INTRODUCTION
SUMMARY
The defining clinical features of a mononucleosis syndrome are fever and reactive lymphocytes in the blood. The two most common causes of mononucleosis are Epstein-Barr virus (EBV) and cytomegalovirus (CMV) infection. The clinical manifestations of EBV and CMV mononucleosis depend on a vigorous host response to the viral infection. Patients who become infected without a host response develop antibodies to the virus but no or minimal clinical manifestations. Several clinical similarities exist between EBV and CMV mononucleosis. Both infections have a febrile prodrome before the mononucleosis phase develops. Both infections can induce fever, an enlarged spleen, and an erythematous skin rash—the mononucleosis phase. The disease is self-limited in the vast majority of patients, although resolution may take several weeks, especially in older individuals. In both viral infections, lymphocytes represent greater than 50 percent of blood cells, and at least 10 percent are reactive lymphocytes. Differences in clinical and laboratory findings are observed. Severe pharyngitis and tender lymph node enlargement, often in several lymph node groups, occur in infection with EBV and perhaps with some unknown agents, but not to the same degree in infections with CMV. The majority of cases of EBV mononucleosis occur in teenagers and young adults, whereas CMV-induced disease occurs most commonly in adults in their 30s to 60s. A much larger percentage of adults have unrecognized primary infection with CMV than with EBV. EBV results in the development of heterophile antibodies, active against sheep and horse red cells among others, but this development does not occur in CMV. The pathway leading to lymphocytosis and reactive lymphocytes differs between the two agents. The B cell is infected in EBV infection which eventually may lead to hematologic malignancy, whereas the macrophage is infected in CMV. This may explain its important role after allogeneic transplantation. In both infections, the T lymphocyte is the reactive cell. Other agents, including Toxoplasma gondii, human immune deficiency virus type 1, and several other viruses, can cause a mononucleosis-like syndrome with reactive lymphocytes in the blood.
DEFINITION AND HISTORY
The first clinical description of what was probably infectious mononucleosis was published in 1885 when Pfeiffer1 described a disorder termed Drüsenfieber (glandular fever). In 1920, Sprunt and Evans2 introduced the term infectious mononucleosis for an acute, self-limited syndrome of mononuclear leukocytosis in febrile patients. In 1932, Paul and Bunnell3 showed that the sera from patients with infectious mononucleosis agglutinated red cells from sheep and horses, a reaction that was termed the heterophile antibody test. Paul was investigating heterophile antibodies in human sera that reacted with sheep red blood cells. These antibodies were unrelated by phylogenetic features to the antigen with which they reacted, the so-called Forssman antigen. He found that the highest titer had developed in the serum of an individual recovering from infectious mononucleosis. Davidson showed that serum, after absorption by guinea pig kidney cells, no longer reacted with sheep or horse cells. This absorption of these antibodies made this test very specific for Epstein Barr virus (EBV) infection.4 In 1964, Epstein, Ashong, and Barr reported the isolation of a virus from the cells of a patient with African Burkitt lymphoma and hence the derivation of its name, EBV. The etiologic role of EBV in infectious mononucleosis was discovered serendipitously in the laboratory of Gertrude and Werner Henle.5 A technician in their laboratory whose serum had been negative for EBV antibodies and was used as a seronegative control was discovered to be EBV antibody-positive after she recovered from infectious mononucleosis. The association later was confirmed in seroepidemiologic studies of college students.6,7,8,9
Acronyms and Abbreviations:
CMV, cytomegalovirus; EA, early antigen; EBNA, Epstein-Barr nuclear antigen; EBV, Epstein-Barr virus; NK, natural killer; PCR, polymerase chain reaction; PTLD, posttransplantation lymphoproliferative disease; VCA, virus capsid antigen.
Much of the clinical nature and the incubation period of mononucleosis were documented by Hoagland10 in studies of cadets at West Point. He established that oral transmission was the principal route of viral transmission, which led to mononucleosis being dubbed the “kissing disease.” He also noted that cadets developed the disease approximately 6 weeks after they returned from their vacation.11
Although EBV is the most common cause of infectious mononucleosis, other agents produce a febrile syndrome with a blood lymphocytosis that mimics some aspects of EBV mononucleosis.
