Fig. 7.1
Position of MCPH1 gene on chromosome 8p.
7.1.2 MCPH1 Protein
BIRT1 (BRCT–repeat inhibitor of human telomerase reverse transcriptase expression) was reported as a repressor of TERT transcription by 2003 (Lin and Elledge 2003) . It encodes for a protein with 835 amino acids with 110 kDalton weight. It has homolouges in chimpanzee, dog, rat, mouse, zebrafish and drosophila. MCPH1 protein has demonstrated rapid evolution from simian ancestors to chimpanzee and then human, tending toward positive selection favoring 45 advantageous amino acids (Evans et al. 2004) . This evolution may be related to enlargement of brain size in human. As a surprising tote, Rimol and his colleagues have shown that the brain size is controlled only by MCPH1 gene in females whereas in men this critical task is fulfilled upon the expression of CDK5RAP2 gene (Rimol et al. 2010) . However, study on 2393 volunteers have revealed no correlation between the recent statement on evolution and normal discrepancies which are present in IQ (Mekel-Bobrov et al. 2007) .
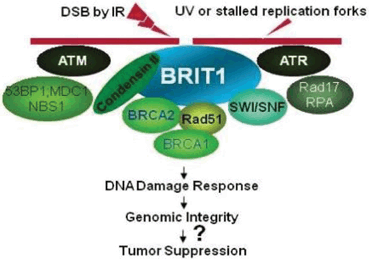
There are three BRCA1 carboxyl-terminal (BRCT) domains in the MCPH1 protein structure including one N-terminal BRCT (N-BRCT) and two C-terminal BRCT (C-BRCTs) domains. One large central IMPDH domain and nuclear localization signal motif constitute the other parts of MCPH1 protein localizing mostly in nucleus (Fig. 7.2).
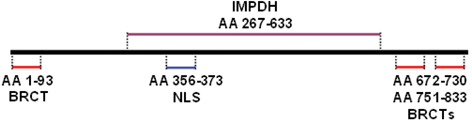

Fig. 7.2
Schematic representation of MCPH1 protein structure.
N-BRCT domain is necessary for changing localization of MCPH1 protein toward centrosome within cells exposed to radiation and it is also important to prevent premature chromosome condensation (PCC) mediated by Condensin II (Yang et al. 2008) . The C-BRCT domains are required for MCPH1 oligomer and foci formation after radiation which will be more discussed later. However, C-BRCTs are also attachment sites of phosphorylated proteins engaged for DNA damage response pathway similar to other BRCT containing proteins involved in DNA damage response (Yu et al. 2003; Wood et al. 2007) γH2A.X is one of the known direct target of MCPH1 which is phosphorylated upon the DNA damage (Wood et al. 2007). The function of IMPDH domain has been poorly studied, but it seems that it is indirectly involved in homologous recombination through binding to Condensin II (Fig. 7.2).
Although, MCPH1 gene is one of the six genes that its mutation is associated with microcephaly, the role of this gene remains to be described in brain development and mental disabilities (Woods et al. 2006; Rushton et al. 2007) . Both male and female knock out mouse bearing homozygote MCPH1 gene deletion have demonstrated infertility, moderate hearing loss, chromosomal instability and cataract (Gerdin 2010) . Although MCPH1 is mutated in patients with microcephaly, no higher incidence of cancer and tumor has been reported in them. It may imply on this fact that MCPH1 gene mutates in different domains or segments of protein lead to cancer and microcephaly (O’Driscoll and Jeggo 2006) . For example, S25X mutation changes the start codon of translation and finally disrupts the N-BRCT domain which is necessary for the role of MCPH1 in DNA repair (Leung et al. 2011) .
7.2 Cellular MCPH1 Protein Functions
The MCPH1 protein has two main cellular contributions including cell cycle control and DNA damage response. It is involved in the latter by being involved in both major DNA damage response pathways, ataxia telangiectasia mutated (ATM) and ATM and Rad3-related (ATR). All of the components of DNA damage response system with an exception of γ-H2AX are unable to reside on site of damage without MCPH1 owing to failure in phosphorylation of ATM and its downstream proteins. Phosphorylated H2AX stimulates accumulation of MCPH1 on the break site of double strand DNA break (DSB) to bind N-terminal of BRCA2 and Rad51 and thereby control their localization. Although, MCPH1 is not an obligatory element for localization of BRCA2 and Rad51, its absence leads to great diminished recruitment of them to damaged site (Wu et al. 2009) . In ATR pathway, MCPH1 regulates cdk1 phosphorylation and thereby prevents the premature chromosome condensation and also marks the damaged DNA to be fragmented instead of running out from S to G2 phases (Alderton et al. 2006) . It was shown that MCPH1 mutation was associated with aberrant G2-M checkpoint and extra mitotic centrosomes leading to genomic instability and various chromosomal abnormalities (Rai et al. 2006) . MCPH1 has pivotal role in regulation of cell cycle through modulating the expression of BRCA1 and ChK1 to impede premature start of mitosis after radiation exposure. It impels activation of p73 and renders it to make a complex with E2F1 to upregulate expression of genes which mediate E2F1 dependent apoptosis including Apaf1, caspase 3 and caspase 7 (Yang et al. 2008; Urist et al. 2004) . It was shown that under-expression of MCPH1 gene was associated with decreased in success rate of chemotherapy due to impairment in activation of p73 to induce apoptosis (Yang et al. 2008).
Moreover, MCPH1 binds chromatin and changes its configuration through recruitment of MDC1 complex, p53 binding protein (p53BP) and NBS1 around the chromatin structure of damaged DNA (Wu et al. 2009) .
MCPH1 by accompanying 101 other genes play important role in centrosome architecture and regulates its function. It was demonstrated that centrosome aberrations had great impact on progression and development of in situe and pre-invasive breast carcinoma. Centrosome duplication leads to inappropriate attachment of polar spindle fibers to kinetochores residing on centromere of each chromosome. Therefore, chromatids are no longer able to separate from each other and move on towards opposite poles in a proper manner resulting in numerical chromosomal abnormalities and aneuploidies which is one of the putative hallmarks of cancer. Recent studies have received great attentions to the role of proteins and other elements of mitotic checkpoints including centrosome in the etiology of aneuploidies, development and expansion of tumor cells. Centrosome controls progression of cell cycle from S-G2 and G2-M indicating that centrosome signaling may be the major governing key in cell division (Liang et al. 2010) .
Given the importance of MCPH1 in cell cycle regulation, maintaining the genomic stability and centrosome structure and regulation in addition to its decreased level in various cancers and metastasis, it was introduced as a tumor suppressor gene (Rai et al. 2006) .
In the following sections of current chapter, we were aimed to describe about the main functions of MCPH1 in details which have been reported in recent years. After that, we will discuss in detail regarding the role of aberrations of MCPH1 activity in development of brain tumors and some neurocognitive disorders.
