Abstract
Introduction: The surgical techniques used in mastectomy are in constant evolution because of advancement in knowledge and the needs of patients.
Methodology: Literature review of different types of mastectomy.
Results: Halsted radical mastectomy (RM), the first effective surgery in treating breast cancer, was later modified by Patey, Madden, and others to preserve the pectoralis major muscle. Studies showed comparable survival outcomes between the two types of mastectomy. The modified radical mastectomy (MRM) became the standard treatment for women with stage I and II breast cancer in the 1970s. However, the axillary lymph node dissection (ALND), a part of modified radical mastectomy, was associated with significant side effects. Hence, the simple mastectomy (SM) was developed to spare the ALND and focus on treating the local disease only. Studies showed that survival after SM with or without radiation was comparable to those with RM.
Recently, adjuvant systemic treatment has been shown to significantly improve disease-free and overall survival in patients with node-positive breast cancer, which requires nodal staging to guide therapy. Sentinel lymph node biopsy (SLNB) was invented to provide adequate pathologic nodal status in clinically negative axilla. Today, SM coupled with SLNB has largely replaced the MRM.
Additional modifications to mastectomy by sparing the skin and the nipple areolar complex further increased its popularity.
Discussion: The evolution of surgical treatment of breast cancer is governed by the principles of controlling the local disease and providing adequate pathology with minimal adverse effects. The validity of any new procedure requires confirmation.
Keywords
modified radical mastectomy, simple mastectomy, contralateral prophylactic mastectomy, prophylactic mastectomy, irradiation
Modified Radical Mastectomy
Historical Evolution of the Surgical Technique
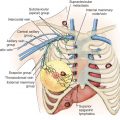
The rationale for the Halsted radical mastectomy was largely to achieve local and regional control of the breast cancer. The mastectomy techniques invented by Halsted’s predecessors in surgery and pathology allowed him to achieve unprecedented success in obtaining this objective without the availability of irradiation or chemotherapy. The techniques now known as the Halsted radical mastectomy and the modified radical mastectomy are an evolution of these methods, which used varying degrees of breast extirpation and lymphatic dissection ( Table 31.1 ).
| Study | Year | Surgery |
|---|---|---|
| Moore a | 1867 | Segmental breast resection, selective axillary dissection |
| Volkmann b | 1875 | Total breast extirpation, with removal of pectoralis major fascia, preservation of pectoralis major muscle |
| Gross c | 1880 | Total mastectomy and complete axillary dissection |
| Banks d | 1882 | Modified radical mastectomy, with pectoralis preservation |
| Sprengel e | 1882 | Total mastectomy and selective axillary dissection |
| Kuster f | 1883 | Total mastectomy and routine axillary dissection |
| Halsted g | 1894 | Radical mastectomy |
| Meyer h | 1894 | Radical mastectomy |
| Murphy i | 1912 | Radical mastectomy, modified by pectoralis preservation |
| McWhirter j | 1948 | Modified radical mastectomy with radiotherapy |
| Patey k | 1948 | Modified radical mastectomy with resection of pectoralis minor |
| Madden l | 1965 | Modified radical mastectomy with pectoralis preservation |
a Data from Moore CH. On the influence of inadequate operations on the theory of cancer. R Med Chir Soc Lond. 1867;1:244.
b Data from Volkmann R. Geschwülste der mamma (36 Fälle) Beitrage zur Chirurgie. Leipsig; 1895:310.
c Data from Gross SW. A Practical Treatment of Tumors of The Mammary Gland Embracing Their Histology, Pathology, Diagnosis and Treatment. New York: Appleton; 1880.
d Data from Banks WM. On free removal of mammary cancer with extirpation of the axillary glands as a necessary accompaniment. BMJ. 1882;2:1138.
f Data from Küster E. Zur behandlung des brustkrebses verhandlungen der deutschen gesellschaft für Chirurgie. Leipsig; 1883:288.
k Data from Patey and Dyson and Patey.