ETIOLOGY AND PATHOGENESIS
The infectious mononucleosis syndrome is most commonly caused by one of two members of the herpes virus family: EBV or cytomegalovirus (CMV). Occasionally the HIV and, less commonly, the parasite Toxoplasma gondii produce a febrile illness with lymphocytosis. Other viral agents produce a febrile syndrome with a blood lymphocytosis, but only infrequently (Table 82–1). For both EBV and CMV mononucleosis, the T-lymphocyte response to the infected target cell, resulting in reactive lymphocytosis, is a hallmark of the disease. The difference is that for EBV, the B lymphocyte is the cell that becomes infected and thus the target of the responding T lymphocyte, whereas it is the macrophage/monocyte lineage that is infected by CMV, which engenders the T-lymphocyte response.13,14
EPIDEMIOLOGY OF EPSTEIN-BARR VIRUS AND CYTOMEGALOVIRUS
Some similar and several distinct epidemiologic and clinical differences exist between EBV and CMV infection. Both EBV and CMV infect young children.15 Those who develop EBV infection at a very young age (1 to 5 years) have an illness similar to other illnesses occurring during their youth. CMV infection presents similarly, but in the young patients there is low-grade fever, mild elevation in liver function, and, quite often, lymphadenopathy. The latter seldom occurs in older people with primary CMV infection.
If an individual escaped EBV infection when he or she was young, it is very common for the individual to develop infection during the teen and young adult years, that is, between the ages of 12 and 25 years.8 By contrast, CMV infection is uncommon in that age range but begins to increase in frequency after the age of 25 years.16,17 Primary CMV infection is also far more common than EBV in those older than 50 years of age.16 Between the ages of 12 and 25 years, the classic mononucleosis illness develops in the majority of those with primary EBV infection.8 In the few older individuals in whom EBV occurs, clinical illness that develops resembles the clinical illness that occurs in those with new CMV infection.18 Congenital infection with EBV occurs,19 but it occurs only in babies of mothers who were infected with EBV during pregnancy and it is very uncommon. By contrast, it is estimated that 1 to 2 percent of all livebirths have CMV congenital infection. Furthermore, it occurs associated with primary infection in mothers; it also occurs in babies of mothers who were seropositive preconception.20 CMV infection of the monocyte/macrophage21 helps explain its role in disease following solid-organ transplant. Because latent CMV is present in all solid organs of those with previous CMV infection, when immunosuppression is administered latent CMV is reactivated. EBV infection posttransplantation occurs only in those recipients who have not been infected before transplantation and, as with CMV, the EBV is reactivated in the donor organ.22
EPSTEIN-BARR VIRUS MONONUCLEOSIS
EBV is a DNA virus of the Gammaherpesvirinae subfamily. The virus is estimated to infect 90 percent of the world’s population. In the process of this infection, the EBV intercalates itself primarily into the long-lived memory B cell, not the naïve B cell,23 and thereafter establishes lifelong residence in its host. Early after infection, the virus is continuously shed into oral secretions. The virus then usually undergoes latency, but it may be reactivated periodically.24
Following primary infection, varying severity of disease ensues. Infection occurs via virus attachment to the cell surface CD21 glycoprotein, a 140-kDa complement receptor type 2. Infection induces polyclonal proliferation of infected B cells in the nodes in the pharynx. There is increasing evidence that host genetic factors predict the severity and duration of disease following primary EBV infection.25,26 Specifically, the interferon (IFN)-γ +874T/A or the interleukin (IL)-10 –592C/A polymorphisms are the important genetic factors. Using measures of severity of illness such as fatigue, myalgia, and height of fever, individuals who have the IFN-γ +874TT (high interferon production) allele have a striking increase in the risk of experiencing these severe manifestations compared to those with IFN-γ +874 A and IL-10 –592.24 When a subject’s blood monocytes are tested in vitro, the high-risk group has a higher production of IFN-γ in stimulated cells.27 Other factors in host response, which include the height of the virus load in the blood, the number of CD 8+ cells, and the T-cell granzyme expression in the CD8+ cell also have been described as contributing to disease severity.28 As a corollary to this, variability in the symptoms, signs, physical findings, and laboratory abnormalities occurs in primary infection.28,29 At the time serology confirms evidence of primary infection, some, but not all individuals with primary infection, have classic symptomatic disease.28,29 In those who develop the classical syndrome, T lymphocytes recognize viral replicative antigens on the infected B cell as foreign, and an exuberant polyclonal cytotoxic T-cell response ensues. The proliferative rate of CD8+ T cells is estimated to be approximately 50 percent of this population of cells proliferating per day. This translates into a population doubling time of 1.5 days so that 5 × 109 CD8+ T cells per day appear in the blood. The later rate of appearance is two orders of magnitude greater than normal.30 The surface activation marker SLAM (signaling lymphocyte activation molecule)-associated protein (SAP) on T lymphocytes stimulates cell activation in response to a signal from CD244 and CD150 (SLAM) on the T-cell surface.31 In the healthy individual, this process subsides over days to weeks. In parallel, the signs and symptoms of the infection subside, although fatigue may persist for a longer period of time.