7.2.1 MCPH1: An Important Accessory Member of Cell Cycle Checkpoints
Most of the neural cells are derived from neural progenitor cells (NPCs) lining the ventricles of human brain. NPCs follow two major particular patterns in their division including symmetric and asymmetric cell divisions (Brand and Rakic 1979) . The first pattern results in two identical NPCs which are switched on to the latter type of division at specific time of brain development to generate one NPCs and one post mitotic neuron. It was found that every deviation at the proper time of switch between these two types of neural cells divisions can affect the number of neuron pools. It was also assumed that deregulations in asymmetric and symmetric cell divisions lead to micro- and macro-cephaly, respectively (Doe and Bowerman 2001) . Functional studies on the orthologous of MCPH1 gene in Drosophila have shown that it may have pivotal role in control of asymmetric cell division and also determining the right time of switching from symmetric to asymmetric cell divisions. Moreover, the important role of MCPH1 gene in regulation of centrosome activity which itself plays crucial role in mitosis of neurons, would be a further evidence of determining activity of MCPH1 in brain development (Yamashita et al. 2003) . As mentioned above, centrosome governs two major cell cycle checkpoints before and after getting entry into the G2. There is an intra S checkpoint in which DNA synthesis is prohibited when the cell is exposed to radiation. This task is mediated by activation of ATM which induces CHK1 to stall the replication process through inactivation of Cdck25A and subsequently Cdk2-cyclin complex (Fig. 7.3) (Abraham 2001; Margolis and Kornbluth 2004) . In this way, co working of MCPH1 seems to be necessary for the proper activity of the major cellular proteins including ATM and BRCA1 which guarantee the G2/M checkpoint to be intact. Cdc27 and Cdc16 which are the elements of anaphase promoting complex (APC) has been shown to be phosphorylated with C-terminal BRCT domains of MCPH1 to drive the separation of chromosome during mitosis. MCPH1 can alternatively binds to another component of APC complex called Cdc26 when the Cdc27 has been mutated. Cdc26 differs from Cdc27 at the residue residing on + 1 amino acid relative to phosphoserine (Singh et al. 2012) . These interactions of MCPH1 are necessary for APC complex stability and may shed light on this finding that loss of MCPH1 can mimics the loss of APC complex function.
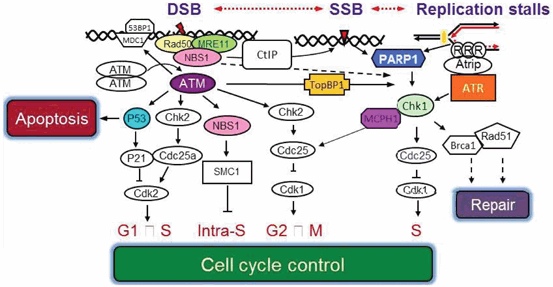

Fig. 7.3
The critical role of MCPH1 protein in cell cycle control in bridging between two main ATR and ATR DNA repair pathways.
Determination of MCPH1 expression in seckel syndrome patients with severe microcephaly revealed that normal level of MCPH1 transcription is required for active centrosome Chk1 expression and eventually induction of centrosome cyclin B-Cdk1 (Tibelius et al. 2009) . It was demonstrated that directed mutagenesis of MCPH1 gene was associated with arrest of manipulated cells in G2 phase and premature condensation of chromosomes (Alderton et al. 2006) . Moreover, directed mutagenesis of MCPH1 led to decrease in tyrosine phosphorylation of Cdk1 in S and G2 phases and consequently formation of PCC in manipulated cells. It also was associated with the aberrant G2-M checkpoint and nuclear fragmentation when DNA has been damaged (Alderton et al. 2006). It is obvious that MCPH1 has a major contribution in controlling the main cellular checkpoints throughout the cell cycle.
7.2.2 MCPH1 Activity in DNA Repair System
Presence of BRCT domains within the structure of MCPH1 protein would be a good predictor of its role in DNA repair system like other proteins carrying two carboxyl- terminal BRCT domains (PTCB). BRCT domain actually provides the suitable context for peptide and phosphopeptide binding activities (Rodriguez et al. 2008; Manke et al. 2003) . Localization of MCPH1 in the DNA damage site and DNA repair complex could be a convincing evidence of its contribution in DNA repair pathway (Fig. 7.4) (Rai et al. 2006; Alderton et al. 2006; Lin et al. 2005) . Loss of MCPH1 prevents activation of other DNA damage proteins including Rad51, BRCA2 and Chk1/Chk2 dependent pathways (Tibelius et al. 2009) . It was shown that biallelic knock out of MCPH1 gene in mice was associated with decreased length of life and genomic stability (Trimborn et al. 2010; Liang et al. 2010) . It was described that the C-terminal domains of MCPH1 protein play pivotal role in DNA repair process (Yu et al. 2003) . It is in line with the capability of N-terminus defective MCPH1 protein to avoid promotion of damaged cell to get entry in to the mitosis (Gavvovidis et al. 2010) . Study on the role of MCPH1 aberrations in endometrial cancer have revealed that deletion of one nucleotide within the repeated tracts of adenine in exons 4, 5 and 8 was compatible with mismatch repair (MMR) deficiency and microsatellite instability (MSI). It was proposed that this type of deletion is associated with non-functional C-terminal domains. The same inefficient MCPH1 protein had been reported prior that wherein deletion of 38 bps within the exon 10 was found in breast cancer (BC) (Alderton et al. 2006). Of note, there is a similar A tract in exon 10 of ATR gene as the major cooperators of MCPH1 in DSB that any monoalleleic alteration of it was reflected by poor prognosis of endometrial cancer patients carrying MSI (Xu et al. 2004). It could be extrapolated that haplo-sufficiency of major components of DSB system is essential for maintaining the stability of genome and prevention of tumor progression in particular in endometrial cancer.
7.3 MCPH1 Gene Aberrations in Various Cancer
It was demonstrated that the chromosome 8p was the fragile site and focal region for deletion in hepatocellular carcinoma (HCC) patients in particular, in larger tumors with poorer differentiation (Chan et al. 2002) . This result motivated further group to search for the genes whose their deletion was associated with more aggressive tumors in two separate studies. They have found that the frequency of loss of heterozygosity (LOH) within the 8p23.1 was significantly more in metastatic (68 %) versus primary (19 %) tumors in HCC patients (Lu et al. 2007; Lu and Hano 2007).
Amongst 782 single nucleotide polymorphisms (SNPs) within the 101 centrosome related candidate genes which were sought in 798 BC patients and 843 controls, 40 SNPs demonstrated significant association with BC risk. Two SNPs of these including 3’UTR and non-synonymous variants (rs24433149 and rs1057091) were identified in MCPH1 gene with equal odds ratio (OR) of 1.23 (Olson et al. 2011) . In the next study of MCPH1 in BC patients, low level of MCPH1 expression was associated with T allele of rs2912010 in almost 50 % of BC patients, however the frequency of this variant was not significantly differs from healthy controls. In addition, T allele was meaningfully higher in patients with higher grades of tumor. Of note, MCPH1 protein was more localized in cytoplasm in higher grades of tumor (Jo et al. 2013) .
Thirty four SNPs whose their association was previously found with BC risk, were screened in 1189 patients affected with pancreatic adenocarcinoma (Couch et al. 2010) . They were undergone genotyping in comparison with 1126 healthy normal individuals through illumina next generation sequencing. The rs2433149 polymorphism within the MCPH1 gene was found to be in significant association with pancreas cancer in patients with low body mass index (BMI). In addition, meaningful correlation was found between higher risk of pancreatic cancer and ever or former smokers carrying that polymorphism.
Expression assay of MCPH1 and ASPM proteins was extended on primary cultured cells derived from benign and epithelial ovarian cancer (EOC) tissues (Bruning-Richardson et al. 2011) . In that study, cytoplasmic localization of MCPH1 and ASPM proteins and their expression level were determined through protein slot blotting and immunofluorescence. In contrast to the higher expression of ASPM protein in lower tumor’s grades, the expression of mutant MCPH1 protein was correlated with higher grades of ovarian tumors and it was localized more in cytoplasm. Association between better survival of patients and nuclear localization of normal MCPH1 protein may indicate that mutant MCPH1 moves out from nucleus and unable to be participated in DNA repair and cell cycle checkpoint leading to higher tumor’s grades. This finding is in line with the prior study which demonstrated that mutant MCPH1 was unable to reside the BRCA2 on DNA damage site. BRCA2 and MCPH1 proteins become connected together through peptide binding. Mutation in MCPH1 gene prevents this connection and also recruitment of BRCA2 and Rad-51 repair proteins to the double strand break site (Wu et al. 2009). In summary, mutation in MCPH1 shifts the localization of it towards cytoplasm and keeps it away to be available for residing of BRCA2 and Rad-51 proteins on DNA damage site.
The expression level of MCPH1 gene was determined in 55 non-small cell lung cancer (NSCLC) patients whom were undergone chemotherapy with EGFR inhibitor or erlotinib (Garcia-Campelo 2012) . It was carried out due to the critical inhibitory effect of Erlotinib on DNA damage response proteins including BRCA2 and Rad51 which are localized at the damage site by MCPH1. The mRNA level of MCPH1 gene was measured using a previously introduced NanoString nCounter gene expression system in which even one mRNA transcript is captured during detection procedure. The most and least median survivals were determined in patients who had the highest and lowest levels of MCPH1 gene expression, respectively. It is worth to note that the efficacy of Erlotinib was improved when the expression of MCPH1 was increased. It was proposed that it may be pertinent to the negative regulatory role of MCPH1 on 53BP and MDC1 proteins. It was also suggested that identification of MCPH1 expression level could be a potential biomarker for clinical response to Erlotinib.
Study on patients affected with chronic myeloid leukemia (CML), and K562 cell line demonstrated under expression of MCPH1 gene (Giallongo et al. 2011) . No association was found between BCR/ABL fusion protein activity and the level of MCPH1 expression. In addition, keeping on cell growth and proliferation in spite of treatment of cells with hydroxyurea is implying on the direct correlation between under expression of MCPH1 and aberrant G2/M checkpoint.
In the first MCPH1 methylation study on BC, expression analysis of MCPH1 and ATM genes and their proteins were performed through qRT-PCR and IHC on MCF7 cell line and 126 BC patients in comparison with 123 healthy volunteers. The promoter methylation statue of MCPH1 and ATM genes were determined by means of methylation specific restriction analysis (MSRA) and methylation specific PCR (MSP). Moreover, single strand conformation polymorphism (SSCP) was implicated to screen the MCPH1 gene of 30 randomly selected fractions of BC patients. They also assessed both MCPH1/ATM genes for the presence of deletions including intragenic micro deletions. Down-regulation in expression of either ATM or MCPH1 gene was further replicated and frequent promoter methylation and deletions was found within the MCPH1 gene. All the molecular alterations including methylation, deletion and expression of MCPH1 and ATM proteins and their genes were significantly more in ER/PR negative versus ER/PR positive patients. In addition, the lower expression and methylation of MCPH1 promoter gene were in significant association with higher grades of BC. The strong expression of MCPH1 and ATM proteins in both nucleus and cytoplasm was detected in BC cells without any molecular defect. This is a further proof on that sufficient expression of intact MCPH1 gene allows its protein product to be translocated from cytoplasm to nucleus wherein DNA repair process takes place.
Recently, a broad study was reported on 93 fresh oral squamous cell carcinoma (OSCC) tissue samples and three A549 (human lung adenocarcinoma), Hela (human cervical carcinoma) and KB (OSCC) cell lines (Venkatesh et al. 2013) . The promoter methylation analysis of MCPH1 gene was carried out in this study throughCombined Bisulfite Restriction Analysis (COBRA) and determination of the effect of 2’-deoxy-5-azacytidine (AZA) treatment on the expression of MCPH1 gene. The MCPH1 gene analysis was included loss of heterozygosity (LOH) assessment using three STR markers nearby the MCPH1 gene locus, mutation screening and real time RT-PCR. The level of MCPH1 protein expression was determined through western blotting and immunohistochemistry (IHC). In addition, the tumor progression and apoptosis were tracked in an OSCC mouse model whom was transfected with an expression pcDNA vector to have high copy numbers of MCPH1 gene.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree