Ultimately determining whether a new pulmonary nodule in a patient with history of cancer is a metastasis requires histopathologic confirmation. In specific circumstances where surgical removal will not be pursued, nonoperative biopsy is preferable. Techniques such as image-guided percutaneous biopsy, fluoroscopic-guided transbronchial biopsy, endobronchial ultrasound, or navigational bronchoscopy are all reasonable approaches and should be individualized to the location of the nodule, institutional expertise, and patient-specific concerns. Differentiating site of origin can be confounded by the histologic similarity between primary lung cancer and metastatic lesions, most commonly differentiating between primary squamous cell cancers from lung and metastatic head and neck tumors. Molecular analyses may be useful in these circumstances.8 In patients who cannot be diagnosed by nonoperative means, surgical exploration and biopsy, preferably by VATS or by thoracotomy, is reasonable.
Criteria for Surgical Resection
Analysis from numerous retrospective studies has led to the following generally accepted criteria for selection of patients for surgical pulmonary metastasectomy: 1) definitive control of the primary tumor has occurred (or is possible), 2) absence of (or ability to control) extrathoracic metastatic disease, 3) the lung metastases are amenable to complete resection, 4) the patient can tolerate the planned procedure, and 5) there is no better treatment alternative.9 Using these criteria, however, long-term survival after metastasectomy remains uncommon, reflecting the fact that in only a subset of patients does the resected disease represent the entirety of their metastatic burden. Ultimately, the greatest management difficulty is differentiating patients that might benefit from surgical extirpation from those whose disease has already, or will soon, spread beyond the means of surgical control. Studies have used retrospective analyses to help clinicians predict which patients will have good outcome after surgery. By far, the largest such analysis of outcomes after metastasectomy comes from the International Registry of Lung Metastasis (IRLM), which was published in 1997.10 That project retrospectively analyzed over 5,000 cases of surgical metastasectomy for curative intent for a variety of histologies from 18 institutions around the world. Actuarial overall survival was significantly higher among patients who underwent complete (R0) surgical resection, with 5-, 10-, and 15-year survival of 36%, 26%, and 22%, respectively, compared to 13% at 5 years and 7% at 10 years among patients with incomplete resections. While not as striking as the impact of complete resection, other factors that had a positive effect on long-term survival in the IRLM analysis were disease-free interval >36 months (5-year survival 45% versus 31% <36 months) and single metastasis (5-year survival 43% versus 34% for two or three and 27% for four or more lesions). Based on their analysis, the outcomes could be differentiated into four groups based on the following risk factors: (1) whether their disease was resectable, (2) single or multiple metastases, and (3) if the disease-free interval was >36 months. Patients with favorable risk in all three criteria could anticipate 50% survival at 5 years compared to <15% for those with unfavorable criteria for each risk factor (Fig. 124.2).
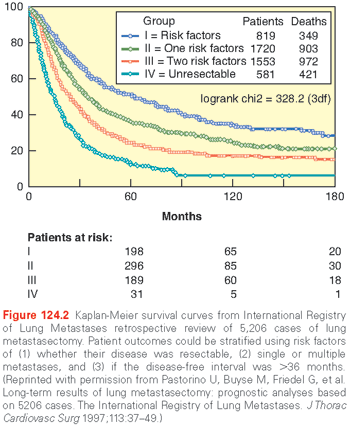
The need to perform a second metastasectomy in the IRLM analysis was associated with remarkably good 5-year survival (44%), which suggested that patients who recur and are still physiologically able should be offered re-resection. Other studies of outcomes after metastasectomy have corroborated the IRLM results and continue to demonstrate the prognostic importance of complete resection, prolonged disease-free interval, and fewer numbers of metastases. Other prognostic factors that have since been proposed include the presence of lymph node metastasis, response to chemotherapy, and size of lesions.
These risk factors alone, however, are not sufficient to solely determine whether a patient should undergo surgery. The decision to operate must be individualized because the risk/benefit relationship is so closely tied to a patient’s specific histology, tumor location, and physiologic state. Furthermore, the role of metastasectomy must be tailored to current nonsurgical treatment options, which continue to advance and may offer similar benefit to resection. Breast and renal cell carcinoma exemplify this phenomenon. Several large but older retrospective studies have demonstrated a benefit from pulmonary metastasectomy in these histologies, but patients with pulmonary metastasis from these tumors now have prolonged response to newer systemic therapies and only selected cases are reserved for surgery. As targeted and molecular therapies continue to improve the treatment of solid tumors, the selection criteria for pulmonary metastasectomy will change. The decision to proceed to surgical removal of pulmonary metastases must therefore be part of a multimodality discussion between patients, surgeons, medical oncologists, and radiation oncologists.
Large randomized trials are thought to provide the best evidence to direct efficacious treatment. A large trial of pulmonary metastasectomy for colorectal cancer is under way in Europe and will likely have a significant impact on clinical decision making.11 Unfortunately, randomized data matures slowly and can be nearly impossible for all clinical scenarios, so the decision to operate often relies upon results of large retrospective series and databases. However, ongoing advances in the treatment of metastatic disease should temper the use of this retrospective data to direct clinical practice.
Open Versus Minimally Invasive Surgery
The surgical approach to metastasectomy is controversial with retrospective studies supporting both open and VATS approaches. The tension between these approaches is based on decreased pain, shorter hospital stay, and increased tolerability in patients with poor pulmonary function associated with VATS12 relative to improved detection of small metastases afforded by manual palpation of the lung, which is not possible with a minimally invasive approach. As thoracoscopic techniques gain popularity for a wide range of thoracic procedures, surgeons have been keen to apply this technique to metastasectomy. Some historical studies suggested that the lack of manual palpation of the lung leads to small metastatic lesions being missed, and is therefore increasing the risk for incomplete resection. Convincing evidence in favor of an open approach and manual palpation of the lungs came from a series from the Memorial Sloan Kettering Cancer Center in 1996.13 In this prospective evaluation, 18 patients underwent a VATS resection immediately followed by thoracotomy. Ten patients (56%) had additional malignant lesions found at thoracotomy that were not identified on preoperative imaging or during thoracoscopy. The major findings of the trial were as follows: (1) CT scan of chest underestimated the number of lung metastases (and therefore would be unable to be found thoracoscopically) and (2) thoracotomy after VATS identified nodules missed during VATS. Although this study is criticized for not using “high-definition” imaging and modern thoracoscopic techniques, other studies have subsequently corroborated these results with a similar percentage of “missed” nodules.14–16 The rebuttal from VATS supporters is that patients who undergo VATS have similar long-term outcomes relative to thoracotomy.
Modern high-resolution CT scan affords precise diagnostic imaging of patients with pulmonary disease. However, numerous modern series continue to suggest that CT underestimates the number of pulmonary metastases identified during thoracotomy. In a recent study from the John Wayne Cancer Institute in California, additional lesions were identified in 26% of patients undergoing thoracotomy that were not identified by preoperative CT chest scan.17 In this series, “missed” nodules were more common if multiple nodules were identified preoperatively, and if the preoperative identified nodules were <1.5 cm. Similar results were reported by Cerfolio et al.16 in a prospective cohort of 152 patients, in which 51 patients (34%) had pulmonary nodules detected at surgery that were not imaged preoperatively and over half were malignant.
An additional concern with minimally invasive approaches is the increased difficulty with intraoperative localization of nodules identified by CT. In a study from Denmark, 37 patients underwent sequential thoracoscopy and thoracotomy for resection of pulmonary metastases. CT scan identified 55 lesions preoperatively, but only 51 were identified using VATS. An additional 29 lesions were found during subsequent thoracotomy, 7 of which (24%) were malignant.14
Despite evidence that nodules may be missed by VATS resection, multiple retrospective series have demonstrated that both short-18,19 and long-term survival are similar between patients undergoing the open and VATS approaches.20–24 A study that compared VATS to thoracotomy for treatment of sarcoma metastases demonstrated nearly identical 3-year survival without ipsilateral recurrence (44.4% versus 45%).19 Proponents of the minimally invasive approach argue that ultimately, tumor biology is the factor that most profoundly affects survival after metastasectomy and that patients should be offered the least invasive procedure.
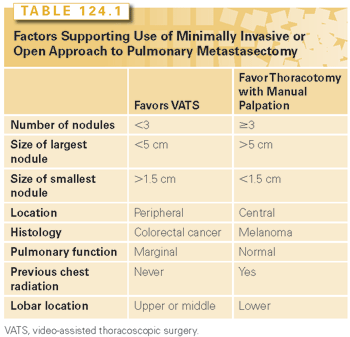
No large or randomized studies exist, or are likely to be performed, to determine which surgical technique is better. Currently, the majority of thoracic surgeons favor the use of thoracotomy for treatment of pulmonary metastases, but minimally invasive techniques are frequently being used with therapeutic intent. This is based on findings from a 2008 survey of the European Society of Thoracic Surgeons (ESTS). A total of 65% of respondents felt that “palpation is considered necessary,” relative to 29% who use thoracoscopy with therapeutic intent.25 There has been a dramatic increase in use of VATS since the 1997 IRLM series, in which only 2% of cases were performed thoracoscopically.10 An algorithm proposed from Cerfolio et al.16 to determine which surgical approach to use offers some insight as to the relative strengths and limitations of each technique offer (Table 124.1).
Approach to Open Metastasectomy
The controversy over surgical approach goes beyond the open versus minimally invasive debate. There is also controversy as to which open approach is ideal. In the setting of unilateral nodules, most advocate use of thoracotomy, but the decision is less clear in the setting of bilateral disease. Some advocate a single surgical procedure with a median sternotomy or clamshell for patients with bilateral metastases. Ideologically, it is appealing to limit the patient to one anesthetic and one recovery, but “one-stage” approaches have their drawbacks. Sternotomy affords poor exposure and restricts manual palpation of the posterior aspect of the lower lobes, especially on the left side. The clam shell incision offers improved access to both lung fields, but is associated with increased pain and postoperative recovery. In the 2008 ESTS practitioner survey, only 26.9% of respondents preferred a one-stage sternotomy approach to bilateral disease and only 7.6% preferred the clamshell approach.25 The most popular approach to bilateral pulmonary metastasectomy is sequential thoracotomy, with contralateral disease being approached by a subsequent thoracotomy after a 3- to 6-week interval.25,26
Role of Lymph Node Dissection
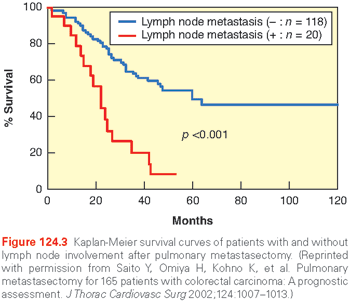
Another controversial aspect of pulmonary metastasectomy involves the decision to perform lymph node dissection or sampling at the time of surgery. The use of systematic lymph node evaluation during metastasectomy varies by institution; no consensus exists. The evidence for lymph node evaluation is supported by a study from Pfannschmidt and colleagues,27 where 245 patients with metastases from colorectal carcinoma, renal cell carcinoma, and sarcoma underwent routine mediastinal lymphadenectomy. Approximately one-third of patients had lymph node involvement, half were limited to pulmonary and hilar metastases, and the other half had mediastinal nodal disease. Patients with more than one pulmonary metastasis or metachronous disease were more likely to have thoracic lymph node metastases, as were patients with renal cell cancer (42%) and colorectal carcinoma (31%) relative to sarcoma (20.3%). Patients without lymph node involvement demonstrated improved median survival (63.9 months) relative to patients with pulmonary nodal involvement (32.7 months) or mediastinal nodal involvement (20.6 months). Subsequent studies support the premise that intrathoracic nodal disease in conjunction with pulmonary metastasis is a poor prognostic indicator. This message was particularly striking in a Japanese cohort of patients undergoing resection of colorectal metastases; patients without intrathoracic lymph node disease had a 5-year survival of 53.6% compared to 6.2% at 4 years among patients with lymph node involvement (Fig. 124.3).28
While the increased prognostic information received from lymphadenectomy appears to support its routine use, many surgeons believe that the primary role for lymphadenectomy is to determine the need for additional oncologic treatment; there are no widely accepted strategies for implementation. The lack of consensus is evident in the ESTS survey, where only 19% of respondents reported routinely performing complete lymphadenectomy, 55% perform lymph node sampling, and 32% do no nodal sampling.25
Extent of Resection
In general, the goal of metastasectomy is complete removal of all gross tumor. Oncologic resection is defined further by the “completeness” of resection: An R2 resection involves a partial resection with gross residual disease, an R1 resection is removal of gross disease but residual microscopic disease, and an R0 resection is a complete resection with no microscopic evidence of disease. In general, an R0 resection is essential for successful metastasectomy, because debulking of disease carries no clinical benefit. A second, but equally important, goal is maintenance of adequate pulmonary function and with that comes the consideration for extent of surgical metastasectomy. As approximately 50% of patients will present with recurrent disease after initial metastasectomy, an important objective of pulmonary metastasectomy is to remove as little functional lung tissue as possible in order to preserve function for the patient’s physiologic needs and for possible subsequent resection(s).10 Nonanatomic wedge resections are ideal for removal of peripheral nodules and are generally associated with improved postoperative lung function relative to a larger anatomic resection (segmentectomy, lobectomy, or pneumonectomy). Lobar and segmental anatomic resections can be safely performed with mortality of <1%, but are usually associated with removal of more functional lung parenchyma than a wedge resection. Although lobectomy has been associated with lower rates of local recurrence in the treatment of primary NSCLC,29 no such data exists for patients receiving resections for metastatic disease. On the contrary, because patients with pulmonary metastases frequently undergo multiple procedures (and additional other therapies), lung conservation is particularly essential.
Studies of lung function after surgical metastasectomy suggest that these resections are associated with a 10% to 15% decrease in pulmonary function on average. Pulmonary function was significantly reduced among patients receiving bilateral operations and postoperative chemotherapy.30 For the purposes of metastasectomy, multiple wedge resections are frequently performed and patients can experience a cumulative decrease in lung function that is similar to a lobectomy.31 The use of VATS/thoracoscopy has been associated with less significant decrease in pulmonary function and fewer complications in patients with marginal lung function.32,33
In patients with multiple lesions in a single lobe, central lesions, or involvement of airway or vascular structures, lobectomy may be the only therapeutic option, and therefore appropriate in patients with acceptable physiologic reserve. Pneumonectomy is rarely indicated for the treatment of metastatic disease, and many believe that the morbidity of a pneumonectomy is a relative contraindication to metastasectomy.25 There are, however, select reports of long-term survival and acceptable morbidity in small case series.34 Pneumonectomy should therefore be reserved for patients with ideal circumstances: long disease-free interval, single central lesion, and excellent performance status.
Image-guided ablative therapies are being used with great frequency in the lungs and are a popular alternative to surgical metastasectomy. Stereotactic body radiotherapy (SBRT) or stereotactic ablative body radiotherapy is highly precise and dose-intensive form of external beam radiation therapy that has been used to treat metastases in the thorax. Percutaneous technologies include radiofrequency ablation (RFA), microwave ablation, or cryoablation, which uses heat or cold to create cellular destruction with necrosis. Patterns of the use of ablative therapies vary significantly according to practitioner and institutional expertise, but have been applied most consistently in patients whose performance status prevents thoracic surgery, or in whom complete surgical resection is not possible. Studies in those populations have been promising, and accordingly the use of ablative techniques to treat metastatic pulmonary lesions has increased. While, the “gold standard” treatment of pulmonary metastases remains surgical, the growing success of ablative techniques has led to more widespread use of these techniques. Rather than compare the techniques to determine which is superior for all cases, prudent complementary use of all available technologies is appropriate. For example, a patient who has undergone multiple previous throacotomies for resection of metastases and has decreased lung function may benefit from an ablative technique to reduce toxicities associated with reoperative surgical resection. The specific strengths and weaknesses of each technique underscore the need for a multidisciplinary approach to patients with pulmonary metastases.
Radiofrequency Ablation
RFA is the most widely used and reported of the percutaneous image-guided technologies. It uses current transmitted through CT-guided, percutaneous probes to heat tissues to temperatures >60°C, resulting in coagulative necrosis. The pattern of tissue injury is related to the tissue’s specific electrical impedance and is modulated by the cooling effect of adjacent tissues such as airways and large blood vessels. In the lung, air-filled pulmonary parenchyma can act as a thermal insulator that protects nearby tissue. RFA is most efficacious for tumors <3.5 cm in diameter, with larger tumors requiring multiple applications. RFA is also less efficacious in areas adjacent to large blood vessels and is not recommended adjacent to central airways; therefore, application is typically limited to the middle and outer third of the lungs. Pneumothorax is the most frequent complication of percutaneous pulmonary RFA and is reported in up to 30% to 50%, less than a third of which require a chest tube for management.35–37 Although radiographic evidence of fibrosis and/or cavitation is common after RFA, serious, symptomatic complications (including bronchopleural fistulae formation, hemoptysis, hemorrhage, infection, and pleurisy) are rare. Cutaneous metastases (tumor seeding) at the electrode insertion site have been documented, but are also uncommon.
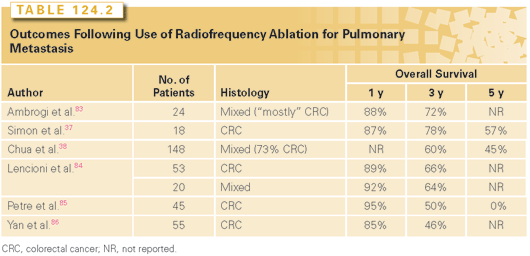
Percutaneous ablative treatments of pulmonary metastases are gaining in popularity because of evidence of therapeutic similarity to surgical resection. In 2010, a prospective trial of percutaneous RFA of lung metastases demonstrated impressive long-term results among a group of patients with mixed tumor histology.38 Of 148 patients treated, 26% had complete radiographic response, 20% had partial response, 39% had stable disease, and 16% showed disease progression. The median overall survival was 51 months, and in multivariate analysis, disease-free interval and response to treatment were independent predictors of overall survival. Other small studies have also shown good results of RFA ablation in pulmonary metastases of esophageal, colorectal, breast, and renal cancer39–41 (Table 124.2).
Stereotactic Body Radiation Therapy
SBRT is another ablative technique being used to treat metastatic lung lesions. Unlike conventional fractionated radiation therapy, SBRT uses highly focused and intensive radiation to deliver ablative doses of radiation to specific areas with less radiation injury to adjacent tissues. The techniques originated for use in the brain. Significant technologic advances were necessary to adaptation to the lung, especially due to the need to compensate for the normal physiologic movement associated with breathing in order to prevent radiation injury to normal tissue without compromising tumor control. Theoretically, the high-dose and tightly focused nature of SBRT provides an ablative response in the target area, which is of particular concern for treatment of pulmonary metastases. In a trial from Kyoto University in Japan, a higher dose of radiation was necessary to treat metastatic pulmonary lesions due to higher rates of local failure (relative to primary NSCLC).42
Much of the experience with the use of SBRT for lung tumors was derived from the treatment of medically inoperable NSCLC. Studies in this population have demonstrated successful treatment of early stage NSCLC among patients unfit for surgery with few complications.43
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