There is an apparent seasonal pattern with its peak incidence in the summer. Close person-to-person contact is required for transmission to a susceptible individual.29 Subclinical primary infection28 or frequent asymptomatic reactivation of EBV in the previously infected individual provides an opportunity for transmission at all ages. Although, transmission from breast milk or from the cervix occurs, it is very uncommon.32 Nonetheless, nearly everyone in the developing world is infected by age 5 years and mononucleosis is rarely seen. Similar rates of infection occur in the lower socioeconomic class in the developed world. In the upper socioeconomic strata of the developed world, the majority of persons avoid infection when they are young. However, between the ages of 12 and 25, infection is very common. Characteristically, primary infection occurs in an individual a few months after the individual develops a relationship with someone who has latent infection.24,28 Transmission occurs from a virus-positive asymptomatic individual who transmits his or her virus to a previously uninfected person. Individuals who are raised in more protected environments may reach their 30s before they are infected. If both individuals in an initial relationship are seronegative, years may pass before they become infected. Individuals who are seropositive usually do not develop clinical disease upon reexposure, although a second infection with a different strain may occur.33,34,35
Clinical manifestations vary by age.15,18,36,37,38,39,40,41 When young children acquire illness they develop a typical childhood illness of upper respiratory infection (43 percent), otitis media (29 percent) pharyngitis (21 percent), gastroenteritis (7 percent), or typical mononucleosis (<10 percent). Rashes and/or periorbital swelling occur more frequently in younger children than in the teens. In the age group 12 to 25 years, many, but not all, present with the classical presentation of infectious mononucleosis. However, there are a substantial proportion of newly infected individuals who have minimal or very mild disease.28,29 Furthermore, evidence of virus in the blood may be present for several days before disease appears.28 For those who present with the classical disease of infectious mononucleosis, the earliest manifestations of disease develop 35 to 42 days after the individual develops infection (Table 82–2). Infection occurs via virus attachment to the cell-surface CD21 glycoprotein, a 140-kDa complement receptor type 2. Infection induces polyclonal proliferation of infected B cells in the nodes in the pharynx. Initial symptoms are lassitude and fever with no evidence of lymphocytosis or pharyngitis. Fever results from infection and proliferation of the B lymphocytes. From the nodes in the pharynx, the infected cells make their way into the circulating lymphocyte pool.42,43 Although the duration of virus in the blood is much shorter than it is in the secretions, it is the movement into the blood that leads to the manifestation of the disease. Subsequent, massive T-cell response to the neoantigens on the infected B lymphocyte is evident by the lymphocytosis with reactive blood lymphocytes and other disease manifestations. The pharyngitis that develops is a result of the T-cell response to the infected B cells that are found in Waldeyer ring in the tonsils. Sometimes enlargement of the tonsils occurs to the extent that they touch each other in the midline. Blood lymphocytosis occurs in response to the virus in the blood. Periorbital swelling, which occurs in mononucleosis, is an important clue to the diagnosis, even in young adults. Other manifestations include lymphadenopathy, hepatitis and splenic enlargement. (Table 82–2).8,28 Although the liver is not an organ rich in lymphocytes and hepatocytes are not damaged by the infection, CD4+ and CD8+ lymphocytes are trapped in that organ and their release of cytokines contributes to the inflammation in the liver and the changes in liver function that occur.44 However, hyperbilirubinemia is very uncommon. The frequency of each clinical finding of the typical syndrome in newly infected patients is variable (Table 82–3).28,29 The disease abates with the occurrence of a T-cell–mediated counterresponse to the virus-induced initial polyclonal B-cell proliferation. During this time, dramatic clinical improvement can occur in 24 to 48 hours. Subsequently, EBV remains in the patient’s B cells throughout life, but expresses only Epstein-Barr nuclear antigen-1 (EBNA-1), which does not elicit a T-cell response because of a glycine-alanine repeat that inhibits its processing.45
| Signs and Symptoms | EBV (Age 14–35 Years*) | EBV (Age 40–72 Years†) | CMV (Age 30–70 Years‡) |
|---|---|---|---|
| Fever | 95 | 94 | 85 |
| Pharyngitis | 95 | 46 | 15 |
| Lymphadenopathy | 98 | 49 | 24 |
| Splenomegaly | 65 | 33 | 3 |
| Hepatomegaly | 23 | 42 | N/A |
| Jaundice | 8 | 27 | 24 |
| Complication | Epstein-Barr Virus | Cytomegalovirus |
|---|---|---|
| Hemolytic anemia | ++ | + |
| Thrombocytopenia | + | + |
| Aplastic anemia | + | – |
| Splenic rupture | + | – |
| Jaundice (age >25 years) | ++ | ++ |
| Guillain-Barré syndrome | + | ++ |
| Encephalitis* | ++ | +/– |
| Pneumonitis* | +/– | + |
| Myocarditis* | + | – |
| B-cell lymphoma | + | – |
| Agammaglobulinemia | + | – |
Group A streptococcus is found in the pharynx occasionally (3 to 4 percent of cases) in concert with an EBV primary infection. Although treatment of the streptococcus eradicates the organism, the severe pharyngitis changes little, and the disease follows its usual course. Thus, treatment should be administered only if the test result for β-streptococcus is positive. If a penicillin congener is used, quite often, but not always, a rash develops46,47 and the patient is labeled “penicillin allergic.” The patient should be reevaluated after the mononucleosis resolves to determine if the patient has a true allergy.
There are exceptions to the usual situation of most people becoming infected by age 25 years. The first is found in a woman who is protected by her family from intimate male contact. She avoids infection until she is married at which time she may become infected from her husband. The second situation occurs when a long-term relationship is established when a couple is young. If they are both seronegative, infection does not occur. When they reach parenting age or older, they become infected by a child or a grandchild. When that occurs, the presentation is less likely to include lymphadenopathy and pharyngitis (see Table 82–3).18,40,41 Fever almost always occurs and abdominal pain, hepatomegaly, and abnormal liver function develops in most. Older adults are less likely to develop lymphocytosis, they have fewer reactive lymphocytes, and splenomegaly is less evident. This leads to the clinical impression of infiltrative hepatic disease or cholecystitis. The illness at this age may be very protracted.
By week three of a mononucleosis caused by primary EBV infection, an heterophile antibody response will occur in approximately 85 percent of patients. The test is called the monospot test and may be falsely negative, especially in young children.36,48
The infection of B cells results in their production of a variety of antibodies against uninvolved infectious agents. The B-cell clones expanded, non-specifically, result in antibodies against Chlamydia, Borrelia burgdorferi, the yellow fever virus, and many other infectious agents. If the patient’s febrile illness is considered a fever of unknown origin, diagnostic conclusions may be misleading. A variety of other antibodies against antigens that are not those of infectious agents also are produced because of polyclonal B-cell activation. These include antiplatelet, anti–red cell (anti-i cold agglutinin), and antinuclear antibodies.
At the time clinical disease is evident, both immunoglobulin (Ig) G and IgM antibodies to Epstein-Barr virus capsid antigen (VCA) usually are detectable. Later, antibody to early antigen (EA) develops. A small proportion of individuals will also have developed antibody to EBNA-1 on presentation. However, usually EBNA-1 antibody does not appear until the recovery phase of the illness. For those patients suspected of having infectious mononucleosis but who do not develop heterophile antibody, detection of IgG and IgM antibody to VCA with the absence of EBNA-1 antibody leads to the diagnosis.49 A real-time positive polymerase chain reaction (PCR) is sometimes useful.50
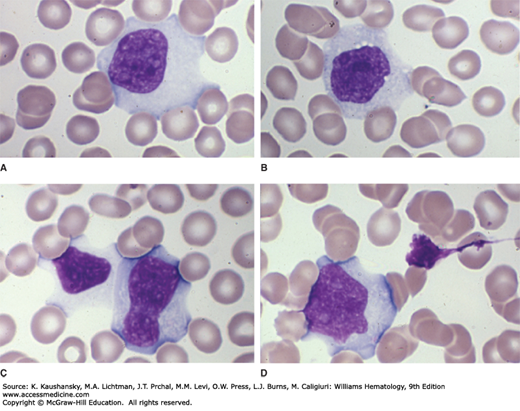
Expansion of cytotoxic T lymphocytes produces lymphocytosis. Reactive lymphocytes are larger than the lymphocytes normally found in the blood (Fig. 82–1). They may have a vacuolated cytoplasm, lobulated and eccentrically placed nucleus, and a cell membrane that often is indented by neighboring erythrocytes. A more darkly staining peripheral cytoplasm, called “skirting,” occurs. Reactive lymphocytes are a hematologic hallmark of infectious mononucleosis, but they are not always found29 and are not pathognomonic. They also are found in CMV infection, roseola (caused by human herpes virus-6), viral hepatitis, toxoplasmosis, rubella, mumps, and drug reactions.
Figure 82–1.
A-D. Blood films from patients with Epstein-Barr virus–induced mononucleosis. These reactive lymphocytes exhibit the characteristic changes seen in patients with infectious mononucleosis: large lymphocytes with abundant cytoplasm. The cytoplasmic margin often spreads around (is indented by) neighboring red cells and the margin may take on a densely basophilic coloration (skirting). This type of reactive T lymphocyte may be seen in a variety of diseases and is not a specific change but is characteristic and helpful in pointing to the diagnosis in concert with other characteristic clinical findings. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
Sheets of lymphocytes are noted on a stained slide preparation of tonsillar exudate. The immunophenotype of lymphocytes in mononucleosis syndromes assessed by multiparametric flow cytometry has confirmed that lymphocytosis results from CD8+ T cells. CD4+ T cells and B cells are not increased. In EBV mononucleosis, the notable populations increased are CD8+CD57– and CD3+γδ+ T cells.51 If β-streptococcal infection accompanies the EBV infection segmented neutrophils may be present in the tonsillar exudate.
Liver function abnormalities are common, especially elevated serum alkaline phosphatase and γ-aminotransferase. There is no or only slight elevation of bilirubin. Studies in Israel have found a higher frequency of hyperbilirubinemia (15 percent), a lower incidence of leukocytosis (46 percent), and elevated liver enzymes (58 percent) than previously reported. The differences may be geographical or genetic.29
Virtually all subjects with acute mononucleosis develop a mildly decreased platelet count (see Table 82–2). More-severe hematologic complications occur infrequently, but include severe immune thrombocytopenia with petechiae, immune hemolytic anemia, immune-mediated granulocytopenia, and aplastic anemia.52,53,54,55,56,57,58,59,60 Uncommonly, the splenomegaly that accompanies the lymphoid proliferation accentuates an underlying, previously undiagnosed, hereditary spherocytosis.60 Splenic rupture is estimated to occur in 1 to 5 per 1000 cases. It is the leading cause of death from EBV mononucleosis.61,62 Avoidance of athletic activities is prudent until the signs of the disease have disappeared and the spleen has returned to normal size.62
Neurologic complication include Guillain-Barré syndrome, acute encephalitis, acute disseminated encephalomyelitis (Alice-in-Wonderland syndrome), acute cerebellar ataxia, viral meningitis, transverse myelitis, and cranial nerve palsies.57,58,63,64,65 There is evidence that antibody to gangliosides plays a role in the pathogenesis of Guillain-Barré syndrome Neurologic complications may occur in the absence of clinical mononucleosis. Diagnosis of EBV-induced neurologic disease requires obtaining specific antibodies to EBV (see “Antibody Responses” above) and a positive PCR for EBV in the cerebrospinal fluid.63 Neurologic disease can be associated with primary infection, reactivated infection or chronic EBV infection.63 Table 82–2 lists other complications.
Fatigue is a very prominent feature of acute infectious mononucleosis. Most recover from this fatigue fairly quickly but a few remain fatigued for a very long period of time. The source of this fatigue is not certain but there is evidence that dysfunction of the midbrain plays a role.68,69 Furthermore, there are genetic factors that are present in those who remain fatigued. Limited information suggests improvement in some with antiviral treatment.69
There are reports indicating that EBV infection is linked to the development of multiple sclerosis.70,71 Further studies to clarify which molecular mechanisms link the immune response to a natural infection of humans with EBV to the subsequent development of chronic inflammatory damage to the CNS are required to explain this relationship
There are epidemiologic links between previous infection with EBV and development of systemic lupus erythematosus (SLE).72 Not unexpectedly, virtually everyone with SLE has had previous infection with EBV. Therefore, the link may be fortuitous. Alternatively, a history of infection with EBV might lead to induction of autoimmunity.73,74 There has also been a suggested relationship between increased viral load in rheumatoid arthritis leading to expansion of CD8+ cells and its consequences.75
Chronic Progressive Epstein-Barr Virus Infections, T-Cell or Natural Killer–Cell Lymphoproliferation, Lymphoma, and Hemophagocytic Syndrome
Chronic EBV infection is a rare outcome of primary EBV infection, notable in persons with an immunodeficiency state.76,77 In chronic EBV infection, fever, marrow hypoplasia, interstitial pneumonia, hepatosplenomegaly, persistent hepatitis often to the point of hepatic failure, lymphadenitis, and uveitis are frequent clinical manifestations. These findings may persist for months or years and eventuate in a high fatality rate.75 EBV antibodies may be very elevated (VCA in excess of 1:5120, anti-EA greater than 1:640) but with no detectable EBNA-1 antibody. A persistently elevated blood PCR for EBV is a feature. The more-severe form of this manifestation may evolve into a natural killer (NK)- or T-cell lymphoproliferative disease that ranges from chronic to fulminant.78
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree