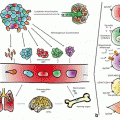
Fig. 5.1
TGF-β signaling prevents cancer development, but promote carcinogenesis and angiogenesis in advanced carcinomas. TGF-β regulates immune system by the mediation of FOXP3, which is over-expressed in tumor cells in pancreatic adenocarcinoma, melanoma, leukemia, hepatocellular carcinoma, bladder cancer, thyroid carcinoma and cervical cancer. TGF-β induces the expression of FOXP3, which is inversely regulated by smad7. Smad7 induces IκBα expression leading to NF-κB inhibition. Smad4 binds phosphorylated Smad2/Smad3 to create the Smad complex, which translocate into the nucleus to regulate target genes. Activation of PI3K and Akt leads to the phosphorylation of FOXO3A and departure of FOXO3A from the nucleus to the cytosol, and hence prevent its DNA binding and transcriptional activity. Smad7 inhibits the phosphorylated Smad2/3 complex formation with Smad4. Lithium inhibits Smad3/4-dependent transcription activity of TGF-β signaling through sequestration of transcriptional co-activator p300, which leads to increased CREmediated activation and expression of cell growth factors like BDNF and Bcl-2. Growth factors facilitate FOXO3A nuclear translocation. Lithium inhibits FOXO3A transcription activity. Phosphorylation of FOXO3A is increased by BDNF. Promoter region of CXCR4 is hypermethylated (associated with reduced gene expression) in SCZ as well as in pancreatic cancer and low levels of CXCR4 has been reported in > 20 types of cancers. miRNA-126 has inhibitory effects on DNMT1 and negatively regulates VEGF signaling pathway through inhibition of SPRED1 and PIK3R2. Metformin, Venlafaxine, SSRIs, Imipramine, Lithium and Antipsychotic drugs increase FOXO3a phosphorylation leading to its decreased nuclear localization. An activated FOXO3a could regulate transcription or cytoplasmic accumulation of p53, modulating its apoptotic activity. Green lines and arrows indicate facilitating or activating roles and red arrows and symbols represent inhibitory roles
In addition to RELN, the promoter region of CXCR4, a marker for cancer cell invasion is also hypermethylated (associated with reduced gene expression) in SCZ (Aberg et al. 2014) as well as in pancreatic cancer (Sato et al. 2005). CXCR4 is overexpressed in most cancers, including prostate and lung cancers (Sun et al. 2003; Singh et al. 2004; Lu et al. 2013; Spano et al. 2004), which is consistent to a reduced risk of these types of cancers in SCZ patients (Table 5.1). In prostate cancer the expression of CXCR4 (as well as BCL2) is decreased by ampelopsin, an anti-cancer flavonoid, associated with reduced cell proliferation of prostate cancer cell lines, but to a much lesser extent in the normal prostate cell line (PrEC). An inhibition of growth of PC-3 tumors and invasion to lymph node and metastasis was also observed with ampelopsin in animal studies using an orthotopic prostate cancer model in mice associated with increased apoptosis, inhibition of proliferation, reduced angiogenesis and reduced CXCR4 expression (Ni et al. 2012).
These observations along with the fact that the incidence of cancer is remarkably low in SCZ patients including lung cancer (Mortensen 1994; Dalton et al. 2005; Barak et al. 2005), despite a higher frequency of smoking (Vanable et al. 2003; de Leon and Diaz 2005) indicate that; epigenetic aberrations as key mediators for the pathogenesis of complex diseases, including cancer and SCZ may be responsible for the general reduced cancer risk and increased risk of certain types of cancer in SCZ patients.
5.2.2 Dysregulation of GABAergic Genes in Cancer and SCZ
Dysregulation of GABAergic system well known to be involved in SCZ pathogenesis is also linked to carcinogenesis. For instance, while epigenetic down-regulation of GAD1 is shown in SCZ patients (Bharadwaj et al. 2013), over-expression of GAD1 is observed in oral squamous cell carcinoma and it has been associated with a higher degree of invasion and migration of cancer cells (Kimura et al. 2013). In breast cancer, the metastatic cells entering into the brain exhibit GABAergic phenotype (higher levels of GABA-A receptors and RELN) in order to co-opt to the brain microenvironment (Neman et al. 2014). Notably, the expression of GABA-A receptor subunits is also increased in human liver cancer, and GABA could inhibit cell migration and invasion mediated by GABA-A receptors (Chen et al. 2012c). Considering that GABAB ligands also directly interact with the CXCR4 receptors (Guyon et al. 2013), the drugs known to modulate GABAergic system may be of use for treating specific cancers with higher levels of CXCR4 expression (Fig. 5.1).
The higher expression of GAD1 in benign and malignant prostatic tissue led to the conclusion that; GAD1 may be a prostate-specific tissue biomarker. However, GAD1 expression decreases as the Gleason score increases in prostate cancer (Jaraj et al. 2011) suggesting that in addition to RELN, the reduced risk of prostate cancer in SCZ patients may also be due to epigenetic down-regulation of GAD1 in these patients. These observations lend additional support for therapeutic utility of GABAergic drugs as well as epigenetic modifiers in specific types of cancers.
5.2.3 Involvement of Dopaminergic Genes in Cancer and SCZ
Catechol-O-methyltransferase (COMT) plays a major role in the metabolism of dopamine as well as the carcinogenic catechol estrogen. A recent meta-analysis concluded that the Val/Val polymorphism of COMT, the overactive genotype, is linked to a higher risk for endometrial cancer during the post-menopausal period in women (Lin et al. 2013b). However such an association was not found in an earlier meta-analysis (Qin et al. 2012), another meta-analysis confirmed that the Val158Met polymorphism of COMT is involved in breast cancer risk in Caucasians (He et al. 2012). An association with the Met/Met (AA) genotype for breast cancer risk has been confirmed by a different meta-analysis in Chinese population (Tian et al. 2014). The reduced activity of membrane-bound catechol-O-methyltransferase (MB-COMT) due to promoter DNA hypermethylation has also been observed in endometrial cancer cell lines as well as in endometrial cancer tissues (Table 5.1) (Sasaki et al. 2003).
We examined DNA methylation and the corresponding expression of MB-COMT in post-mortem brains as well as saliva samples from the SCZ and BD patients versus control subjects (Abdolmaleky et al. 2006; Nohesara et al. 2011). In contrast to cancer, the MB-COMT promoter DNA was significantly hypomethylated, and MB-COMT gene expression was significantly increased in SCZ and BD patients, compared to the control subjects (Abdolmaleky et al. 2006). Interestingly, while the rate of pancreatic cancer is higher in SCZ patients, an increased expression of COMT was also observed in pancreatic cancer cell lines as well as pancreatic ductal adenocarcinoma compared to the normal pancreatic tissues (Wu et al. 2012).
Notably, beside COMT, monoamine oxidase A (MAOA), another gene encoding an enzyme involved in the degradation of dopamine as well as serotonin was found to exhibit increased expression in advanced prostate cancer (Flamand et al. 2010). However, its expression is epigenetically silenced in cholangiocarcinoma due to promoter DNA hypermethylation (Huang et al. 2012). Hypermethylation of specific CpG sites of MAOA was also reported in male SCZ patients (Chen et al. 2012a). These findings, although diverse, support that epigenetic modifications of genes related to dopaminergic system might serve as targets for cancer therapy. This idea gains additional support from the observations that the inhibitory effects of Epigallocatechin 3-O-gallate (EGCG) on the growth of lung cancer cell lines is synergistically potentiated by COMT inhibitor drugs such as entacapone and tolcapone (Forester and Lambert 2014) and MAOA inhibitors exhibit antioncogenic effects in advanced prostate cancer (Zhao et al. 2009; Flamand et al. 2010).
5.3 Immune System Dysregulation in Cancer and SCZ
5.3.1 Maternal Infections and Dysregulation of Interleukins in Cancer and SCZ
While a defective immune system is a well-known causal factor in cancer development, over-activity of immune system is linked to higher rate of autoimmune diseases in SCZ as well as in relatives of patients with SCZ (Benros et al. 2011; Eaton et al. 2006; Chen et al. 2012b). Maternal infection as a factor in immune system activation also increases the risk of SCZ in the offspring. Hospital contacts resulting in viral or bacterial infections in childhood or adolescence can also increase the risk of SCZ (Nielsen et al. 2013). Maternal infections, as an environmental risk factor has been indicated as a contributor for the pathogenesis of at least one third of SCZ patients (Brown and Derkits 2010). It has been shown that maternal immune activation could induce a number of abnormalities in the offspring, such as alterations in the pattern of gene expression, neurochemistry and cortical connectivity as well as other neuropathologies similar to those observed in SCZ, including decreased cortical thickness and enlarged ventricles which are characteristic of SCZ pathology (Garbett et al. 2012; McAllister 2014). There is also strong evidence for a relationship between maternal immune system activation and the development of autism in offspring, both due to viral as well as bacterial infections (Abdallah et al. 2012a, b). One of the well-studied potential mechanisms is an increased level of maternal cytokines, especially IL-6, which may cause an “expanded adult forebrain neural precursor pool” following maternal infection perturbing the “olfactory neurogenesis in the offspring months after fetal exposure”. This observation suggests that an acute and transient hyper-activation of IL-6 may have long-term impacts in the- IL6-dependent self-renewal pathway of neural stem cells altering the characteristics of neural precursors throughout the life of the offspring (Gallagher et al. 2013).
Table 5.1
The shared factors implicated in Psychiatry, with main focus on SCZ, and Cancer. The table summarizes the connected activities of factors in SCZ, BD, MDD and AD with Cancer. Symbols: (↑) indicates increase and (↓) indicates decrease
Factor | Activity in Psyc/Cancer | Mechanisms/activities | References |
|---|---|---|---|
TGF-β | ↑ in SCZ, ↓ by antipsychotics in SCZ a ;BD;↓ in Cancer | A disease state marker in SCZ. Lithium inhibits Smad3/4-dependent TGFβ signaling. Induction of apoptosis involving SMAD or DAXX pathways. Regulation of immune system by FOXP3. Up-regulates miR-182 expression to promote gallbladder cancer metastasis by targeting CADM1. Acts as a tumor suppressor in normal epithelial cells, silenced by epigenetic mechanisms in some types of carcinomas | |
TGFβ1 | ↑ in SCZ, ↓ in AD ↓ in Cancer (↓in early, ↑in late Cancer) | Cell growth, cell proliferation, cell differentiation and apoptosis. Inhibits interferon-γ, TNF-α. Suppresses RELN expression and RELN is a negative regulator of TGFβ1 induced cell migration in esophageal carcinoma cells. Promoter DNA hypermethylation in gastric cancer | |
TGFβ2 | ↑ in SCZ/BD ↓ in Cancer (↓in early, ↑in late Cancer) | Increased in SCZ and associated with decreased Wnt10A expression. Suppresses the effects of interleukin dependent T-cell tumors and disruption of the TGFβ/SMAD signaling is involved in diverse human cancers. Promoter DNA hypermethylation is linked to prostate cancer progression | |
FOXP3 | ↑ in SCZ/ BD ↓↑ in Cancer | Potential tumor suppressor in gastric cancer. Down regulated in melanoma, breast, prostate, ovary and brain tumor cells but elevated level of expression reported in pancreatic adenocarcinoma, leukemia, hepatocellular carcinoma, bladder cancer, thyroid carcinoma and cervical cancer | |
FOXO | ↑ in SCZ and BD ↓ in Cancer; Diabetes | Transcription factor; involved in cell metabolism, proliferation and apoptosis. Increased expression of FOXO3 in the brains of SCZ and BD patients. FOXO3A is linked to longevity. Loss of functions in several types of human cancers and diabetes. Some FOXO3A genotypes are linked to cancer, cardiovascular disease and deficit in cognitive functions. Metformin, Simvastatin, DRD2 blockers, Venlafaxine, SSRIs and ImipramineincreaseFOXO3a phosphorylation leading to its decreased nuclear localization | |
TNF-αb | ↑ in SCZ (in drug naïve and treated) and Depression; ↓ by Anti-depressants; Cancer | Role in cancer treatment. Specific polymorphisms are associated with paranoid SCZ and others with cervical and gastric cancer. Mediates acute myeloid leukemia treatment response | |
IL-1βc | Serum ↑ in SCZ (drug naïve and treated); ↓ by anti-depressants and antipsychotics; Cancer | Has pro-inflammatory property. IL-1β expression is induced by NF-κB after activation of immune cells. Through Wnt signaling, stimulates metastatic behavior and growth of colon and gastric cancer cells | |
INF-γ | CSF and serum ↑ in SCZ (drug naïve and treated); Reported ↓ in SCZ; ↓ by Anti-depressants; Cancer (anti-tumor effects) | Inhibits the growth of nasopharyngeal carcinoma and induces the cytotoxicity of daunorubicin against leukemic cells | |
IL-2 | ↑ in SCZ (first episode psychosis and schizophrenia in relapse); Serum ↓ in stable SCZ; ↑ in plasma by antipsychotics; Cancer | Genotype TT and allele T is associated with paranoid SCZ. GG genotype and the G allele of the same polymorphism (rs2069762) are associated with higher risk of childhood lymphoma and gastric cardia cancer. IL2 is used in cancer immunotherapy | |
IL-6 Th2 prototype cytokine | Serum ↑ in SCZ (in drug naïve and treated) and Depression; ↓ by Anti-depressants in MDD and antipsychotics in SCZ; Cancer | Blocks apoptosis in cancer cells during inflammatory process. Induces tumor growth in colorectal cancer. Increases proliferation, migration, invasion and/or survival and chemoresistance in ovarian cancer cells. Th2 predominance hypotheses by meta-analysis in schizophrenia. Associated with paranoid SCZ | |
IL-8 | Serum ↑ in chronic SCZ; ↑ in Cancer | Increased in kidney and breast cancers. Elevated level of IL-8 during pregnancy increases likelihood of SCZ in offspring. High levels of IL-2 and IL-8 are indicators of poor treatment response in SCZ | |
IL-17 | Serum ↓ in stable SCZ; Serum ↑ in first episode psychosis and schizophrenia in relapse; ↑ in Cancer | High levels of IL-17+FOXP3+CD4+T cells is associated with colon carcinoma. Increased level of IL-17+regulatory T cells is reported in blood and in ovarian and breast cancers, melanoma, and renal cell carcinoma. Prognostic factor in colorectal carcinoma | |
RELN | ↓ in SCZ; ↑ in prostate cancer; ↓ in pancreatic and breast cancers | Neuronal migration, positioning and synaptic plasticity. RELN is a cell migration suppressor. RELN gene/protein expression is increased in prostate cancer. RELN promoter DNA hypermethylation in SCZ, pancreatic and breast cancers | |
CXCR4 | ↑ DNA methylation in SCZ; ↑ expression in Cancer (23 types) | Chemokine receptor. Decreased expression and promoter DNA hypermethylation in SCZ. Marker of metastasis and cancer invasion. Promoter DNA hypermethylation in pancreatic cancer | |
miRNA-126 | ↑ in SCZ (DLPFC#); ↓ in Diabetes; ↓ in Cancer | Angiogenesis control. Acts as a tumor suppressor and down regulated in various cancers: breast, gastric, prostate, colorectal, clear-cell renal and osteosarcoma. Down regulates CXCR4 and VEGF. Up-regulated in SCZ | |
VEGF | ↓ in SCZ (DLPFC); ↓ in AD; ↑ in Cancer | Involved in cancer progression and metastasis, Neuronal survival, neuroprotection, regeneration, growth and differentiation. Inhibited by miR-126 | |
GAD1 | ↓ in SCZ (PFC); ↑ in benign and malignant prostatic tissue; ↑ in oral squamous cell carcinoma, liver cancer | Key enzyme for GABA biosynthesis. Decreased expression and increased DNA methylation in SCZ. Over-expressed in oral squamous cell carcinoma and is associated with a higher degree of invasion/migration of cancer cells | |
COMT/MB-COMT | ↑ in SCZ and BD patients ↓ in endometrial cancer | Monoamines, catecholamines and estrogen degradation. Hypomethylation of MB-COMT promoter DNA and increased gene expression in SCZ. Hypermethylation of promoter DNA and reduced expression in endometrial cancer. Increased expression in pancreatic cancer | |
MAOA | ↑ DNA methylation in SCZ and cholangiocarcinoma; ↑ expression in advanced prostate cancer | Monoamines degradation. DNA Hypermethylation in male SCZ patients. MAOA inhibitors restrain prostate cancer |
Since the genes encoding cytokines are among the main mediators of crosstalk between the immune system and brain, aberrations in a specific region of chromosome 6 which harbors immune genes, and specific haplotypes of immune genes especially those within the major histocompatibility complex (MHC) are highly relevant to cancer (Urayama et al. 2013) as well as SCZ pathogenesis (Shi et al. 2009; Stefansson et al. 2009; Purcell et al. 2009; Li et al. 2010; Jia et al. 2012). While deletion of this chromosomal region and down regulation of the associated genes are linked to cancer development (Feenstra et al. 1999), the expression of at least two out of five of the MHC class I genes, including Butyrophilin 2A2 (BTN2A2) and antigen HLA-B, which may have roles in synaptic development are increased in SCZ patients (Sinkus 2013).
The link between SCZ and the chromosome 6 region, containing MHC genes has also been confirmed by genetic and genome-wide association studies (Shi et al. 2009; Stefansson et al. 2009; Purcell et al. 2009; Li et al. 2010, Jia et al. 2012; Debnath et al. 2013). Interestingly, the TT genotype and the allele T of interleukin-2 (IL-2) are associated with paranoid type of SCZ (Paul-Samojedny et al. 2013). On the contrary, the GG genotype and the G allele of the same polymorphism of IL-2 (rs2069762) are associated with higher risk of childhood lymphoma (Song et al. 2012) as well as gastric cardia cancer (Wu et al. 2009). There are also several reports on cancer immunotherapy with IL-2 in diverse types of cancers (Antony and Dudek 2010; Kolitz et al. 2014; Guma et al. 2014).
It has been proposed that MHC I negatively regulates the synaptic densities and bidirectionally controls glutamatergic and GABAergic synaptic densities in the developing brains and modulates the region and age-specific gene expression patterns, and these alterations apparently dysregulate the immune response in SCZ patients. Furthermore, changes in MHC I expression in neurons of SCZ patients may also change channel properties of glutaminergic genes such as NMDA receptors, and may alter activity-dependent synaptic strength by limiting the NMDA-mediated AMPAR trafficking (McAllister 2014). These observations along with the fact that NMDA affects the growth of malignant glioma cells in vitro (Panchanathan et al. 2013) provide support that drugs acting on GABAergic system and/or NMDA receptors could have potential value in cancer therapy.
In addition to IL2, the serum levels of IL-6 and IL-8 are also elevated in chronic SCZ patients (Table 5.1) (Zhang et al. 2002; Song et al. 2013a, b). Although all studies do not support a role for IL-1β in SCZ susceptibility (Shibuya et al. 2014), higher serum levels of IL-1β as well as TNF-α and adiponectin have been reported in drug naïve SCZ patients (Song et al. 2013a, b) that might play a role in the maintenance of cancer stem cells. Interestingly, risperidone (an antipsychotic drug widely used in SCZ patient) decreases the serum levels of IL-1β (Fig. 5.1), however it may reach to the baseline level after 6 months. Notably, while the serum level of IL-6 is decreased during the first 3 months of risperidone treatment, it may also reach the baseline level in 6 months. Nevertheless, the serum level of TNF-α increases by risperidone treatment during this time period. Hence, risperidone treatment seems to be associated with an initial anti-inflammatory effect that is neutralized with long-term treatment (Song et al. 2014). A recent meta-analysis also concluded that antipsychotic drugs exhibit anti-inflammatory effects in SCZ through increasing the soluble IL-2 receptor and decreasing the IL-1β and interferon-γ plasma levels (Tourjman et al. 2013).
Beside expression changes of these cytokines in SCZ, there are reports indicating aberrant DNA methylation of genes coding for some of these cytokines in different kinds of cancer (Table 5.1). For instance, hypomethylation of IL-8 in human astrocytoma and clear cell renal cell carcinoma, aberrant DNA methylation of IL1β, IL6, and IL8 in non-small cell lung cancer, hypermethylation of TGF-β1 promoter DNA in gastric cancer have been reported (Venza et al. 2012; Tekpli et al. 2013; Wang et al. 2013; Yoo et al. 2013). Therefore, further investigations for the identification of these and other possible epigenetic mechanisms that could complete the missing part of this important line of evidence in SCZ and cancer is warranted.
5.3.2 TGF-β Signaling in Cancer and SCZ
The TGF-β super family of cytokines are involved in the regulation of cellular processes, including cell division, differentiation, motility, adhesion and death. TGF-βs and BMPs signal through binding to the TGF-β membrane receptors leading to transphosphorylation of R-Smads such as Smad1, Smad2, Smad3 and Smad5/8, which along with Smad4 (the Co-Smad) translocate to the nucleus and form transcriptional complexes with DNA binding factors and co-activators/co-repressors modulating the expression of many genes (Massague 2000; Papageorgis et al. 2015). In animal studies, high TGF-β1 and TGF-β3 expression were observed in cerebral cortex, hippocampus, central amygdaloid nucleus, substantia nigra and the brainstem reticular formation. In contrast, TGF-β2 is reported to be highly expressed in deep cortical layers, dentate gyrus, cerebellum and areas of monoaminergic neurons (Vincze et al. 2010) known to be affected in SCZ and BD.
Hypo-activity of TGF-β signaling is known to be involved in the early stage of cancer development. However, high level of TGF-β in an already developed cancer may promote metastasis. For instance, in advanced breast cancer an activated TGF-β-Smad signaling silences the expression of several genes such as CDH1, CGN, CLDN4 and KLK10 by altering the binding capacity of DNMT1 to the CpGs in regulatory regions of these genes and play a role in epithelial to mesenchymal transition (Papageorgis et al. 2010; Papageorgis et al. 2015). Therefore, in the advanced breast cancer, the disruption of TGF-β signaling could decrease DNMT1 binding activity minimizing the malignant phenotype. On the other hand over-expression of SMAD7 which inhibits R-SMADs could reverse the malignant mesenchymal phenotype to epithelial-like phenotype (Papageorgis et al. 2010).
A recent study reported an increase in the production of TGF-β in first episode psychosis , psychotic patients and in relapsed SCZ patients suggesting that TGF-β could be a valuable marker for psychosis (Borovcanin et al. 2012). More importantly, a meta-analysis also concluded that TGF-β is a state marker in SCZ patients (Miller et al. 2011). These observations indicate that, while a reduced activity of TGF-β signaling is involved in early stage cancer development, an increased TGF-β expression in SCZ could help to reduce cancer risk. However, it may increase the risk of invasion and/or metastasis after the development of cancer in these patients. Although, the symptoms of SCZ are apparently linked to an accelerated gear for apoptosis of neuronal cells (Catts and Catts 2000; Jarskog et al. 2004; Glantz et al. 2006; Jia et al. 2010) and cortical atrophy (Francis et al. 2012), an increase in TGF-β signaling associated with a decrease in Wnt signaling may have a different effect and promote adult neuronal differentiation and migration, and inappropriate insertion into the neuronal network in SCZ patients (Kalkman et al. 2009). In support of this finding our recent gene expression profiling of post mortem brain samples uncovered an increased TGF-β2 expression associated with a decreased Wnt10A expression in the frontal lobe of SCZ patients (Abdolmaleky and Thiagalingam, manuscript in preparation).
Collectively, these findings support the hypothesis that an increased activity of TGF-β signaling which has a significant role in neuronal cell fate and apoptosis as well as SCZ pathogenesis might be the underlying mechanism for the reduced risk of cancer in these patients. However, antipsychotic drugs through modulating this pathway, combined with epigenetic silencing of RELN signaling may increase the risk of certain types of cancers such as the breast and pancreatic cancers. While these findings suggest that genes involved in SCZ may promote apoptosis reducing the risk of cancer, studies to compare genetic and epigenetic aberrations in cancer versus patients with SCZ may help to find targets for the prevention and treatment of both of these complex diseases. In this line, the use of metformin which is known to inhibit TGF-β signaling (Cufí et al. 2010) might be helpful in advanced cancer as well as SCZ treatment. Indeed, several recent in vitro and in vivo as well as clinical studies have shown anti-cancer activity of metformin in different types of cancers such as esophageal (Xu and Lu 2013), ovarian (Dilokthornsakul et al. 2013) breast (Zhu et al. 2014b; Hadad. et al. 2014) hepatic (Miyoshi et al. 2014; Lin et al. 2013a), bladder (Zhang et al. 2013), endometrial (Ko et al. 2014; Nevadunsky et al. 2014) and other cancers, in general (Yin et al. 2013; Beck and Scheen 2013). Lithium, a drug widely used for the treatment of BD and refractory SCZ, also inhibits Smad3/4-dependent TGF-β signaling in neurons (Fig. 5.1) through increasing the activity of cAMP/PKA signaling (Liang et al. 2008). It is likely that antipsychotic drugs that block DRD2 receptor and increase cAMP level may also have the same effect.
5.4 Adiponectin and Body Weight in Cancer and SCZ
Adiponectin is a protein hormone exclusively secreted from the adipose tissues and in contrast to SCZ, is found at reduced level in plasma of patients with several types of cancers as well as obesity linked to insulin resistance and type 2 diabetes (Kishida et al. 2014; Hebbard and Ranscht 2014). The use of adiponectin receptor agonists such as AdipoRon, which is an orally active small molecule and act on both AdipoR1 and AdipoR2 receptors, to activate AMPK and PPAR-α pathways, respectively, has been proposed for the treatment of obesity-related disorders such as type 2 diabetes and cancer (Okada-Iwabu et al. 2013). In fact, in a study that used human and mouse colon cancer cell lines, both adiponectin and metformin additively reduced the malignant potential of colon cancer. The major mechanism proposed for this effect is that; adiponectin and metformin inhibit the IL-1β signaling and decrease malignant potential through their effects on the expression of p53 (a tumor suppressor), p21, p27, and cyclin E2 (genes regulating cell cycle) involving AMPK/LKB1 pathways (Moon and Mantzoros 2013).
As mentioned above, in the first five years of SCZ diagnosis and before the use of antipsychotics, the rate of cancer is lower in SCZ compared to the general population. However, because of the poor nutritional state of SCZ patients and more importantly the use of atypical antipsychotic drugs with common side effects such as weight gain and metabolic imbalance, the innate lower risk of cancer is found to decline in this group of patients (Ji et al. 2013; Chen et al. 2013; Manzanares et al. 2014). It is important to note that, in SCZ patients, treatment with atypical antipsychotic drugs decreases the circulating adiponectin at levels comparable to patients with diabetes. Adiponectin is reported to exhibit anti-angiogenic and tumor growth-limiting properties during in vitro studies and its level is inversely correlated with several malignancies that occur later in life (Adachi et al. 2012; Tsai et al. 2011; Song et al. 2013a, b; Dalamaga et al. 2012). While the circulating levels of adiponectin with insulin-sensitizing, anti-inflammatory, proapoptotic, anti-proliferative properties and cancer protectiveness declines with the use of atypical antipsychotics in SCZ (and bipolar disorder patients), current data, considering the higher levels of TGF-β in SCZ patients indicates that; metformin may not only be useful in the treatment of metabolic syndrome and the increased cancer risk, but also psychotic symptoms of SCZ patients as an inhibitor of TGF-β signaling pathway. In fact, excess weight gain and/or obesity not only is an emerging worldwide heath problem in general population, it is also an important issue in psychiatric patients, especially in SCZ patients under atypical antipsychotic drug treatment which have well known weight gain as the side effect (Subramaniam et al. 2014). Excess body weight is also considered as a risk factor for postmenopausal breast cancer, endometrial and ovarian cancer, pancreatic cancer, renal cell cancer, esophageal adenocarcinoma, hematological malignancies, high-grade prostate cancer, colon, thyroid, and gallbladder cancers (Dalamaga et al. 2012). There is a tendency for a higher frequency of a number of these cancers in SCZ patients using antipsychotic drugs.
5.5 Vascular Endothelial Growth Factor (VEGF) and mir-126 in Cancer and SCZ
VEGF is a growth factor implicated in cancer progression and metastasis. A decreased level of VEGF mRNA has been reported in the dorsolateral prefrontal cortex of SCZ patients (Fulzele and Pillai 2009). Furthermore, a low level of serum VEGF in Alzheimer’s disease (Mateo et al. 2007), and a significant increase in the VEGF serum level following the clinical improvement of drug resistant depressed patients treated by electroconvulsive therapy (ECT) support an important role for VEGF in neuropsychiatric disorders (Minelli et al. 2011). In an animal study, VEGF was found to mediate the anti-depressive effects of cAMP down-stream events in the adult hippocampus during the treatment by antidepressants (Lee et al. 2009). Chronic treatment with lithium could also attenuate the stress-induced decrease of VEGF expression in the hippocampus in stressed animals, thus the therapeutic efficacy of lithium as a mood stabilizer may be mediated by VEGF (Silva et al. 2007; Guo et al. 2009). VEGF is considered as a neurotrophic factor and has been implicated in neuronal survival, neuroprotection, regeneration, growth and differentiation (Rosenstein et al. 2010). As reported in a mouse model of diabetes, inhibition of the VEGF receptor 2 (VEGFR2) mediated signaling results in endothelial dysfunction and vascular problems, which could be potentially reversed with the use of antioxidants (Warren et al. 2014). It is noteworthy that VEGF is inhibited by miR-126 which its dysfunction is strongly associated with angiogenesis and is especially expressed in endothelial cells, and down-regulated under hypoxic condition as shown in both in vitro and in vivo studies (Ye et al. 2014). There is also supporting evidence that mir-126 is a tumor suppressor, and the reduced level of miR-126 is a significant predictor of poor survival in many cancers (Yang et al. 2013a; Yu et al. 2013; Sun et al. 2013). Mir-126 also suppresses DNMT1 (Zhao et al. 2011) as well as CXCR4 expression (Fig. 5.1), and its tumor suppressing potential is mediated by the AKT and ERK1/2 signaling pathways (Liu et al. 2014). Interestingly, along with the decreased level of VEGF, the expression level of mir-126 is up-regulated in the postmortem dorsolateral prefrontal cortex of the brain in SCZ patients (Beveridge and Cairns 2012). This signifies that multiple aspects of epigenetic alterations are inversely regulated in SCZ patients versus cancer and provide additional support for the idea that; the identification of disease pathogenesis in either illnesses could help the design of novel therapeutics for both of these diseases.
5.6 Cell Maintenance is Impaired in Both Cancer and Neuropsychiatric Diseases
FOXO genes, the O subclass of the forkhead family of transcription factors, mediate the effects of insulin and growth factors and are involved in cell metabolism, proliferation and apoptosis. The FOXO family members in humans are FOXO1, FOXO3, FOXO4 and FOXO6. The shared nature of FOXO protein family members (with the exception of FOXO6, which is exclusively nuclear) is translocated out of the nucleus upon phosphorylation by Akt/PKB proteins of the PI3K signaling pathway (Brunet et al. 1999). The loss of FOXO functions, has been detected in several types of human cancers and diabetes, a known risk factor for cancer (Monsalve and Olmos 2011; Eijkelenboom and Burgering 2013). FOXO3 is widely distributed in the adult brain and exhibits an increased expression during the brain development (Barthel et al. 2005). Activation of PI3K and Akt lead to the phosphorylation of FOXO and translocation from the nucleus to the cytosol, to prevent its DNA binding and transcriptional activity (Fig. 5.1). However, dephosphorylated FOXO can return to the nucleus and induce expression of genes that are involved in cell cycle arrest, apoptosis and resistance to oxidative stress. Human FOXO3 (FOXO3A) has been associated with longevity and some of the FOXO3 genetic variations are also linked to cancer, cardiovascular disease and deficit in cognitive functions (Rodriguez et al. 2013; Jia et al. 2014; Pan et al. 2014; Carbajo-Pescador et al. 2014; Di Bona et al. 2013). Epigenetic modifications of FOXO3 such as methylation of the lysine 270 of histone protein can also inhibit DNA binding of FOXO3 and prevent the neuronal cell death induced by oxidative stress (Xie et al. 2012). The decreased expression of the FOXO3 is associated with poor prognosis of human breast cancer and primary gastric adenocarcinoma (Jiang et al. 2013; Yang et al. 2013b). Interestingly, in C. elegans all antipsychotic drugs activate AKT pathway and inhibit nuclear localization of DAF16, the homologous gene of the human FOXO3 (Weeks et al. 2010) suggesting this mechanism as one of the underlying causes of the increased breast cancer risk in medicated SCZ patients.
The phosphorylation of FOXO3 is also increased by brain derived neurotrophic factor (BDNF) that inhibits its transcriptional activity in differentiated human SH-SY5Y neuroblastoma cells. Treatment with lithium in mood disorders alongside the decrease in the levels of FOXO3, inhibits its transcriptional activity and alleviates the proposed BDNF deficiency in mood disorders based on an in vivo study (Mao et al. 2007). In other in vivo studies, an increased serotonergic activity was found to result in phosphorylation of FOXO1 and FOXO3 in several brain regions, and reduce the nuclear distribution of FOXO1 and FOXO3. Similarly, chronic treatment with imipramine, an antidepressant drug with serotonergic and noradrenergic properties, could also increase FOXO1 and FOXO3 phosphorylation in brain (Fig. 5.1). Additionally, metformin also increases FOXO3 phosphorylation leading to a decreased FOXO3 nuclear localization (Takayama et al. 2014). Of note, the selective deletion of FOXO1 from the brain reduces anxiety level, and the FOXO3a-deficient mice exhibit antidepressant-exposed behavior (Polter et al. 2009). In humans, while the loss of FOXO function, has been linked to cancer (Monsalve and Olmos 2011), we found a highly significant increase in the expression of FOXO3 during gene expression profiling of the post- mortem brains of SCZ and to lesser extent in BD patients (Abdolmaleky and Thiagalingam, manuscript in preparation). Therefore, similar to other genes/pathways mentioned above FOXO also has opposite roles in cancer versus SCZ as well as BD proposing the indication/contraindication of specific psychiatric drugs in cancer therapy and vice versa.
5.7 Huntington’s Disease, Cancer and Dopamine Related Drugs
Similar to SCZ, a decreased rate of cancer has been reported in patients with Huntington’s disease (HD) in different countries (Sørensen and Fenger 1992; Ji et al. 2012). Interestingly, a large fraction of patients with HD exhibit SCZ-like symptoms before or after the appearance of HD symptoms. An increased CAG repeat expansion in the exon 1 of gene coding an expanded chain of glutamines in huntingtin (Htt) protein is responsible for HD as an inherited neurodegenerative disorder. Although the underlying mechanisms of the reduced cancer risk in patients with HD have not been fully explored, it has been attributed to an increased apoptotic capacity of the expanded polyglutamine repeat in an animal study using p53 deficient mice (Ryan and Scrable 2008).
The striatal neurons expressing dopamine receptors predominantly become degenerated in HD patients, and the degree of striatal neuronal loss is inversely linked to the age of death in HD patients. Thus the mutated Htt, which is predicted to exhibit prominent toxic activity in this brain region (Hadzi et al. 2012), may also be involved in the senescence of other cells. Interestingly, low doses of a selective dopamine type 1 (D1) receptor agonist such as 100 μM of dopamine which activate adenylate cyclase, accelerates the formation of mutant Htt nuclear aggregates and increases the rate of cell death in neuroblastoma cell lines (Robinson et al. 2008). Earlier studies also showed an accelerated formation of aggregates and cell death with 1 mM of dopamine in striatal primary cultures containing human HTT gene with expanded CAG repeats (Petersén et al. 2001). However, the dopamine D2 (dopamine type 2) receptor antagonists could prevent these effects of dopamine in primary cultures of striatal neurons transfected with GFP-tagged exon 1 of the mutant HTT. In this experiment, the D2 receptor agonist was also found to enhance the number of mutant Htt aggregates in the dendrites of neurons and increased cell death (Charvin et al. 2005). As dopamine system is involved in the striatal neuropathology, depletion of striatal dopamine by the 6-hydroxydopamine was shown to be neuroprotective in rodents of HD model via reduction of striatal glutamate (Stack et al. 2007). Considering these observations, tetrabenazine, as an inhibitor of vesicular monoamine transporter (VMAT2) was approved for use in the treatment of HD patients. Tetrabenazine acts by reducing dopaminergic input to the striatum and alleviates the behavioral deficits and neuronal death in the YAC128 mouse model of HD (Tang et al. 2007). In addition, treatment with haloperidol decanoate, a potent D2 antagonist, also protects neurons from expanded Htt-induced dysfunction in the rat striatum (Charvin et al. 2008).
These observations suggest that, manipulation of Htt function by the modulation of dopamine receptors activities may help to inhibit cancer progression. This hypothesis is supported by the fact that, other modulators of the normal Htt protein such as Huntingtin interacting protein (HIP) and huntingtin associated protein (HAP1) are also involved in cancerous cell fate. For instance, in-vitro studies on huntingtin-associated protein 1 (HAP1) , the ligand of Htt that binds more tightly to Htt with an expanded glutamine repeat than to wild type Htt, showed reduced expression in human breast cancer tissues compared to the normal breast tissues. Interestingly, over-expression of HAP1 reduces cell growth in breast cancer cell lines (MDA-MB-231 and MCF-7) and suppresses the cell migration and invasion, and promotes apoptosis in these cell lines (Zhu et al. 2013). These lines of evidence suggest that in addition to Htt and the interlinked dopamine signaling pathway, other huntintin associated genes may also be involved in cancer development and progression, thus could be targeted for cancer therapy.
Conclusion
Several lines of evidence provided here indicate that cancer and SCZ are inversely correlated in a disease stage specific manner and the molecular defects involved in SCZ pathogenesis might be protective against the development , especially the early stage of cancer. Certainly, follow up studies should reveal the key dysregulated genes up or downstream of affected genes/pathways such as RELN, dopamine, GABA and TGF-β2 and other genes involved in the pathogenesis of both SCZ and cancer to generate clues to deduce strategies for the prevention and to uncover novel molecular and epigenetic targets for therapeutic applications in SCZ as well as cancer.
References
Abdallah MW, Hougaard DM, Nørgaard-Pedersen B, Grove J, Bonefeld-Jørgensen EC, Mortensen EL (2012a) Infections during pregnancy and after birth, and the risk of autism spectrum disorders: a register-based study utilizing a Danish historic birth cohort. Turk Psikiyatri Derg 23(4, Winter):229–235PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree