence of axillary lymph node metastases |
| pN2b | Metastases in clinically detected internal mammary lymph nodes in the absence of axillary lymph node metastases |
| pN3 | Metastasis in 10 or more axillary lymph nodes; or in infraclavicular lymph nodes; or in clinically apparent ipsilateral internal mammary lymph nodes in the presence of 1 or more positive level 1, II axillary lymph nodes; or in more than 3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy, but not clinically detected; or in ipsilateral supraclavicular lymph nodes |
| pN3a | Metastases in 10 or more axillary lymph nodes (or at least one tumor deposit higher than 2.0 mm), or metastases to the infraclavicular nodes |
| pN3b | Metastases in clinically detected ipsilateral internal mammary lymph nodes in the presence of one or more positive axillary lymph nodes, or in more than 3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected |
| pN3c | Metastases in ipsilateral supraclavicular lymph nodes |
| Distant metastasis (M) | |
| MX | Presence of distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
| AJCC stage groupings | |
| Stage 0 | Tis, N0, M0 |
| Stage IA | T1, N0, M0 |
| Stage IB | T0, N1mi, M0 |
| T1, N1mi, M0 | |
| Stage IIA | T0, N1, M0 |
| T1, N1, M0 | |
| T2, N0, M0 | |
| Stage IIB | T2, N1, M0 |
| T3, N0, M0 | |
| Stage IIIA | T0, N2, M0 |
| T1, N2, M0 | |
| T2, N2, M0 | |
| T3, N1, M0 | |
| T3, N2, M0 | |
| Stage IIIB | T4, N0, M0 |
| Stage IIIC | T4, N1, M0 |
| T4, N2, M0 | |
| Any T, N3, M0 | |
| Stage IV | Any T, Any N, M1 |
Source: Edge 2010.222 Reproduced with permission of Springer.
In general, clinical staging systems underestimate the extent of disease. The inclusion of pathologic information improves staging accuracy and is the basis for most modern clinical trials. In all cases, it is wise to remember that the goal is to define the biologic activity of the tumor. Cox regression statistical models demonstrate that the presence of nodal metastases are the most important factor, and the number of nodes involved can be used to further subset the prognostic groups. It is generally acknowledged that nodal metastases that penetrate the capsule and extend into adjacent perinodal tissue carry a worse prognosis.
In a regression analysis model, tumor size is found to be closely related to axillary lymph node involvement.84, 223 Tumor size is not an important discriminant within axillary node groups, except for patients with more than four involved nodes. Thus, size, which can be a function of either time or growth rate, is less useful than axillary node involvement, which is a more specific indicator of biologic aggressiveness. Some patients with large masses may have slow-growing tumors, which have not metastasized. By contrast, patients are also diagnosed with small but aggressive tumors found by screening mammography, in whom distant metastases are already present. These patients may die rapidly despite the initial favorable local clinical features. A biologic classification would identify these women more accurately than does the TNM system; this is the goal of future modifications to current staging.
Surgery
Over the past three decades, revolutionary changes have occurred in the locoregional management of primary breast cancer. As a result, radical and extended radical mastectomy has been relegated to the archives of surgical history. The publication of a series of randomized controlled clinical trials comparing radical mastectomy to less-extensive surgical interventions led a National Institutes of Health (NIH)-sponsored consensus development conference (1990) to recommend that breast preservation is the preferable treatment for women with stages I and II breast cancer, because it provides survival figures equivalent to those of total mastectomy and axillary dissection while, at the same time, preserving the breast.224
Information from various sources indicates that many patients with breast cancer have disseminated disease by the time a clinical diagnosis is established. This is not surprising, because a breast tumor of 1 cm has already progressed through 30 of the theoretical 40 doublings that result in a tumor of approximately 1 kg, a size that could be lethal to the patient. However, at present, we know that breast cancer is a heterogeneous disease, and that some patients have tumors with a low potential for metastatic spread, and that some, even when small and confined to the breast, have a high risk for metastatic spread.86, 89 Molecular profiling is likely to add much to our ability to distinguish these types of tumors and tailor treatment accordingly.
Treatment failed in three of four patients with positive axillary nodal involvement and in almost nine of 10 patients with four or more involved nodes 10 years after radical mastectomy without adjuvant chemotherapy for what were considered clinically “curable” breast cancers. These findings emphasize the systemic nature of some breast cancers and indicate the inadequacy of extensive local and regional surgery when used as sole modalities of treatment in those women at high risk to develop metastases.
Breast-conserving treatments
The goal of breast conservation is to remove the tumor and a rim of normal tissue while preserving the contour and shape of the breast. Tumor left at a margin will increase the ROR. Recht and colleagues, in a study of 533 patients, showed that recurrence risk depends on the amount of tumor left at the margin: grossly positive margins, focally positive margins, and close margins were associated with a recurrence risk of 27%, 14%, and 7%, respectively. Close margins did not materially change the recurrence risk in this study where all women received radiation therapy. The presence of extensive intraductal carcinoma, once thought to be a contraindication to breast conservation, has now been shown not to be associated with higher risk of local recurrence if all of the DCIS has been excised. After breast conservation, 90% of all in-breast recurrences, in the first decade after diagnosis, are in the same quadrant and genetically identical to the primary breast cancer. Long-term follow-up studies of BCS versus mastectomy, however, show that there is an ongoing risk for developing new cancers in the breast after 10 years, but these often occur in other quadrants.
With the advent of lumpectomy, there was a need to adopt a two-stage approach to biopsy and then to definitive surgery. Preoperative core needle biopsies, either clinically or radiologically directed, should be considered standard, so that the surgeon has a definite diagnosis before any surgery takes place and can better plan the operation. A detailed algorithm describing an optimal surgical strategy for the management of primary breast cancer has been described and is presented in Figure 14.
Figure 14 Recommended surgical strategy for management of primary breast cancer.
If an open breast biopsy must be carried out, it should be done as if a lumpectomy were being performed. Attention must be focused toward ensuring that specimen margins are likely to be free of tumor should a malignancy be encountered. In all circumstances where breast conservation is feasible, the operation carried out to establish the definitive diagnosis of a breast lesion becomes the definitive treatment, regardless of the time at which axillary surgery was performed. Most breast cancer operations—biopsy, lumpectomy, axillary dissection, and even mastectomy—can be performed as outpatient procedures with comparable low levels of surgical complications and equal or better personal and social adjustment to the procedure.
Technique and cosmetic considerations
In order to achieve the best cosmetic results, incision should be planned based on the extent of tumor in the breast, the location of the tumor, and the original shape of the breast. Many options now exist for patients who require breast surgery. The extent of resection of tissue depends on breast size. Plastic surgery techniques can be used to improve symmetry, both with and without cosmetic surgery on the contralateral breast. Once breast tissue is removed, undermining the breast tissue at the level of the fascia provides the opportunity to arrange the closure of the breast tissue in a medial to lateral direction, thereby avoiding the displacement of the nipple either superiorly or inferiorly. Mastopexy or reduction can be performed on the contralateral breast to improve symmetry. If there is a significant degree of ptosis in the breast, a partial mastectomy can be accomplished by performing a breast reduction, thereby combining a cosmetic enhancement with the oncologic procedure of removing the breast tissue (Figure 15). In a breast reduction procedure, more than half of the breast tissue can be removed; thus, this technique can be used for larger tumors or when there is scattered in situ disease in a single quadrant. Figure 15a and b shows a patient with significant discomfort from her large breast, who had always wanted to undergo breast reduction surgery.
Figure 15 Example of a breast reduction technique to accomplish a breast reduction and partial mastectomy in a woman with extremely large, pendulous breasts. (a) Preoperative and (b) postoperative images.
Clear margins were easily obtained by removing over a quadrant of the breast tissue, leaving medial and lateral flaps of at least 1 cm, and achieving a very nice cosmetic result. Preoperative MRI can be very useful for planning the surgical approach. High-resolution scans should be used to maximize the anatomic detail provided by MRI. When breast surgery is required, it provides an opportunity to change the shape and size of the breast, which, if desired by the patient, may make the surgical resection easier and turn an unpleasant procedure to a more tolerable one.
In order to perform a satisfactory lumpectomy, it is essential that the incision be placed directly over the tumor. The use of a circumareolar incision for removing a lesion that is not in proximity to the areola is not optimal, nor is tunneling through breast tissue to remove a lesion that is not beneath the incision. Tumor-free specimen margins are difficult and often impossible to obtain when such an incision is made. Reexcision of the tumor site to obtain free margins through such an incision is equally difficult. If there is concern that a mastectomy may eventually be necessary, the incision should be made with some thought as to what type of incision would be made if a mastectomy were required. In instances where lumpectomy cannot be carried out because of the inability to obtain tumor-free margins, mastectomy incisions can be modified to accommodate the lumpectomy incision. Preoperative diagnosis with needle biopsy can be of significant importance in planning the incision and should be the standard.
Skin removal is not required for lumpectomy. If a prior biopsy was performed, skin encompassing the biopsy scar can be removed when lumpectomy is done, but it is not essential. The quality of cosmesis is inversely related to the amount of skin removed. A special point to be emphasized is that skin edges should not be undermined when the excision is being performed, that is, thin skin flaps are not desirable. Undermining of skin can result in an unfavorable cosmetic result, and hence removal of skin may be the better cosmetic option in some cases.
Tumor removal and examination of specimen margins
The tumor is removed so that it is completely enveloped in normal fat and/or breast tissue. This procedure does not necessitate the removal of a predefined amount of normal tissue around the lesion, just the amount adequate to achieve specimen margins grossly free of tumor. Orienting the specimen is particularly important. Adopting a standard procedure to orient and ink specimens in your institution will improve communication among pathologists, surgeons, and radiation oncologists. A typical standard is to use a long stitch for the lateral margin and short stitch for the superior margin, secured to a piece of Telfa posteriorly to improve orientation. This is particularly beneficial for specimens requiring specimen radiography, by preserving the shape and improving the chance to identify and further resect a specific margin that may be close to the radiographic lesion (Figure 16).
Figure 16 (a) Image of gross specimen. (b) Radiograph of gross specimen showing calcifications.
If a prior excisional biopsy was performed and margins were not evaluated, it is obligatory that, at the time of node dissection, a reresection be performed to ensure tumor-free margins.
The specimen is immediately delivered to the pathologist, who must confirm or establish the diagnosis of cancer (if a needle biopsy was not done), aid the surgeon in deciding intraoperatively whether the specimen margins are grossly free of tumor, and take an aliquot of tumor for any special studies.
The pathologist receives the specimen and carefully orients it by means of the suture tags that the surgeon has placed. After measurement, the uncut specimen is inspected for gross margin involvement. If there is evidence that the tumor has been transected, the surgeon is immediately apprised of the precise location of the margin involvement so that additional tissue can be removed from that area while the pathologist is completing inspection of the specimen. The pathologist then coats the entire surface of the specimen with India ink, blots it dry, and then bisects the tumor and specimen transversely. Some pathologists use multiple colors (e.g., for the posterior, anterior superior half, and the anterior inferior half) to improve the ability to pinpoint the location of an involved margin. If tumor is found on gross examination to be close to the resected tissue margin, the pathologist may do a frozen section to determine margin involvement. Additional breast tissue can be removed to obtain a new true margin any time that margin involvement is considered uncertain. A multiplicity of frozen sections should not be carried out to determine whether the margins are tumor free. Margin assessment is better done with permanent sections in a detailed manner.
If the margin is later found to be involved microscopically following re-resection and no evidence of transaction of gross tumor is found, breast removal may not be recommended. In such circumstances, when it is clear that gross tumor has not been transected, it is likely that radiation and systemic therapy will provide adequate locoregional tumor control and that the majority of patients will remain free of local recurrence. An update on their series of reports on margin control from the Joint Center in Boston demonstrates that recurrence rates are the same for focally positive margins as for true negative ones.225 Similarly, when a breast tumor recurrence occurs following lumpectomy, it is likely that tumor control will be obtained by repeat lumpectomy if such tumors are small and can be removed with tumor-free specimen margins.
Pathologic criteria used for making a decision about whether the tumor involves specimen margins can vary. Many pathologists infer margin involvement by such subjective designations as tumor “very close” to margin. A review of cases showed that there was residual cancer in only 12% of total mastectomy specimens removed because the margin was close. Thus, it is most appropriate to regard lines of resection as involved only when cancer is transected.
For mammographically detected carcinomas requiring radiographic guidance, one or more wires are placed in the breast to either bracket calcifications or pinpoint the center of the lesion or calcifications. Specimen mammography is required once the lesion is removed to confirm the presence of the lesion as well as the location of the mammographic abnormality relative to the margins. Whenever possible, nonpalpable lesions that can be identified using ultrasound should be localized using that technique, because these procedures are better tolerated by patients and do not use compression required for mammographic localization. Some surgeons are being trained to use ultrasound as it can better improve the targeted excision of solid lesions in the operating room. This reduces the need and time for communication between the operating room and radiology suite. However, it requires that surgeons be specially trained, very skilled in the technique, and able to determine which lesions they can accurately identify to avoid missing the lesion intraoperatively.
Mastectomy
Patients who undergo mastectomy can opt reconstruction or not. Cosmetic considerations are important, regardless of a woman choosing reconstruction. It is important to try to leave a flat surface on the chest wall so that wearing a prosthesis is possible and comfortable. Avoiding skin folds in the axillary line can be accomplished through fishtail incisions at the axillary portion of the axilla or contouring the incisions in the axilla using plastic surgery techniques to avoid excess skin.
Immediate reconstruction has been shown to be safe,226 even in the setting of locally advanced disease (Figure 17), when appropriate multimodal therapy is used. Therefore, any woman considering mastectomy should also be told about options for reconstruction, both immediate and delayed. Complications after reconstruction are common and should be expected by both the surgeon and patient. Expectations about outcomes should be appropriately set, and women should be prepared for the possibility of multiple surgical procedures to optimize the reconstructive outcome.
Figure 17 Preoperative and postoperative images of a skin-sparing mastectomy with immediate reconstruction with TRAM flap delayed nipple reconstruction in a 37-year-old women (a, b) and 65-year-old woman (c, d). Postoperative images at 12 weeks (b) and 8 weeks (d) show appearance 5 days after nipple reconstruction.
If the decision to use chemotherapy does not require additional tissue or definitive resection of the primary tumor, and if chemotherapy if going to be part of multimodal treatment for a given woman, and if she is strongly considering mastectomy, based on the extent of disease or patient preference, neoadjuvant chemotherapy should be recommended (see systemic adjuvant therapy: neoadjuvant therapy). Sequencing the surgery and reconstruction after systemic therapy minimizes the risk that a complication would delay adjuvant therapy and gives women much more time to consider surgical options and adjust to the diagnosis. Neoadjuvant systemic therapy increases breast conservation rates. In a recent study of carboplatin and bevacizumab added to anthracycline- and taxane-based chemotherapy, treatment before surgery led to a conversion rate of 42% from ineligible to eligible for breast conservation therapy.227 Breast conservation was successful in 93% of those who chose it. However, about 30% of women who were eligible for breast conservation chose mastectomy. Neoadjuvant chemotherapy allows many patients with large breast tumors to have clinically meaningful tumor reduction, indicating response that would affect ability to undergo breast conservation. However, response varies by imaging and tumor subtypes. In the ISPY-1 trial, concordance between tumor size on MRI and surgical pathology was higher in well-defined tumors, particularly those with a triple-negative subtype, and was lower in HR+ diffuse tumors.228
For women who are ambivalent about reconstruction, delayed reconstruction may be optimal. However, cosmetic outcomes are better when reconstruction is performed immediately, and scars can be minimized and tailored to the type of reconstruction that has been chosen. It is difficult to make reconstruction decisions within days, and women who are deciding should be reassured that an extra week or two to study their options and make good decisions would not affect their survival outcome. They will likely live for decades with the consequence of this decision, and hence the investment of a few weeks, if necessary, to ensure that they are comfortable with their decision is important.
Two basic types of reconstruction can be offered: implant reconstruction and autologous tissue reconstruction. Implants can be placed as expanders or permanent implants. Expanders are the most commonly used form of reconstruction. They are placed under the pectoralis muscle and the pocket is gradually expanded until is it larger than the desired breast size. Then the expander is exchanged for a permanent implant and the breast shape is contoured. The entire process can take 6–8 weeks. An alternative technique, used in conjunction with skin-sparing mastectomy (SSM), is the placement of permanent implants. The majority of implants used for reconstruction are saline. Although silicone implants were pulled off the market because of a concern that they increased the risk for developing autoimmune disease, several large studies have failed to show a definitive connection; as a result, silicone implants are again being used.128 There are specific instances where silicone products may be preferred (e.g., if a permanent implant is placed immediately, but the ability to expand the implant is desired). Autologous tissue flaps include TRAM, DIEP, or latissimus dorsi flaps (Figures 18 and 19).
Figure 18 Examples of total skin-sparing technique combined with various reconstruction techniques. (a) Placement of immediate implant using mastopexy incision and contralateral mastopexy. Mastectomy is on left; images are 3 weeks postoperative. (b) Bilateral mastectomy with placement of immediate implants in a woman at high risk after recurrence subsequent to prior lumpectomy for subcentimeter DCIS. Preoperative MRI demonstrated single small focus of lateral recurrence. (c) Mastectomy on right followed by immediate reconstruction using TRAM flap.
Figure 19 Latissimus dorsi reconstruction with bilateral augmentation in a 39-year-old woman. Pre- (a) and (b) 12-week postoperative image.
Decision about the type of reconstruction depends largely on patient preference, although treatment considerations can also play an important role. If radiation is anticipated, complications are less if autologous tissue is used. There is controversy about whether TRAM flaps can tolerate radiation, and several published reports suggest that radiation causes significant deleterious effects on the flap. It may be that free flaps, requiring an anastomosis, tolerate radiation as well as pedicle flaps, or more likely, institutions that use higher doses of radiation (up to 6500 Gy including the boost) may experience more complications. An institution from Dundee, Scotland, that limits radiation to the chest wall to 4500–5000 Gy recently reported excellent results in a large series of patients.
A new technique for mastectomy is the total skin sparing mastectomy (TSSM), which has become a standard procedure in many centers.229, 230 This technique removes all of the breast tissue, including the tissue of the areola and nipple, but preserves the overlying dermis. A report of 171 cases shows that over time, the technique has become reliable and that various incisions can be used to achieve excellent results. This technique results in 99% preservation of nipple and areola skin. Although follow-up is limited, early results show that the local recurrence rate is extremely low (<2%), and we anticipate that the total skin sparing technique will not affect recurrence risk. The key is the complete removal of the nipple duct tissue (Figures 18 and 19). This technique can be combined with any reconstructive technique except the use of permanent implants. The immediate expansion of the skin significantly increases the risk of skin necrosis. This exciting development in technique, while challenging, offers safety associated with the removal of all of the breast tissue combined with superior cosmetic results. The opportunity to use TSSM is particularly important when considering prophylactic mastectomy for women at highest risk and may enable them to feel comfortable about the cosmetic result to undergo the surgery.
Axillary dissection
Axillary dissection is not used with the intent of enhancing curability, because regional lymph nodes are regarded as indicators of distant metastatic disease rather than instigators of such tumor.
The incision for axillary dissection should be separate from that used for the removal of tumor in the breast. A longitudinal incision placed along the posterolateral margin of the pectoralis major muscle or a transverse incision just below the axillary hairline may be used. At present, an axillary dissection includes nodes from axillary levels I and II. Anatomic delineation of this dissection is the latissimus dorsi muscle laterally, the axillary vein superiorly, and the medial border of the pectoralis minor muscle medially. Removal of the pectoralis minor muscle is not required. The nerves to the serratus anterior and latissimus dorsi muscles should be identified and preserved. The axillary vein should be visualized and followed under the pectoralis minor muscle to the medial border. These are the minimal limits for the dissection. The average number of nodes removed is about 15. Although the lumpectomy site in the breast is not drained, a suction drain is present in the axilla for several postoperative days.
The management of the axilla has changed significantly since the introduction of sentinel node dissection. This procedure limits considerably the extent of surgical procedure in the axilla and, for majority of patients with negative axillary lymph nodes, precludes the need for formal axillary dissection while providing similar (and in some cases superior) diagnostic and prognostic information. The identification of a single (or a few) sentinel node(s) also permits the pathologist to perform a more detailed assessment of multilevel sections of the sentinel node. The detection of micrometastases is significantly increased by combining light microscopy, IHC, and even more sensitive molecular techniques. However, the prognostic significance of identifying isolated metastatic cells in histologically negative lymph nodes by more sensitive techniques is still undetermined; therefore, at this time, it is not considered sufficient to constitute stage 2 disease. Sentinel node biopsy and assessment are under extensive evaluation by large cooperative research groups, including the NSABP and ACOSOG. ACOSOG Z-010 trial required clinicians not to use immunohistochemical analysis in their hospital, so that all nodes could be centrally stained and the results blinded to assess the prognostic significance of microscopic metastases to the lymph nodes. NSABP B-32 randomized 5611 women with negative sentinel lymph node (SLN) to either SLN biopsy only or SLN biopsy followed by ALND. Women who were found to have positive SLN also proceeded to full ALND. The strength of the study is that it is the largest randomized trial of SLND and that it is representative of a cross section of surgeons across the country with 232 surgeons participating across 80 centers. Although longer-term outcomes, regional control, and survival are not yet known, the technical results are. On average, three SLNs were removed per patient; the SLN identification rate was 97.2% and improved with surgeon experience, and 26% of patients had a positive sentinel node. In 61.5% of patients with a positive sentinel node, the additional nodes were negative (38.5% had additional positive nodes). The false-negative rate (axillary node involvement when the SLN was negative) was 9.7%, which did not change with experience, but was significantly affected by the type of biopsy used for diagnosis; the false-negative rates were 8.0%, 14.3%, and 15.2% for FNA/core, incisional biopsy, and excisional biopsy, respectively. The number of SLNs identified also affected SLN false-negative rates. The confidence in a negative result is higher if more than one SLN is identified, or if the prior probability of having a negative node is high, but may be less for the woman with a higher prior probability of having a positive node.
Sentinel lymph node dissection
The use of radiotracer material or visible blue dyes to locate and remove the SLN (the node that drains the tumor site most directly) is becoming a standard technique for evaluating the axilla. This technique was initially described for melanoma and then studied in breast cancer patients and has a high degree of accuracy once the operator has become proficient with the technique. The ability to identify a sentinel node ranges from 85% to 97%. The ACOSOG, in selecting surgeons to participate in a sentinel node registry trial as well as a trial to determine if full node dissection after SLN removal was beneficial, required proof that the sentinel node could be identified in 90% of cases and that the false-negative rate for SLN was less than 5%. Lymphedema rates after SLND are reported to be <7% compared with 17–25% with ALND.231 SLND has been shown to exhibit similar performance after neoadjuvant chemotherapy232; although the identification of the SLN may be somewhat lower, the false-negative rate is very similar. Although still somewhat controversial, the potential benefits of using SLND after neoadjuvant therapy are that the patient is spared an additional operation before starting chemotherapy—they may avoid an axillary dissection as tumor in the nodes may disappear with chemotherapy—and that the information about the presence of tumor in the nodes after chemotherapy is beneficial in predicting local recurrence rates and determining the need for radiation therapy.151 Intraoperative frozen section has not been as accurate in detecting nodal metastases as is specific analysis done later in the laboratory. Imprint cytology has recently been reported to have a very low false-negative rate, but the sensitivity is not as high as frozen section. In NSABP B-32, cytology was found to indeed have a low false-positive rate, 0.4%, but a sensitivity of 61.5%. Intraoperative detection of LN metastases is beneficial as it enables the surgeon to proceed to a full axillary dissection at the time of SLND, thereby avoiding a second procedure. Clinicians should use the technique in their own institution that yields the lowest false-positive rate to avoid further surgery.
Radioisotope injection with a gamma isotope-labeled colloid and scanning with a handheld probe is replacing blue dye injection, which provides a visible clue of blue lymphatics, leading to the blue sentinel node. Although some authors strongly favor one or the other of these, the majority of reports indicate that the combination of both results in the highest level of successful identification of the sentinel node. In NSABP B-32, the radioisotope had a higher SLN identification than blue die. There is a small but real risk (<1%) of anaphylaxis with Lymphazurin blue dye. The risk of severe anaphylaxis was shown to be 0.2% in NSABP B-32. Anesthesiologists should be aware of this possibility if blue dye is used.
Need for axillary dissection
It is frequently asked whether all lumpectomy patients require axillary staging. If the need for systemic therapy, as well as the type of systemic therapy, can be determined by patient and tumor characteristics other than the status of the axillary nodes, then the need for axillary staging becomes less clear. Furthermore, if all node-negative and node-positive patients were to be given the same systemic adjuvant therapy, as is the case in trials of preoperative chemotherapy, there would seem to be no reason to know the nodal status, except for predicting patient outcome. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overview suggests that adjuvant chemotherapy benefits women with node-negative and node-positive cancers and that the proportionate reduction in risk of treatment failure is the same for both groups, and many oncologists offer adjuvant chemotherapy to all women except those that have such a low proportionate reduction as to be of little real gain. However, modified decision-making tools such as adjuvantonline.com, a well-validated tool for predicting absolute benefit of chemotherapy,233 or molecular tools that provide a recurrence score (RS) such as the Oncotype DX234 have increased the ability of oncologists to refine recommendations for systemic therapy. The molecular characteristics of the tumor may be more important than stage in determining whether to perform adjuvant therapy and which type to use. Two molecular tests used in the United States (trial assigning individualized options for treatment (TAILORx) trial) and Europe microarray in node-negative disease may avoid chemotherapy (MINDACT) are in clinical trials to better answer these questions. As these data mature, the roles of sentinel node dissection and axillary dissection will need to be clarified. In the molecular age, there are still situations where removal of all possible locoregional tumor may be very important: for those women in whom surgical excision is the primary treatment, for example, in a setting with a low predicted ROR, or for women who have undergone neoadjuvant chemotherapy and have residual disease and possibly resistant to best available therapies. In the setting of complete pathologic response after neoadjuvant therapy, the probability of performing surgery may be low, and the increasing use of sentinel node dissection after neoadjuvant chemotherapy in women with clinically N0 disease, regardless of nodal status pretreatment, is an example of modifying the extent of surgical treatment to response to therapy. The need for axillary dissection may also be questioned, particularly in elderly women with ER-positive disease who are planning to undergo adjuvant hormonal therapy. In this setting, the status of the axillary nodes would not likely alter the decision about administration of adjuvant therapy. In almost all cases with clinically negative axilla, however, sentinel node dissection is a standard part of staging and surgical management and can be successfully performed in most women with very low morbidity. In the setting of positive sentinel nodes, level I and II axillary dissections are considered standard management. Although it is generally accepted that axillary dissection provides optimal local control of the axilla, a randomized trial comparing total mastectomy with and without axillary dissection suggested that not all patients (∼50%) with positive axillary lymph nodes would develop an axillary recurrence in the absence of an axillary dissection.
The multicenter ACOSOG Z011 trial randomized women having breast-conserving surgery with a positive sentinel node to either full axillary dissection or not.235 The target accrual was 1900; 991 were actually enrolled. There was no difference between the two arms between 5-year axillary or in-breast recurrence, and most importantly, there was no difference in either disease-free or OS. These important results have already had a significant impact on standard management of the axilla, sparing many women from the potential long-term toxicities of a full axillary dissection. Management of the axilla in women undergoing mastectomy with a positive sentinel node still mandates further axillary sampling unless radiation is planned for the primary tumor.
Since the routine use of mammographic screening, the vast majority of patients diagnosed with invasive breast cancer have T1 or T2 primary tumors that are amenable to a breast-conserving locoregional treatment. Breast conservation therapy has currently been firmly established as an appropriate standard of care for women with early-stage breast disease. This therapy consists of three important components: removal of the tumor with achievement of negative margins (often called a lumpectomy, tylectomy, or a segmental mastectomy), surgical assessment of axillary lymph nodes with either a sentinel lymph node surgery and/or a level I/II ALND, and breast irradiation. Most women treated with modern breast conservation approaches achieve excellent outcomes. When combined with systemic therapy, the annual local recurrence rate after appropriate breast conservation treatments has been reported to be less than 0.5% for women with favorable disease.236, 237 Furthermore, the complication rates of breast conservation therapy are very low and continue to improve with advances in surgical and radiotherapy techniques.
Despite these facts, breast conservation remains underutilized in the United States. In a recent multinational randomized trial comparing two hormonal therapies for women with early stage disease, the rate of breast conservation in the United States was only 49%.238 By contrast, the rate of breast conservation in the United Kingdom was 58%, and even higher rates were observed in Sweden, Germany, or Australia/New Zealand. Previous data had also indicated that the use of breast conservation varies within regions of the United States, with Southern and Midwestern women less likely to be treated with breast conservation than those from either the East or West Coast.239 The reasons for the underuse of breast conservation are likely multifactorial. Unfortunately, some women undergo mastectomy based on a misperception that mastectomy is likely to achieve a superior outcome. It is critical, therefore, that breast cancer providers understand the data concerning breast conservation, so that patients with newly diagnosed disease can be given the option of this treatment if appropriate.
Breast conservation therapy versus mastectomy
Breast conservation has been studied as an alternative locoregional treatment to mastectomy for over 40 years. After initial successful results were obtained in single institutions, a number of phase III clinical trials were initiated, which directly compared the outcome of patients treated with breast conservation versus those of patients treated with mastectomy.240–242 The NSABP B-06 study was one of the most important trials.234 This trial began in 1976 and enrolled 1843 women. Patients with T1 or T2, N0, or N1, M0 breast tumors of 4 cm or less were randomly assigned to one of the following three treatment groups: (1) modified radical mastectomy, (2) lumpectomy and axillary dissection, and (3) lumpectomy, axillary dissection, followed by radiation therapy. With 20 years of follow-up, this trial demonstrated that breast conservation therapy provides survival equivalent to mastectomy.
Contraindications for breast conservation therapy
Selected patients with ESBC are not suitable for breast conservation therapy, because the primary tumor cannot be successfully removed with a lumpectomy. For example, patients presenting with diffuse suspicious calcifications that cannot be resected with a lumpectomy that leaves an acceptable aesthetic result are best treated with a mastectomy. Similarly, patients in whom repeated attempts of BCS did not achieve negative surgical margins are best managed with a mastectomy. Increasingly, however, women who present with stage II and III cancers can be treated with neoadjuvant chemotherapy or hormone therapy, and over 50% have sufficient shrinkage of the primary tumor to enable breast conservation. A second reason why some patients are not candidates for breast conservation is that they are at high risk for radiation complications. Specific examples of such patients include those previously treated with radiation, women who are pregnant, and patients with certain connective tissue diseases. For women early in the course of their pregnancy, internal radiation scatter from irradiation of the intact breast can reach lethal and teratogenic dose levels.243 Certain collagen vascular diseases, such as systemic scleroderma, polymyositis, dermatomyositis, lupus erythematosus, and mixed-connective tissue disorders have been associated with significant risks, including breast fibrosis and pain, chest wall necrosis, and brachial plexopathy.244
Radiation therapy
For more than 100 years, radiation has had a vital function in the effective treatment of breast cancer. From its crude and early history as the first adjuvant therapy to radical surgery to its role as the vehicle that drove the breast conservation revolution of the 1970s, radiation oncologists and surgeons formed the first “multidisciplinary” cancer teams. Radiation therapy is a component of care for the overwhelming majority of breast cancer patients at some point during the course of their disease. Therefore, it is crucial that radiation oncologists become involved at the time of diagnosis to collaborate with medical oncologists and breast surgeons in the prospective design of each patient’s care path. At present, radiation therapy has been in the midst of a technological renaissance, creating challenges and controversies in the balance of evidence-based medicine and personalized, modified therapy.
Role of radiation in BCS for early-stage invasive disease
Defining the role of radiation in breast conservation therapy has been the focus of numerous randomized prospective clinical trials conducted over the past 30 years.241, 242 In conclusion, these trials have demonstrated that radiation after breast-conserving surgery significantly improves local control, minimizes the risk of subsequent distant metastases, and decreases breast cancer death rates.
Benefits of adjuvant radiation
The NSABP B-06 trial was one of the first randomized studies to evaluate the benefit of radiation after lumpectomy. This trial showed that for patients treated with breast conserving surgery, whole-breast radiation therapy (WBRT) offered significant clinical benefits. After 20 years, the recurrence rates in the breast were 40% and 14% for those treated with lumpectomy only and lumpectomy plus WBRT, respectively. The almost two-thirds reduction in the ROR was very similar to reductions observed in other similarly designed trials.234 The data from NSABP B-06 and the other prospective clinical trials assessing the role of radiation for patients with invasive disease treated with breast conservation have been analyzed by the EBCTCG. In this important meta-analysis, the individual patient data from 7300 women were studied. Breast irradiation after lumpectomy reduced the 10-year rate of in-breast recurrence from 29% to 10% for patients with negative lymph nodes and from 47% to 13% for patients with positive lymph nodes. More importantly, radiation use significantly decreased the 15-year risk of dying from breast cancer. For patients with negative lymph nodes, breast cancer mortality decreased from 31% to 26% and for patients with positive lymph nodes, it decreased from 55% to 48%.242
Use of a “boost” after WBRT
A strategy to reduce local recurrence after WBRT even further is dose escalation via an additional boost of radiation to the tissue at the highest risk in the postoperative tumor bed. The first randomized trial investigating the impact of a 1000-cGy boost following 5000-cGy WBRT was performed in Lyons, France. The use of a boost led to a small but statistically significant reduction in the rates of local recurrence at 5 years (3.6% vs 4.5%, p = 0.04).245 Subsequently, the EORTC completed a much larger trial that randomized over 5000 patients to 5000-cGy WBRT with or without an additional tumor bed boost of 1600 cGy. This trial again demonstrated a reduced breast recurrence rate in patients treated with a boost (10.2% vs 6.2% at 10 years; p < 0.001).246 In a subset analysis, this benefit was noted across all ages but most pronounced in younger women.
Omission of radiation for selected patients
Although the data are conclusive that radiation is beneficial for the majority of patients treated with breast-conserving surgery, there remains an interest as to whether subsets of patients may do well with surgery only. Therefore, some trials have selected patients with more favorable disease characteristics to investigate the omission of radiation. An early trial conducted in Milan, Italy, randomized patients who underwent quadrantectomy and axillary clearance for tumors ≤2.5 cm to undergo WBRT versus no further therapy. The 10-year results from this trial showed that radiation reduced the breast recurrence rate from 24% to 6% (p < 0.001).247 For patients with even smaller primary tumors (stage I), separate randomized trials in Sweden and Finland showed that radiation reduced the 5-year breast recurrence rate from 18% to 2% (p < 0.0001) and from14.1% to 6.2% (p = 0.029), respectively.248, 249
Data from early trials have also indicated that the use of systemic treatment (either chemotherapy or tamoxifen) does not require breast radiation. In the aforementioned NSABP B-06 trial, chemotherapy was used in patients with lymph node-positive disease, and the 20-year breast recurrence rate for these patients was 44% when no radiation was used versus 9% when radiation was used.234 A trial from Scotland that required systemic treatment for all of the 589 enrolled patients yielded a 6-year breast recurrence rate of 6% in the surgery, systemic therapy, and radiation arm versus 25% in patients randomized to forego radiation.250
Four more modern trials conducted among very low-risk subgroups of patients diagnosed in the mammographic era have further refined the selection criteria.215, 216, 251, 252 In all four of these studies, radiation achieved a statistically significant reduction in breast recurrence rate, although the absolute benefit for some subcategories of patients treated with surgery only was relatively small. The NSABP B-21 trial enrolled only patients with lymph node-negative breast cancers whose primary tumors measured ≤1.0 cm. All patients underwent lumpectomy and ALND and were randomized to tamoxifen only, radiation only, or tamoxifen plus radiation.249 A contemporary Canadian trial had very similar eligibility criteria and results. Finally, two trials recently tested the omission of radiation for older women with early stage, biologically favorable disease. Long-term results of the CALGB study showed that for patients over the age of 70, the 10-year recurrence rates were 10% and 2% in the tamoxifen only and tamoxifen plus radiation arms, respectively.215 Furthermore, there were no differences in the rates of salvage mastectomy, distant metastases, or OS between the two groups. The similarly structured Scottish PRIME II trial enrolled over 1300 patients with early-stage disease of age ≥65 and found 5-year local recurrence rates of 4% and 1% for those on tamoxifen only and tamoxifen plus radiation (p = 0.0002), respectively.216
In conclusion, almost all of the clinical studies to date have indicated that breast radiation reduces local recurrences following BCS and therefore should be considered a standard component of treatment for all women with early-stage invasive disease. Thus far, most attempts to define favorable subsets of ESBC patients who may not require radiation have been unsuccessful with the exception of older women with biologically favorable disease planning to undergo hormonal adjuvant therapy. In this cohort, the ROR is low, the overall benefit is small, and hence the addition of radiation should be based on the patients’ life expectancy and personal preferences.
Radiation as a substitute for radical axillary surgery in early-stage node-positive patients
In the era of mammographic diagnosis, ubiquitous systemic therapy, and SLNB, there has been an enormous interest in reducing the use of axillary dissection for not only node-negative patients but also low-volume node-positive patients. The interest in eliminating radical axillary surgery is particularly important for patients in whom radiation is planned, as the risk of lymphedema and its impact on quality of life (QOL) are higher when these modalities are combined. The previously referenced ACOSOG Z0011 trial showed that for patients undergoing BCS with WBRT for early-stage disease with one to two positive nodes, the addition of axillary dissection to SLNB did not improve disease-free or OS at 5 years.233 The EORTC 10981-22023 (AMAROS) analyzed a similar patient population, includingpatients with more than two positive nodes (5%) and patients who had undergone mastectomy (18%).253 Over 1400 patients with a positive SNLB were randomized to receive either axillary dissection or axillary irradiation, and at 6 years there was no statistical difference in axillary recurrence. Although the trial was underpowered because of the low overall number of events, the rate of upper extremity lymphedema was significantly higher for patients who underwent dissection. These two trials taken together have had a broad, practice-changing effect on the locoregional treatment of early-stage node-positive patients, directing therapy away from radical surgery. Given that axillary recurrences are rare events, typically occurring within 2 years,234, 254 longer-term follow-up of these two trials is unlikely to alter their significant impact on clinical practice.
Role of radiation after mastectomy for locally advanced disease
A strong rationale exists for combining the beneficial effects of radiation and mastectomy for selected women with locally advanced breast cancer, in whom areas of subclinical disease extend beyond the operative field. The initial studies that investigated radiation after mastectomy began in the 1950s and represented some of the first controlled clinical trials in oncology history. After five decades of study, there still remain controversies over the selection criteria and value of postmastectomy radiation therapy (PMRT), mainly due to two factors: the late-term morbidity of early radiation techniques and the addition of systemic therapy as a routine component of care. In general, however, as PMRT techniques have improved and systemic therapies decrease the competing risk of distant metastases, improvements in locoregional control gained by radiation have had a consequential positive effect on survival.
Modern data supporting PMRT
There have been several meta-analyses conducted to quantify the value and risk of PMRT, the most comprehensive and important being the work of the EBCTCG. This group was able to obtain the raw data from every randomized prospective trial of PMRT, including nearly 10,000 randomized to radiation versus observation following mastectomy and axillary clearance. For node-positive patients, PMRT reduced the risk of locoregional recurrence threefold (29–8%), which led to a 5% absolute decrease in breast cancer mortality (60% vs 55%) at 15 years. For patients with node-negative disease, the locoregional benefit was smaller (8–3%) and not associated with a difference in survival.242 Overall, data from this meta-analysis suggested that a long-term survival benefit was only manifested in the trials where there was a ≥10% improvement in 5-year locoregional control.242 As such, there is a clear indication that the role of PMRT in eradication of persistent postsurgical microscopic disease is to prevent the development of a subsequent local source for distant metastasis, which eventually can lead to death.
The three PMRT randomized trials that most heavily influenced the EBCTCG meta-analysis all involved relatively modern radiation techniques and the judicious use of systemic therapy. The Danish Breast Cancer Cooperative Group (DBCCG) 82b trial, perhaps the most important study, randomized 1708 premenopausal women with stage II or III breast cancer to mastectomy and CMF-based chemotherapy with or without PMRT.255 The majority of patients entered in this trial had one to three positive lymph nodes; however, the median number of axillary lymph nodes resected was only seven, rather than a formal level I/II axillary dissection. Patients randomized to PMRT had an improved OS rate at 10 years (54% vs 45%, p < 0.001), likely a consequence of decreased locoregional recurrence (9% vs 32%, p < 0.001). Simultaneously, investigators in Vancouver, British Columbia, conducted a similar, albeit smaller trial in which 318 premenopausal women with lymph node-positive disease were randomized to receive mastectomy and CMF with or without PMRT.256 The results were nearly identical with PMRT providing a 10% absolute benefit in long-term OS (20-year rates: 47% vs 37%, p = 0.03) and an even higher benefit in locoregional control (87% vs 61%; p < 0.0001).
Finally, the DBCCG 82c randomized 1300 postmenopausal patients to mastectomy and tamoxifen with or without PMRT.257 The stage of disease and extent of axillary surgery were also very similar to the 82b trial. The magnitude of benefits in OS and local control was similar to the two previous studies in terms of 10-year OS (45% vs 36%, p = 0.03) and locoregional control (92% vs 65%; p < 0.001). At almost 20 years of follow-up, updates of the DBCCG 82b and 82c combined data continue benefits of PMRT for both locoregional control (86% vs 51%, p < 0.0001) and distant metastasis-free survival (47% vs 36%, p < 0.0001).258
Current indications for postmastectomy radiation
The American Society of Clinical Oncology (ASCO) consensus guidelines for PMRT, published almost 15 years ago, recommended treatment for patients with stage III disease (defined by T3 with involved lymph nodes, T4 primaries, or four or more involved lymph nodes)259 after mastectomy, standard axillary dissection, and chemotherapy due to the magnitude of survival benefit for these locally advanced patients. However, the landmark publication left the role of PMRT for patients with stage II disease with one to three positive nodes unsettled and hence controversial. The reason for this is that although most of the patients in the Danish and British Columbia trials had one to three positive lymph nodes, the benefit of PMRT for this subset has been inquired by surgeons who indicate the inadequate clearance of the axilla in the standard arm of the larger Danish trial. Indeed, the long-term locoregional recurrence rate for these patients in the Danish trials, who were treated without PMRT, was 41% versus 21% in similar patients who underwent a more complete axillary clearance in the British Columbia trial.256, 260 It is important to note that inadequate axillary surgery may have led to an underestimation of the true number of positive lymph nodes and that many of these patients would likely have had four or more lymph nodes who already underwent more extensive surgery.
The findings and critiques of the Danish and Canadian Studies heavily influenced the ASCO consensus guidelines for PMRT (published in 2001), which in turn have guided clinical practice patterns for over a decade. More recently, a meta-analysis by the EBCTCG published in 2014 has sought to clarify the utility of PMRT for the most controversial patients, those with small primary tumors and one to three positive nodes.258 Data from 22 randomized trials including over 3700 patients treated with PMRT after axillary dissection to at least level II were analyzed for differences in 10-year locoregional recurrence and 20-year survival. For patients with one to three positive nodes in whom systemic therapy was followed (n = 1133), PMRT significantly reduced locoregional recurrence by more than three-quarters (20% vs 4%; p < 0.0001) and improved breast cancer-specific mortality by approximately 8% (50% vs 42%; p = 0.01). Interestingly, the magnitude of benefit in this controversial subgroup was nearly the same as that for patients with four or more positive nodes for both locoregional recurrence (32% vs 13%; p < 0.00001) and breast cancer mortality (80% vs 70%; p = 0.04).
Therefore, there should remain no doubt regarding the utility of PMRT for all patients with positive lymph nodes and a >10-year life expectancy. In addition to the number of positive lymph nodes, other adverse pathologic features such as the volume of nodal disease, the presence of extra nodal extension, and the percentage of nodes involved should be used to guide clinical decision making. In conclusion, the EBCTCG update,261 together with the aforementioned AMAROS trial,253 indicated a trend of expanding the application of PMRT at a time when the role of axillary dissection is diminishing. It is likely that a more complete understanding of tumor biology and genomic profiling will play a role in further guiding clinical practice in the not-to0-distant future.
Integrating PMRT with breast reconstruction
Two trends on a collision course over the last decade have been the expanding indications for PMRT, particularly in younger patients, versus the increasing use of elective mastectomy combined with reconstruction in this population.262 While breast reconstruction, both implant- and tissue flap-based, poses certain challenges for the treating radiation oncologist, it should never be considered a contraindication to PMRT. While plastic surgery practice patterns are highly variable and guided more by experience than randomized trials, there are certain common principles that have developed over time. In general, immediate reconstruction (in a patient requiring PMRT) is best accomplished with a tissue expander (TE)/implant-based technique.259 This is the most popular modality as it consolidates surgical procedures and allows full-tissue expansion through the month long course of adjuvant chemotherapy. The TE may be swapped for the permanent implant before PMRT263 or left in place through the course of PMRT, allowing its deflation to improve radiation dosimetry.264 Alternatively, autologous flap techniques are the procedures of choice for delayed reconstruction, where vascularized tissue is brought into the irradiated field for improved wound healing and cosmetic results.262 While breast reconstruction after mastectomy proves vital to many patients in terms of QOL posttreatment, it is important that patients not short change their oncologic therapy for fear of consequences that are cosmetic and rectifiable.
Role of radiation after neoadjuvant chemotherapy
Modified radical mastectomy followed by adjuvant chemotherapy and PMRT has been the historical standard locoregional therapy for patients with locally or regionally advanced primary tumors. However, over the past three decades, the use of neoadjuvant chemotherapy (chemotherapy before surgery) has significantly increased in patients with advanced disease. This strategy was first adopted for patients with unresectable or marginally resectable disease, and the initial clinical data from such patients indicated that chemotherapy achieved high rates of tumor response. Subsequently, this approach has also been investigated in patients with large, resectable breast cancers at diagnosis.
Breast conservation after neoadjuvant chemotherapy
One of the original main reasons to investigate sequencing chemotherapy before surgery was to determine whether breast conservation could be offered to selected patients with larger primary tumors whose residual disease would then be amenable to a breast-conserving operation. In order to investigate this, the NSABP and EORTC independently conducted clinical trials that compared neoadjuvant chemotherapy with adjuvant chemotherapy for patients with stage II or III breast cancer. Although both trials found that breast conservation rates were higher in the neoadjuvant chemotherapy arms,265, 266 the NSABP study showed that this increase was largely due to a near tripling of breast conservation use for patients with T3 disease postchemotherapy (22% vs 8%).264
The goal of breast conservation therapy for patients with advanced disease is to achieve an aesthetically acceptable cosmetic outcome and a low risk of breast recurrence. Accordingly, for patients with large initial primaries, resection must be directed at the postchemotherapy tumor bed rather than the initial volume of residual disease. However, one concern with resecting only the postchemotherapy volume of residual disease is that some advanced breast cancers do not shrink concentrically to a solitary nidus in response to neoadjuvant chemotherapy, but rather break up into nests of residual disease over the initially involved volume.193 In such cases, surgery directed at the primary core may exert more pressure of microscopic disease around the tumor bed site, which may theoretically be associated with higher rates of breast cancer recurrence.
In the NSABP B-18 trial, the overall rate of breast cancer recurrence did not statistically differ in the patients treated with neoadjuvant chemotherapy compared with those treated with adjuvant chemotherapy (16-year rates of 13% vs 10%, respectively).267 However, breast cancer recurrence rate was higher in patients with large primary tumors in whom a response to neoadjuvant chemotherapy permitted a breast conserving surgery. In this subset of patients treated with neoadjuvant chemotherapy, the breast recurrence rate at 8 years was 16%, which was more than twice the rate in the patients with smaller tumors who were treated with breast-conserving surgery first.264 Other multicenter series have also shown relatively high breast cancer recurrence rates in patients who receive neoadjuvant chemotherapy.268, 269
In contrast to these data, single-institution studies with careful selection criteria and a high degree of multidisciplinary coordination have reported excellent rates of local control. Investigators at the University of Texas MD Anderson Cancer Center have recently published the results of one of the largest studies investigating breast conservation after neoadjuvant chemotherapy.267 In this study, 340 carefully selected patients who had a favorable response to chemotherapy were treated with breast-conserving surgery and radiation. Despite the fact that 72% of patients in the study had clinical stage IIB or III disease, the 5- and 10-year breast cancer recurrence rates were only 5% and 10%, respectively. Four tumor-related factors were associated with breast cancer recurrence and locoregional recurrence: clinical N2 or N3 disease, lymphovascular space invasion, a multifocal pattern of residual disease, and residual disease higher than 2 cm in diameter.270 These investigators subsequently developed a prognostic index from these factors and found that for patients with 0 or 1 index, breast conservation will offer similar excellent outcome as mastectomy and radiation. However, mastectomy and radiation were associated with a lower risk of locoregional recurrence in the patients with three or more adverse factors.271
PMRT after neoadjuvant chemotherapy
While data indicate that breast conservation therapy is appropriate for patients with advanced primary tumors that have a favorable response to neoadjuvant chemotherapy, much less is known about the indications for and efficacy of PMRT for such patients. As previously discussed, for patients treated with up-front mastectomy, the decision to administer radiation therapy is made based on the pathological extent of disease. It is clear that neoadjuvant chemotherapy changes the extent of pathological disease in 80–90% of cases, and this may hold implications for locoregional recurrence.268 Therefore, both the pretreatment clinical stage and the extent of pathologically defined residual disease need to be considered when assessing the risk of locoregional recurrence in patients treated with neoadjuvant chemotherapy and mastectomy. Indeed, in an analysis of the patients treated with neoadjuvant chemotherapy, mastectomy, and no radiation, a multivariate analysis of locoregional recurrence predictors found that both pre- and posttreatment factors were independent predictors.272 One large retrospective study of PMRT after neoadjuvant chemotherapy compared outcomes of 579 patients who received radiation following neoadjuvant chemotherapy and mastectomy with 136 patients who were treated with neoadjuvant chemotherapy and mastectomy only.273 Despite the fact that the PMRT cohort had more extensive disease than those treated without radiation, irradiated patients had a significantly lower locoregional recurrence rate at 10 years (8% vs 22%, p = 0.001). In the subgroup of patients with clinical T4 tumors, clinical stage IIIB/C disease, and in those with four or more positive lymph nodes after chemotherapy, the absolute improvement in locoregional recurrence risk was approximately 30–40%. The use of radiation in these same subgroups was associated with an approximately 15–20% improvement in overall and cause-specific survival. In a subsequent analysis from this group that focused only on patients who achieved a pathologic complete response (pCR), the locoregional recurrence rate for them with clinical stage III disease was improved with radiation therapy (33% vs 7%, p = 0.04).274
Whether to use PMRT for patients with clinical stage II disease who have positive lymph nodes after chemotherapy remains less clear. A report of 132 patients with clinical stage II disease who did not receive radiation therapy after neoadjuvant chemotherapy and mastectomy indicated that patients with clinical T3N0 disease or four or more positive lymph nodes had high rates of locoregional recurrence.275 The 5-year local recurrence rates in the remaining patients were less than 10%. In the patients with clinical stage II disease who had one to three positive lymph nodes after neoadjuvant chemotherapy, the 5-year rate of locoregional recurrence was 8%. It is important to recognize that this study had a small sample size and that the studied population was prone to selection biases, in that they represented only those patients for whom the treating physicians elected not to use radiation.
On the basis of the available data, it is reasonable to recommend PMRT for all patients with clinical stage III disease at initial presentation. It is also reasonable to infer that patients with clinical stage I or II disease who have four or more positive lymph nodes after chemotherapy or a primary tumor size >5 cm should receive radiation. The role of PMRT is currently controversial for patients with clinical stage I or II disease who have one to three positive lymph nodes after neoadjuvant chemotherapy276 and node-positive disease at diagnosis, but proceed toward a pCR. Fortunately, these clinical conundrums are the subjects of two ongoing complementary prospective randomized trials in the United States. The NSABP B-51 trial is open to pathologically confirmed node-positive patients who convert to node-negative after neoadjuvant chemotherapy. Patients are then randomized to WBRT with or without regional nodal irradiation (postlumpectomy) or PMRT versus observation (postmastectomy).277 The Alliance A011202 trial randomizes similar patients who remain node-positive after chemotherapy to axillary dissection versus PMRT.278
Details of radiation treatment
The planning and design of the treatment fields and the delivery of radiation treatments for breast cancer are of critical importance and require modern equipment and attention to detail. It is clear that radiation treatments can have serious normal tissue consequences and even cause life-threatening radiation injuries, which can be avoided or minimized with modern treatment techniques.
There have been a number of recent advances in the field of radiation oncology that directly benefit breast cancer patients. One of these major advances has been the use of 3D imaging to assist in field design. Computed tomography (CT)-based simulation allows radiation oncologists to design treatment fields on CT images acquired from patients in their treatment positions, thereby permitting better visualization of the targeted regions and the normal tissues that one wishes to avoid, such as the heart. An example of a field design for a stage I left breast cancer treatment after lumpectomy is shown in Figure 20. In this image, the tumor bed has been contoured on individual CT slices and reconstructed for the image. The breast is treated with a medial tangentially oriented beam, with a deep edge that enters near the midline and transverses the anterior thorax to exit near the midaxillary line. This field is opposed with a lateral tangent beam to match the decrease of dose as the beam travels through the breast and ultimately provides a homogeneous dose distribution. Other regions of interest, such as lymph nodes in the axilla, internal mammary chain, or supraclavicular fossa, can similarly be contoured to visualize their anatomical location with respect to treatment field borders. Figure 21 shows a “beam’s eye view” of a medial tangent beam used to treat the breast (including the tumor bed), the upper internal mammary lymph nodes, and level I and a portion of level II of the axilla.
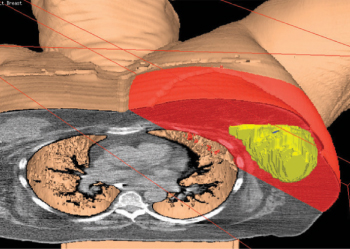
Figure 20 Cross-sectional image of radiation fields used for the treatment of left-sided invasive breast cancer. The red-shaded areas represent the volume included within the fields. The solid yellow object represents the tumor bed, which was reconstructed by contouring this region on sequential computed tomography slices. Two opposed treatment fields are used: a medial tangent that obliquely enters the medial breast and exits the lateral breast and an opposed lateral tangent field. The fields are opposed to match the falloff of radiation dose that occurs as beams travel through tissue.
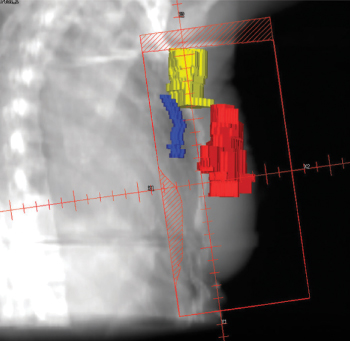
Figure 21 A beams’ eye-view of a medial tangent radiation treatment field designed to cover the breast, low axilla, and upper internal mammary lymph node chain. The reconstructed contours of a tumor bed (red), upper internal mammary lymph node region (blue) and the low axilla (yellow) are shown.
A second major advance has been the development of 3D dose calculation systems, which more accurately calculate and display the dose on the CT images throughout the 3D treatment volume. Finally, the currently used modern treatment tools also allow the dose to be modulated in three dimensions. The goal of such modulation is to provide a homogeneous dose distribution, which minimizes the risk of normal tissues and targeted areas receiving more than and less than the prescribed dose, respectively.
The treatment planning process is the initial critically important step in radiation treatments of breast cancer. Patients are conventionally immobilized in a supine position with the ipsilateral arm abducted and externally rotated. They then undergo a CT scan that acquires the image set used for treatment planning. Reference marks are placed on the patient’s skin of the breast. The planning process, in which the fields are designed and the dose distribution optimized typically takes 2–3 days and does not require the patient to be present. Following completion of planning, daily treatments begin, which typically require 15 min each day in the treatment room. The therapy is painless and the patients’ experience slightly differs from that of a diagnostic X-ray treatment. A number of alternative positioning279 and respiratory management280 techniques have recently been developed to improve dosimetry in large-breasted patients and further minimize heart exposure for left-sided cancers.
The most common course of treatment entails 25–28 treatments of 180–200 cGy per day to the breast or chest wall with or without inclusion of lymphatic regions at risk (total dose 4500–5040 cGy). This course is typically followed by five to eight supplemental treatments of 180–200 cGy per day to the tumor bed region (often called a tumor bed or chest wall boost) for an additional 1000–1600 cGy. In total, treatments are given 5 days a week for approximately 6–6.5 weeks. This strategy of delivering the lowest biologically effective daily dose over a protracted number of weeks is appropriate for patients in the postlumpectomy or postmastectomy setting, with or without inclusion of the regional lymph nodes and is referred to as conventionally fractionated radiation therapy (CFRT).
New radiation treatment approaches for early-stage breast cancer
Some patients treated for breast cancer fail to receive recommended radiation and a number of patients who are excellent candidates for breast conservation therapy elect to be treated with mastectomy to avoid radiation.281, 282 A major factor that contributes to both of these scenarios concerns the inconvenience and expense associated with 5–7 weeks of CFRT. This schedule is particularly burdensome for patients who have significant home or work responsibilities, patients in rural areas who need to travel for access to the nearest radiation facility, and patients who rely on public transportation. In addition, worldwide, there are too few radiation oncologists, facilities, and equipment to offer this type of treatment schedule to all patients with breast cancer who will benefit from radiation.
For these reasons and others, a number of strategies have been developed to shorten the radiation treatment schedule and lessen the burdens of time and out-of-pocket expense. The simplest strategy is called hypofractionated radiation therapy (HFRT), where the amount of radiation delivered per treatment is increased to decrease the overall treatment course by about one-half. As the amount of radiation per fraction increases, the total dose delivered is commensurately adjusted downward to provide radiobiologic equivalence to CFRT dosing. Results of large randomized trials of HFRT are shown in Table 8.
Table 8 Outcomes for selected randomized clinical trials comparing conventionally fractionated radiotherapy (CFRT) to hypofractionated radiotherapy (HFRT)
| Trial | Median follow-up (years) | N | Dose (cGy) | # fractions | IBTRa (%) | LRRa (%) | DFSa (%) | OSa (%) | Cosmesisa (% good or excellent) | Acute toxicitya (% ≥ grade 3) |
| Canada283 | 10 | 612 | 5000 | 25 | 6.7 | — | — | 84 | 71.3 | 3.0 |
| 622 | 4250 | 16 | 6.2 | — | — | 85 | 69.8 | 3.0 | ||
| Royal Marsden284 | 10 | 470 | 5000 | 25 | 12 | — | — | — | 71 | — |
| 466 | 4290 | 13 | 9.6 | — | — | — | 74 | — | ||
| 474 | 3900 | 13 | 15 | — | — | — | 58b | — | ||
| START A285, 286 | 9 | 749 | 5000 | 25 | 6.7 | 7.4 | 77 | 80 | — | 0.3 |
| 750 | 4160 | 13 |