In contrast to the Halsted radical mastectomy, the modified radical mastectomy defines a surgery of complete breast removal, with the inclusion of the tumor, overlying skin, and axillary lymphatics, with preservation of the pectoralis major muscle. Preservation of the pectoralis major muscle provides for better cosmesis of the chest wall.
In 1894, Halsted and Meyer independently reported their individual techniques for the successful therapy of breast carcinoma with radical mastectomy. The initial clinical experience by Halsted suggested that the pectoralis major muscle was removed concomitantly with the axillary node dissection. The pectoralis minor muscle was transected only for technical expediency during the axillary dissection and was thereafter reapproximated to close the posterior superior axillary space. Soon after, however, Halsted advocated Meyer’s concept of the routine resection of both muscles. This method, once advocated by both Halsted and Meyer, became the state-of-the-art operative procedure for decades in treating cancer of the breast.
However, both American and British surgeons began to develop more conservative procedures. By 1912, Murphy had abandoned the Halsted radical mastectomy in favor of preserving both pectoral muscles. This practice was based on the experiences of Bryant of London, who acknowledged only one case of recurrent breast carcinoma in the pectoral muscles in patients followed over a 40-year clinical review period. Furthermore, the practice by Grace to perform only the simple mastectomy in certain patients went unchallenged until the widely acclaimed reports of the modified radical mastectomy by McWhirter, Patey and Dyson, and Patey in the 1940s. This paradigm shift to focus on surgery with muscle preservation has continued since the 1960s. In 1972, 30% of patients with breast cancer were treated with modified radical mastectomy and 50% with radical mastectomy. By 1981, only 3% received radical mastectomy, and 73% had modified radical mastectomy. The Consensus Development Conference on the treatment of breast cancer in 1979 stated that the modified radical mastectomy was the standard of treatment for women with stages I and II breast cancer during that period of time. Any other local or regional treatment developed thereafter must be compared with results of modified radical mastectomy.
Retrospective Studies of the Modified Radical Mastectomy
Multiple retrospective clinical studies were conducted to evaluate the survival outcomes after modified radical mastectomy and are summarized in Table 31.2 . These series clearly demonstrated that the rate of survival decreased as the tumor size increased and the lymph node metastases became evident. In these nonrandomized, retrospective studies, there appeared to be no survival benefit obtained by a complete axillary dissection to include level 3 nodes or removal of the pectoralis minor muscle (Patey vs. Auchincloss-Madden techniques). Although a rigorous statistical comparison was not applied, the survival rates at 5 and 10 years after the modified radical mastectomy were clearly affected by the initial stage of the disease (i.e., stage I vs. stage II), and the survival rates deteriorated with longer follow-up.
| Study | Location/Affiliation | Technique | No. of Patients | Disease Stage | ABSOLUTE SURVIVAL (%) | |
|---|---|---|---|---|---|---|
| 5-yr | 10-yr | |||||
| Handley Thackray, 1969 b | United Kingdom | Patey | 77 | I | 75 | 61 |
| 58 | II | 57 | 25 | |||
| Delarue, 1969 c,d | Toronto | Madden | 75 | I | 61.8 | — |
| 25 | II | 51.4 | — | |||
| Madden, 1972 e,f | New York City | Madden | 94 | I | 81.6 | 63 |
| II | 32.4 | 17 | ||||
| Robinson, 1976 g | Mayo Clinic | Madden | 280 | I | 81 | — |
| II | 54 | — | ||||
| Meyer, 1978 h | Rockford, Illinois | Madden | 175 | I–III | 74 | 43 |
| Baker, 1979 d,i | Johns Hopkins University | Patey | 91 | I | 90 | — |
| 22 | II | 72 | — | |||
| 31 | III | 45 | — | |||
| Leis, 1980 j,k | New York Medical College | Patey | 397 | I | — | 72.2 |
| 333 | II | — | 40.2 | |||
| Nemoto, 1980 d,l | American College of Surgeons | Mixed | 8906 | I | 65.1 | — |
| 7832 | II | 35.1 | — | |||
| Hermann, 1985 d,m | Cleveland Clinic | Madden | 358 | I | 73 | 56 |
| 211 | II | 55 | 28 | |||
a Includes some patients treated with radical mastectomy with equivalent results.
b Data from Handley RS, Thackray AC. Conservative radical mastectomy (Patey’s operation). Ann Surg . 1969;170:880.
c Data from Delarue NC, Anderson WD, Starr J. Modified radical mastectomy in the individualized treatment of breast carcinoma. Surg Gynecol Obstet. 1969;129:79.
j Data from Leis HP Jr. Modified radical mastectomy: definition and role in breast cancer surgery. Int Surg. 1980;65:211.
k Columbia Clinical Classification.
l Data from Nemoto T, Vana J, Bedwani RN, et al. Management and survival of female breast cancer: Results of a national survey by the American College of Surgeons. Cancer. 1980;45:2917.
Further analysis of these series also demonstrated survival rates for operable breast cancer as a function of the status of the axillary lymph nodes at the time of modified radical mastectomy ( Table 31.3 ). Survival was not only inversely related to the presence of a positive lymph node but also decreased proportionate to the number of positive lymph nodes. Similarly, the local and regional recurrence rates after modified radical mastectomy were affected by the stage of disease at the time of treatment ( Table 31.4 ).
| Study and Year | Clinic or Study Group | No. of Patients | SURVIVAL RATE FOR PATIENTS WITH NEGATIVE NODES | SURVIVAL RATE BY NUMBER OF POSITIVE NODES | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ANY | 1–3 | ≥4 | ||||||||
| 5-yr | 10-yr | 5-yr | 10-yr | 5-yr | 10-yr | 5-yr | 10-yr | |||
| Handley and Thackray, 1969 a | United Kingdom | 135 | 75 | 57 | 61 | 25 | NA | NA | NA | NA |
| Madden, 1972 b | New York City | 94 | 82 | 63 | 32 | 17 | NA | NA | NA | NA |
| Robinson, 1976 c | Mayo Clinic | 339 | 80 g (93) | 48 g (55) | 61 g (72) | 37 g (42) | ||||
| Nemoto, 1980 d | American College of Surgeons | 24,136 | 71.8 | 40.4 | 63.1–58.8 h | 51.9–22.2 h | ||||
| Hermann, 1985 e | Cleveland Clinic | 564 | 78 | 62 | 55 | 28 | 66 | 41 | 47 | 25 |
| Martin, 1986 f | Mayo Clinic | 208 | 87 | 74 | — | 56 | NA | NA | NA | NA |
a Data from Handley RS, Thackray AC. Conservative radical mastectomy (Patey’s operation). Ann Surg 170:880, 1969.
d Data from Nemoto T, Vana J, Bedwani RN, et al. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;45:2917.
f Data from Martin JK et al. Is modified radical mastectomy really equivalent to radical mastectomy in treatment of carcinoma of the breast? Cancer. 1986;57:510.
| Study and Year | Clinic or Study Group | No. of Patients | Disease Stage | SITE (%) | |
|---|---|---|---|---|---|
| Chest Wall Scar or Operative Field | Axilla | ||||
| Delarue, 1969 a | Toronto General g (Canada) | 43 | I | 0 | 0 |
| 32 | II | 12.5 | — | ||
| 25 | III | 15 | — | ||
| Madden, 1972 b | New York City h | 94 | I–III | 10 | 0 |
| Handley, 1976 c | United Kingdom h | 77 | A i | 10 | 1.8 |
| 58 | B | 22.6 | 0.1 | ||
| 8 | C | 63.6 | 9.1 | ||
| Baker, 1979 d | Johns Hopkins g | 91 | I | 13.2 | 1.1 |
| 22 | II | 9.1 | 4.5 | ||
| 31 | III | 22.6 | 22.6 | ||
| Leis, 1980 e | New York Medical College h | 116 | 0 | 0 | 0 |
| 397 | I | 5 | 0.08 | ||
| 333 | II | 13.8 | 0.08 | ||
| Crowe, 1991 f | Case Western | 917 | LN– | 6.5 | 2.7 |
| 475 | LN+ | 9 | 8.4 | ||
a Data from Delarue NC, Anderson WD, Starr J. Modified radical mastectomy in the individualized treatment of breast carcinoma. Surg Gynecol Obstet. 1979;129:79.
e Data from Leis HP Jr. Modified radical mastectomy: definition and role in breast cancer surgery. Int Surg. 1980;65:211.
Support for limiting the level of lymph node dissection was demonstrated by Madden and colleagues using the Auchincloss technique, which questioned the need to completely dissect the axillary contents. Madden et al. reported a local recurrence rate of 10%. Auchincloss found that the apical nodes (level 3) should be removed if clinically involved because of the high rate of recurrence if these nodes were found to be positive and not subsequently removed. However, if these level 3 nodes were clinically negative, complete axillary dissection was unnecessary because mastectomy with excision of levels 1 and 2 and pectoralis preservation had control and survival rates identical with more radical approaches. Furthermore, if Rotter nodes were not involved, clearance of these interpectoral nodes was found to be unnecessary. Further retrospective studies by Dahl-Iversen and Tobiassen, Handley, Hermann, and Nemoto and Dao confirmed similar survival results between the classic radical mastectomy and the modified radical mastectomy and that these two procedures were equal in the recovery of axillary lymph nodes.
Baker and associates of Johns Hopkins University compared the results of modified radical mastectomy with radical mastectomy in the treatment of operable breast cancer. For 205 patients with stage I cancer, 60 with stage II disease, and 67 with stage III disease (based on the tumor, node, metastasis [TNM] system), there were no statistically significant differences in 5-year survival when the results of the radical mastectomy were compared with those of the modified radical mastectomy. Furthermore, no statistically significant differences in incidence of locoregional recurrence were evident in patients with stages I and II disease when the results of the two surgical procedures were compared. In contrast, individuals with stage III disease treated with modified radical mastectomy had a statistically significant ( p = . 002) higher incidence of local recurrence (chest wall and axilla) compared with patients treated with radical mastectomy. Baker and colleagues concluded that modified radical mastectomy is the treatment of choice in patients with TNM stages I and II disease. For patients with stage III disease, the radical mastectomy provided a greater probability of locoregional control of disease but did not enhance survival.
Crowe and coworkers reported a 16-year experience of locoregional recurrence in a series of 1392 patients treated for operable breast cancer with modified radical mastectomy. They found that most cases of locoregional recurrence occurred within the first 3 years of treatment. Among patients with locoregional recurrence, distant metastases had developed in 64%. Large size of tumor and positive nodes were associated with rapid, locoregional recurrence. Furthermore, the incidence of developing distant failure was associated with the time of locoregional recurrence. In summary, the size and nodal status of the original cancer, as well as the length of the locoregional disease-free interval, were found to directly affect the likelihood of developing metastasis.
These and other observations support the conclusion that extirpation of the pectoralis major muscle is not essential to provide locoregional control of stages I and II disease (Columbia Clinical Classification A and B). It must be noted that neither procedure was adequate for achieving locoregional control of TNM stage III breast cancer or Columbia Clinical Classification C and D tumors (see Section XV). In properly selected patients with stages I and II disease, these retrospective analyses for the modified radical procedure show survival and control rates comparable to the more radical procedure. Chapters 50 and 51 contain comprehensive discussion of local, regional, and systemic complications that may ensue with the modified radical mastectomy.
Prospective Trials for the Modified Radical Mastectomy
During the 1970s, two prospective randomized trials by Turner in Manchester, England, and by Maddox at the University of Alabama, Birmingham, compared the Halsted radical mastectomy with the modified radical mastectomy. The study by Turner and associates consisted of 534 patients with T 1 or T 2 (N 0 or N 1 ) carcinoma of the breast and demonstrated that after a median follow-up of 5 years, there was no significant difference in disease-free survival, overall survival, or locoregional control rates ( Table 31.5 ).
| No. of Patients Followed Up | Overall Survival (5-yr) | Disease-Free Local Recurrence (5-yr) a | Disease-Free of Distant Metastases (5-yr) a | Overall Disease-Free Survival (5-yr) a | |
|---|---|---|---|---|---|
| All Cases | |||||
| Radical | 278 | 70 | 75 | 63 | 58 |
| Modified | 256 | 70 | 79 | 63 | 58 |
| Clinical Stage I | |||||
| Pathologic Stage I | |||||
| Radical | 119 | 80 | 85 | 79 | 69 |
| Modified | 108 | 79 | 90 | 79 | 71 |
| Pathologic Stage II | |||||
| Radical | 52 | 57 | 57 | 52 | 39 |
| Modified | 49 | 62 | 74 | 62 | 57 |
| Clinical Stage II | |||||
| Pathologic Stage I | |||||
| Radical | 41 | 85 | 91 | 79 | 79 |
| Modified | 38 | 78 | 88 | 71 | 70 |
| Pathologic Stage II | |||||
| Radical | 64 | 55 | 59 | 47 | 38 |
| Modified | 59 | 55 | 56 | 45 | 30 |
a Figures indicate the percentages of patients not experiencing each event regardless of any other outcome.
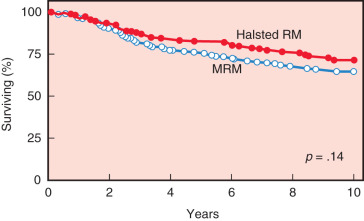
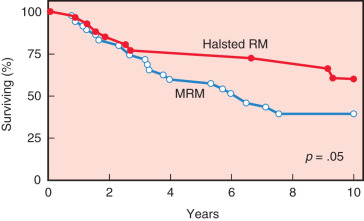
The study by Maddox and associates compared modified radical mastectomy with radical mastectomy in 311 patients with stages I to III breast cancers. Patients with positive lymph nodes were randomized to receive chemotherapy. Results of this study demonstrated no significant difference in the 5-year disease-free survival rate but did show a significant decrease in local recurrence rates after the radical mastectomy ( Table 31.6 ). A trend toward improved overall survival after radical mastectomy versus modified radical mastectomy was evident after 5 years (84% vs. 76%, respectively) and became more evident after 10 years (74% vs. 65%, respectively; Fig. 31.1 ). Although there was no significant difference in the overall survival rate between the procedures in patients with smaller cancers, the ultimate survival rate for patients with T 2 or T 3 tumors was significantly better after the radical mastectomy ( Fig. 31.2 ).
| Disease Stage | MODIFIED RADICAL MASTECTOMY | HALSTED RADICAL MASTECTOMY | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | LOCAL RECURRENCE | No. of Patients | % | LOCAL RECURRENCE | |||||||
| 5-yr | 10-yr | 5-yr | 10-yr | |||||||||
| n | % | n | % | n | % | n | % | |||||
| I | 43 | 13.8 | 4 | 9.3 | NA | NA | 37 | 11.9 | 2 | 5.4 | NA | NA |
| II | 112 | 36 | 8 | 7.1 | NA | NA | 83 | 26.7 | 3 | 3.6 | NA | NA |
| III | 20 | 6.4 | 4 | 20 a | NA | NA | 16 | 5.1 | 1 | 6.3 a | NA | NA |
| Total | 175 | 56.2 | 16 | 9.1 b | 20 | 11.4 c | 136 | 43.7 | 6 | 4.4 b | 8 | 5.8 c |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree