INTRODUCTION
Breast cancer is a significant cause of morbidity and mortality among women. In the United States, it is the most common malignancy among women. It is estimated that approximately 231,840 new cases of invasive breast cancer will have occurred in the United States in 2015 (1,2). Although lung cancer has surpassed breast cancer as the leading cause of cancer death among women, nearly 39,620 deaths were estimated to occur from breast cancer alone in 2013 (1).
Since the 1970s, advances in combined-modality therapies have substantially improved the outcomes of patients with breast cancer. Still, approximately 10% to 60% of patients with initial localized breast cancer will suffer a systemic relapse. Metastatic disease is diagnosed at the time of presentation in 3% to 12% of patients depending on the series (1,3). Bone is the most common site of first distant relapse; other common sites of metastases include lymph nodes, lung, liver, and, less frequently, brain. The 5-year survival rate for localized breast cancer is 99%; for metastatic disease, this rate is only 17% to 28% (1,2,3). As is true with cancers in general, the clinical course for patients with metastatic breast cancer (MBC) varies, but as a group, patients with MBC have a median survival of 2 years (4). Patients with bone-only disease tend to live longer than patients with visceral involvement. Untreated patients with MBC have a median overall survival time of 9 to 12 months. With systemic therapy, the mean survival time is 21 months for patients with visceral disease and as long as 60 months for patients with bone-only disease. Survival and response to therapy are affected by several factors, including estrogen receptor (ER), progesterone receptor (PR), and HER2/neu receptor status; performance status; site of disease; number of disease sites; and duration of disease-free interval (DFI).
The therapeutic objectives and approach to patients with advanced breast cancer is distinct from that of patients with early-stage disease. Treatment for MBC is triaged to endocrine therapy, biological therapy, or chemotherapy, depending on the hormonal and HER2/neu receptor status of the tumor, the severity of symptoms, and the site and extent of disease. Generally, breast cancer can be classified as three molecularly and clinically different syndromes: hormone-receptor positive/HER2/neu negative, Her-2/neu positive (hormone-receptor negative or positive) and triple negative breast cancer. They have different clinical courses, prognoses, metastatic patterns, and responsiveness to available therapies. Systemic treatment prolongs survival, provides palliation of symptoms, and enhances quality of life but, in general, is not considered curative. Therefore, a discussion regarding goals of care is imperative between the patient and treating oncologist. Cure in MBC is rare; less than 2% of patients with MBC may remain disease free after anthracycline-containing therapy. The overall survival of patients has improved in the last few decades due to more effective therapies. This chapter reviews standard care for patients with MBC and discusses some unique approaches used at the University of Texas MD Anderson Cancer Center (MDACC).
DIAGNOSTIC WORKUP
Once metastatic disease is suspected, careful evaluation of the primary disease history, current symptoms, and existing comorbid diseases is essential. The history of the primary disease should include a review of the initial presentation, stage of disease, histology, hormone receptor and HER2/neu status, nuclear grade, and treatment modalities employed. Knowledge of the initial tumor type may yield clues about the sites of disease as well as its biology. For instance, infiltrating ductal carcinoma most commonly involve the lungs, pleura, liver, and brain. Infiltrating lobular carcinoma may metastasize to unusual sites such as the bone marrow, meninges, peritoneum, and retroperitoneal structures, such as the ureters (5).
If possible, a biopsy of the metastatic or recurrence site is required to confirm the histologic type, as well as ER, PR, and HER2/neu status, because there is some evidence of significant discordance in the receptor status between the time of diagnosis of the primary tumor and the time of diagnosis of metastasis (6,7). Changes in ER status occur in 14.5% to 40% of cases, whereas changes in HER2 expression/amplification range from 0% to 37% (8). Pathologic confirmation is also essential in patients suspected of having metastases if the clinical presentation or course is not typical. Such relapse scenarios include single-lesion metastasis, unusual metastatic sites, and long DFI. Solitary lesions should always be biopsied because of the possibility that the lesion may not be not malignant or may be caused by a second different primary malignancy. This occurs in up to 10% of patients with solitary lesions and would have a direct impact on the treatment selection.
In addition to a comprehensive physical examination, basic laboratory evaluation should include a complete blood count with differential, liver and renal function tests, and serum calcium determination. In addition, CA 15-3 and CA 27-29 are potentially helpful in monitoring response to therapy. The CA 15-3 test is a combination of two monoclonal antibodies bearing two reactive determinants directed against DF3 and MAM-6 antigens expressed on mammary epithelial cells (9,10). CA 15-3 and CA 27-29, which are more sensitive than CEA, are elevated in approximately 70% of patients with MBC, but they lack sensitivity and specificity for breast cancer progression. Therefore, their prognostic significance remains indeterminate (9). Carcinoembryonic antigen (CEA) is elevated in 40% to 50% of patients with metastatic disease (10,11). Current recommendations from the American Society of Clinical Oncology are that tumor markers can be used in conjunction with diagnostic imaging, history, and physical examination to monitor patients with metastatic disease during active therapy; however, data are insufficient to recommend their use alone to monitor response to treatment (12). Caution should be used when interpreting tumor marker levels during the first 4 to 12 weeks of a new therapy, because spurious early rises may occur (9,12). Furthermore, the absolute value of a tumor marker measurement does not represent the extent of disease, and no therapeutic decision should be based on a single tumor marker measurement. However, trends over time are helpful to monitor clinical course. In monitoring treatment, the physician should be aware of the coefficient of variation of the assays used for CA 15-3 and CA 27-29: changes <10% fall within the accepted intra-assay variability and do not represent real change. The measurement of circulating tumor cells (CTCs) has been studied in patients with MBC. Several studies showed that high levels of CTCs (>5 cells/75 mL of blood) are correlated with poor survival in MBC and with decreased response to treatment (13,14). However, based on current recommendations, the measurement of CTCs should not be used to make the diagnosis of breast cancer or to influence any treatment decisions. Similarly, the use of the US Food and Drug Administration (FDA)-cleared test for CTCs (CellSearch Assay) in patients with MBC cannot be recommended for routine use until further validation confirms its clinical value. An intergroup trial is under way to determine the implication of changing treatment based on the CTC level (12).
In most cases, we perform a baseline evaluation that also includes a computed tomography (CT) of the chest and abdomen (ultrasonography is less accurate for the chest and abdomen and would be indicated only in patients who cannot have CT or magnetic resonance imaging [MRI]), but occasionally, MRI of the abdomen may be indicated (15). The presence of bone metastases should be evaluated, and in general, we recommend a bone scan to determine the presence and extent of bone metastasis. Only 30% to 60% of patients with true-positive bone scans have increased levels of alkaline phosphatase (16,17). Conversely, only 20% of patients with elevated levels of alkaline phosphatase are disease free (17). Impending fractures in the weight-bearing bones, such as the femur, and an unstable spine must be ruled out. The preferred test for spinal evaluation for metastases is MRI. Monitoring of bone metastases is best done with serial MRI or CT scans. Radiographic evaluations of the brain, leptomeninges, and spinal cord have low yield unless the patient is symptomatic or has abnormal neurologic findings (15). Current guidelines discourage the use of positron emission tomography (PET)/CT except in situations where other staging studies are equivocal or suspicious (15).
TREATMENT
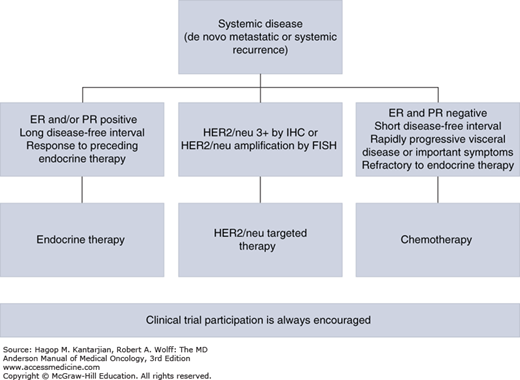
The decision whether to use chemotherapy or biological or hormonal therapy for the initial treatment of MBC should be guided by several factors including hormone receptor and HER2/neu status and the presence of symptomatic visceral disease or life-threatening disease (Fig. 28-1). Patients with moderately symptomatic visceral disease or life-threatening disease should be considered for treatment with systemic chemotherapy regardless of hormone receptor status because systemic therapy offers faster palliation of symptoms. Among women who do not have life-threatening or symptomatic visceral disease, those whose tumors are negative for ER and PR should be also considered for systemic chemotherapy. Those whose tumors are positive for ER or PR should be treated with hormonal therapy. Since the discovery of the importance of HER2 gene amplification in breast cancer and the development of anti-HER2 therapies, patients with tumors positive for HER2/neu overexpression or gene amplification should be treated with anti-HER2 therapy in combination with chemotherapy because this provides a significant survival advantage.
Multiple agents are active against hormone-responsive tumors. Endocrine therapy tends to be associated with fewer side effects and helps maintain quality of life for many patients. If the tumor does not respond to endocrine therapy or becomes unresponsive to hormonal therapy, systemic chemotherapy should be initiated. For patients with hormone receptor–positive breast cancer, endocrine therapy is at least as effective as chemotherapy.
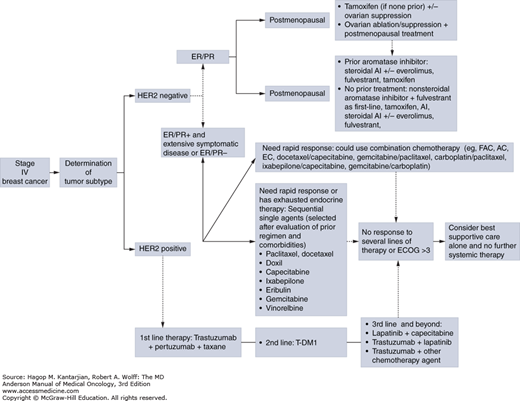
Currently, the primary goals of chemotherapy for MBC should be palliation of symptoms attributable to cancer and prolongation of life. It is the physician’s duty to balance the benefits of therapy with possible toxic effects and to fully discuss therapeutic options with patients. The patient’s multiple previous therapies, decline in performance status, comorbid conditions, and organ function should be taken into consideration in the treatment decision. The MDACC treatment algorithm of patients with MBC is illustrated in Fig. 28-2.
FIGURE 28-2
Algorithm for the treatment of metastatic breast cancer. AC, doxorubicin and cyclophosphamide; AI, aromatase inhibitor; EC, epirubicin and cyclophosphamide; ECOG, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide; PR, progesterone receptor.
Patients presenting with solitary metastases or oligometastases represent a unique subset of patients who are potentially curable and should be approached with combined-modality therapy, including surgical resection of the metastases (or radiotherapy at curative doses), combination chemotherapy before or after local treatment, anti-HER2 agents for HER2-positive MBC, and endocrine therapy for hormone receptor–positive MBC. Such patients have a 20% to 25% probability of long-term cure after such treatment.
ENDOCRINE THERAPY
Endocrine therapy has dramatically improved outcomes in patients with hormone receptor–positive breast cancer. It can result in significant palliation of symptoms and improvement in quality of life in patients with hormone receptor–positive MBC. Manipulation of the endocrine system as a treatment for MBC was introduced in 1896, when Beatson demonstrated objective regression of breast cancer after oophorectomy. Today, a number of endocrine therapies are used in patients with hormone-sensitive MBC; most therapies are directed at reducing the synthesis of estrogen or blocking ERs in hormone-dependent tumors.
Tumors that are positive for ER and/or PR expression do not respond to endocrine therapy, and these patients should be offered endocrine therapy (18,19). Patients who have tumors that are both ER and PR positive have a 50% to 70% probability of receiving clinical benefit from endocrine therapy. Patients with either ER-positive or PR-positive tumors have a 30% probability of receiving clinical benefit from endocrine therapy. Of patients with a prior history of hormonal response in the metastatic setting, 30% to 50% will have a response or clinical benefit from another hormonal regimen. Patients with low-volume disease and better performance status generally have higher response rates. The duration of first response is usually 9 to 12 months, similar to that with chemotherapy. The selection of endocrine therapy depends on the menopausal status of the patient: tamoxifen and/or ovarian suppression/ablation for premenopausal women and aromatase inhibitors or selective ER downregulators (SERDs) for postmenopausal women. The side effect profile also aids in the determination of which hormonal therapy to use, because the efficacy of all agents is nearly equal (20). A substantial minority of patients with hormone receptor–positive MBC will benefit from sequential single-agent endocrine therapy. Some patients might benefit from three or four lines of endocrine therapy, and a few will continue to respond to multiple lines and for many years. Additionally, for patients whose tumors are unusually sensitive to hormonal manipulation, repeated treatment with a previously effective agent may again be effective if a long interval has elapsed since it was discontinued.
The clinical criteria used to determine eligibility for endocrine therapy are longer DFI, no involvement of vital organs, no major dysfunction of the organs involved by the disease, minimal or moderate visceral involvement, and metastases confined to the soft tissue or bone.
Several types of endocrine therapy are available in managing MBC (Table 28-1). They include ovarian ablation (oophorectomy, ovarian radiation), functional suppression (luteinizing hormone–releasing hormone [LHRH] agonists), selective ER modulators (SERMs), ER downregulators, progestins, androgens, estrogens, and nonsteroidal and steroidal selective aromatase inhibitors (AIs). Medical ovarian castration obviates surgery as a first choice. There is no current indication for adrenalectomy or hypophysectomy. There are no conclusive data to support combined hormonal therapies in postmenopausal breast cancer, aside from the use of fulvestrant with AIs in the first-line setting based on a recent trial noting clinical benefit. In most trials of combinations, some minimal increases in response rates were seen, but without a significant improvement in survival. Additional toxicities are usually observed (21,22). As the understanding of the mechanisms of resistance to endocrine-based therapies has improved, the addition of therapies targeting these pathways has resulted in improved clinical outcomes. SERMs (tamoxifen and toremifene) are effective in pre- and postmenopausal patients with breast cancer. The LHRH agonists are effective in premenopausal women only (23), and the combination of tamoxifen and ovarian ablation is superior to ovarian ablation alone (20,23). Estrogen biosynthesis is reduced by inhibiting the aromatase enzyme, which catalyzes the final step in estrogen production in humans. This does not completely block ovarian estrogen production in premenopausal women, and there is concern that the use of an AI as a single agent in this patient population may cause a reflex increase in gonadotropin levels and result in ovarian hyperstimulation. Thus, AIs must be used only in postmenopausal women; they are not effective in premenopausal women (15,24,25,26). The AIs can broadly be categorized as selective and nonselective. The nonselective AIs block not only aromatase but also other enzymes in the cytochrome P450 family. Thus, they alter other steroid hormone levels and are associated with more side effects. Therefore, they are not frequently used.
| Type of Therapy | Examples |
|---|---|
| Ovarian ablation | Surgery, radiation therapy, pharmacologic interventions |
| LHRH agonists | Goserelin acetate |
| Selective estrogen receptor modulators | Tamoxifen |
| Toremifene | |
| Raloxifene | |
| Arzoxifene hydrochloride | |
| Selective estrogen receptor downregulators | Fulvestrant |
| Selective aromatase inhibitors | Anastrozole |
| Letrozole | |
| Exemestane | |
| Fadrozole | |
| Formestane | |
| Nonselective aromatase inhibitors | Testolactone |
| Aminoglutethimide | |
| Estrogens | Diethylstilbestrol Estradiol Megestrol acetate |
| Progestins | Medroxyprogesterone acetate |
| Androgens | Fluoxymesterone Danazol |
Endocrine therapy is better tolerated than cytotoxic chemotherapy. However, several unique complications of endocrine therapy should be anticipated. One such complication, flare, is defined clinically by an abrupt, diffuse onset of musculoskeletal pain, increased size of skin lesions, or erythema surrounding the skin lesion within the first month of endocrine therapy. Flare may also be characterized as the worsening of bone lesions on bone scan, the reason for which bone scans may make assessing response to endocrine therapy difficult. The most serious manifestation of flare is hypercalcemia, which can be seen with several hormonal therapies except AIs and surgical castration. Hypercalcemia usually occurs in patients with bone metastases and manifests itself within the first 2 weeks after treatment. The underlying mechanism is the predominating early agonist effect of hormonal agents. Low doses of prednisone (10-30 mg/d) may abrogate the initial flare of bone pain.
Side effects of endocrine therapy, such as hot flashes and mood disturbances, are related to estrogen deprivation and are common with tamoxifen and AIs, reflecting the mechanism of action of these drugs. Tamoxifen has estrogenic effects that are beneficial in some tissues; it lowers serum cholesterol levels and protects against bone loss and cardiovascular disease but is also associated with potentially life-threatening side effects, such as endometrial cancer and thromboembolic events. AIs are associated with musculoskeletal side effects, such as arthralgias, myalgias, and bone loss. Weight gain is clearly associated with estrogens, androgens, corticosteroids, and progestins. Randomized trials of tamoxifen do not support an association with weight gain because patients on placebo experienced the same degree of weight gain as those on tamoxifen. This side effect is most common with progestins, which can cause both a true increase in weight from their anabolic effect and fluid retention secondary to their glucocorticoid effect. Progestins are the drugs most likely to cause thromboembolism; tamoxifen is the next most likely drug to cause this complication.
The preferred agent for endocrine therapy depends on the menopausal status of the patient. In premenopausal women, tamoxifen is recommended as the initial therapy, although ovarian suppression with an LHRH agonist alone or with tamoxifen can also be used. In patients who are within 1 year of antiestrogen exposure, the preferred second-line therapy is surgical oophorectomy or an LHRH agonist with endocrine therapy (15). In postmenopausal women who are antiestrogen naïve or who are more than 1 year from previous antiestrogen therapy, an AI is recommended as initial first-line therapy, but tamoxifen or the combination of fulvestrant with an anastrozole can be considered. Aromatase inhibitors appear to have superior outcome compared with tamoxifen, but differences are modest (27,28). The use of anastrozole or letrozole as initial therapy in postmenopausal women with ER-positive tumors results in increased response rates and longer disease control (29). Patients who respond to initial therapy have a higher probability of response to second- and third-line endocrine therapies. There is a partial lack of cross-resistance between steroidal and nonsteroidal AIs. These agents may provide palliation of disease if used sequentially in patients with hormone receptor–positive tumors. There is evidence that a steroidal AI (exemestane) is effective in patients who have disease progression after a nonsteroidal AI. Most patients with hormone-responsive breast cancer benefit from the sequential use of endocrine therapies at the time of disease progression. Patients who respond to endocrine therapy with tumor shrinkage or long-term disease stabilization should receive additional endocrine therapy at the time of disease progression (15).
Fulvestrant is an ER antagonist that downregulates ER and has no agonist effects. It was compared with tamoxifen in a large randomized trial involving 587 postmenopausal women with advanced breast cancer or MBC who had not previously been treated with endocrine therapy. Patients were given either fulvestrant 250 mg intramurally monthly or tamoxifen 20 mg orally (PO) daily. At a median follow-up of 14.5 months, there was no significant difference between fulvestrant and tamoxifen in time to progression (TTP; median, 6.8 vs 8.3 months, respectively) (30). Fulvestrant also appears to be as effective as anastrozole in patients whose disease progressed on tamoxifen (31,32). The clinical benefit rates of exemestane and fulvestrant observed in a phase III trial of postmenopausal women with hormone receptor–positive breast cancer who experienced disease progression on prior nonsteroidal AIs were comparable (32.2% vs 31.5%) (33). The CONFIRM trial (n = 736) compared fulvestrant at different doses (250 and 500 mg) in postmenopausal women with advanced disease recurring or progressing after prior endocrine therapy. Response rates were similar in both groups (13.8% vs 14.6%), but TTP and overall survival (OS) were significantly longer for the patients who received 500 mg (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.68-0.94) (33,34).
Based on preclinical studies suggesting that the combination of fulvestrant and an AI was superior compared with either agent alone, three prospective clinical trials evaluated this approach in postmenopausal women with hormone receptor–positive MBC. In the Southwest Oncology Group SO226 trial, postmenopausal women (n = 707) with previously untreated metastatic disease were randomized to anastrozole versus anastrozole and fulvestrant, with crossover to fulvestrant encouraged (35). Median progression-free survival (PFS) and OS were significantly improved with the combination (median OS, 41.3 months with anastrozole vs 47.7 months with the combination; HR, 0.81; 95% CI, 0.65-1), despite the fact that 41% of patients receiving anastrozole crossed over to fulvestrant. Subgroup analysis revealed that those deriving the greatest benefit were patients who received no prior tamoxifen. The other two studies showed equivalent outcomes in patients receiving the combination. In a randomized phase III trial, Johnston et al reported that patients with hormone receptor–positive MBC who relapsed or progressed while receiving a nonsteroidal AIs had similar PFS when treated with fulvestrant plus anastrozole, fulvestrant plus anastrozole-matched placebo, or exemestane (36). Another phase III trial showed no advantages in clinical efficacy for the combination of anastrozole and fulvestrant compared to treatment with anastrozole alone as first-line treatment in postmenopausal women with hormone receptor–positive MBC (37). One possible reason for the difference in outcomes of the three studies is a potential imbalance in the prognostic subgroups in the SWOG study (38). Given the positive phase III findings and especially the significant prolongation in OS, the combination can still be considered, especially in patients who have never received tamoxifen.
De novo and acquired resistance is a known phenomenon. Options in this setting include changing the class of AI or using a drug with a different mechanism of action, such as fulvestrant or tamoxifen. Another approach is the addition of a mammalian target of rapamycin (mTOR) inhibitor, such as everolimus. Activation of the PI3K/Akt/mTOR pathway in breast cancer has been implicated as a mechanism of resistance to endocrine therapy. Preclinical research has evaluated the molecular basis of resistance to endocrine therapy, combatting resistance by incorporating inhibitors of this pathway (39). A phase II study demonstrated similar efficacy and safety with the use of everolimus in combination with tamoxifen in the treatment of of 111 patients with hormone receptor–positive, HER2-negative MBC with prior exposure to AI treatment either in the adjuvant and/or metastatic setting (40). The study fulfilled its primary end point, with a clinical benefit rate at 6 months of 61.1% with everolimus plus tamoxifen versus 42% with tamoxifen alone (P = .045). There was a delay in time to disease progression with the combination; the TTP was 8.6 months in patients treated with everolimus plus tamoxifen versus 4.5 months in those treated with tamoxifen alone, resulting in a significant reduction in the risk of disease progression (HR, 0.54; 95% CI, 0.35-0.81; P = .002).
The BOLERO-2 study, a randomized, phase III, double-blind, placebo-controlled, multicenter trial (41), randomized 724 patients with hormone receptor–positive advanced breast cancer who had recurrence or progression after receiving previous nonsteroidal AIs to receive either exemestane or exemestane plus everolimus. The combination arm resulted in an improvement in the primary end point of PFS (10.6 vs 4.1 months; P < .001). Adverse effects of everolimus reported to affect 30% of more of patients included stomatitis, infections, rash, fatigue, diarrhea, and reduced appetite. The most common grade 3 to 4 adverse reactions affecting 2% or more of patients included infections, hyperglycemia, fatigue, stomatitis, diarrhea, dyspnea, and pneumonitis. In 2012, everolimus in combination with exemestane was approved by the FDA for the treatment of postmenopausal women with recurrent or progressive hormone receptor–positive, HER2/neu-negative disease after failure of therapy with either letrozole or anastrozole.
Enticing new options in the treatment of hormone receptor–positive, HER2-negative MBC are cyclin-dependent kinase (CDK) 4/6 inhibitors (42,43). Together with cyclin D1, CDK4 and CDK5 are kinases that facilitate the transition of dividing cells from the G1 phase of the cell cycle to the S phase. Preclinical studies have demonstrated that breast cancer cells rely on both CDK4 and CDK6 for division and cell growth. Inhibition of these pathways leads to cell cycle arrest at the G1/S phase checkpoint (43). Palbociclib, LEE011 (ribociclib), and LY2835219 (abemaciclib) are three selective CDK inhibitors presently under evaluation for the treatment of hormone receptor–positive MBC. In a phase I trial of LY2835219, 132 patients with five different tumor types, including MBC, received 150- to 200-mg doses of oral drug every 12 hours (44). The overall disease control rate for the 36 patients with hormone receptor–positive breast cancer was 81%, with a median PFS of 9.1 months. Common adverse events included diarrhea, nausea, vomiting, fatigue, and neutropenia. In a phase II trial, the oral CDK4/6 inhibitor, palbociclib, resulted in a near doubling of the primary end point of PFS as compared to control in the first-line treatment of 165 postmenopausal women with hormone receptor–positive MBC (45). Those receiving the combination of palbociclib in addition to letrozole had an objective response rate of 43% compared to 33%, with a PFS of 20.2 months versus 10.2 months (HR, 0.488; P = .0004). Common adverse events included leukopenia, neutropenia, and fatigue. The CDK inhibitors are being studied in phase III trials. The PALOMA-2 trial is testing the combination of palbociclib with letrozole in MBC in the frontline setting, and PALOMA-3 (NCT01740427) is evaluating the combination with fulvestrant in patients who have failed previous endocrine therapy. The MONALEESA-2 study is evaluating the efficacy and safety of the selective CDK inhibitor LEE011 in combination with letrozole in postmenopausal women with hormone receptor–positive MBC who have received no prior treatment for advanced disease (NCT01958021).
After third-line endocrine therapy, little high-level evidence exists to help select the optimal sequence of endocrine therapy. There are other hormonal agents available; progestins, for example, are synthetic derivatives of progesterone that have a progesterone agonist effect. Progestins such as megestrol acetate and medroxyprogesterone acetate are effective in treating MBC. These drugs are thought to have antiestrogenic properties and may result in interruption of the pituitary-ovarian axis. Androgens (eg, fluoxymesterone, danazol) have been evaluated and used in patients with MBC treated with multiple endocrine agents who still have hormone-dependent disease. Prior to the identification of hormone receptors or the development of SERMs, high-dose estrogens were commonly used to treat MBC. Their efficacy is similar to that of tamoxifen, although high-dose estrogens (eg, diethylstilbestrol, ethinyl estradiol) are associated with more severe side effects. Recent data suggest that the use of estrogens can be beneficial for patients with AI-resistant breast cancer (46). In a phase II study, 66 patients with AI-resistant MBC were randomized to receive 6 versus 30 mg of estradiol. The clinical benefit rates were 25% with 30 mg and 29% with 6 mg. The authors concluded that 6 mg of estradiol was as effective as 30 mg with greater safety and that this regimen could be a palliative therapeutic strategy in MBC progressing after other endocrine therapies (46). The treatment algorithm for treating patients with MBC at MDACC is illustrated Fig. 28-2.
CHEMOTHERAPY
No predictive test for response to chemotherapy has been sufficiently validated to use in a standard clinical setting. For patients with MBC not previously treated with chemotherapy, the response rates are 30% to 75%. Predictors of response to chemotherapy include DFI, sites of disease, organ function, and performance status, among others. Different biomarkers have been studied; some correlate with treatment response, but none is sufficiently accurate to help make a decision to treat or withhold therapy (47).
In deciding which cytotoxic regimen to use in the setting of negative ER/PR and HER2 status or in patients who have progressive disease after endocrine therapy, consideration should be given to the previous therapies, organ function, and comorbid conditions. Typically, chemotherapy within the conventional range of doses is associated with higher response rates than “low-dose chemotherapy.” As in the setting of adjuvant therapy, high-dose chemotherapy with peripheral blood/bone marrow stem cell transplantation has not been found to be of clinical benefit in randomized trials (48).
The choice between sequential single agents and combination chemotherapy is controversial. The principle of nonoverlapping mechanisms of resistance and toxicities has been the basis of combination chemotherapy. Multiple randomized trials involving single-agent versus multiple-agent regimens in MBC have generally demonstrated that combination chemotherapy has improved response rates and TTP, but OS is not improved. Fossati et al (49), in a systematic review that included 31,510 patients, estimated that the proportional reduction in overall mortality for combinations versus single-agent regimens is only 18%, translating to an absolute benefit in survival of 9% at 1 year, 5% at 2 years, and only 3% after 5 years. More toxicity was associated with combination therapy. In two randomized trials of combination versus single-agent therapy in MBC, formal quality-of-life analyses favored the single-agent arms, even though response rates were slightly lower (50,51).
A Cochrane review (52) including 28 trials and 5,707 patients with MBC randomly assigned to receive single-agent or combination chemotherapy found that combination therapy was associated with a higher response rate (odds ratio, 1.28; 95% CI, 1.15-1.42), longer TTP (HR, 0.78; 95% CI, 0.73-0.83), and longer OS (HR, 0.88; 95% CI, 0.83-0.94) than single-agent therapy. Most trials included in the Cochrane review did not specifically investigate the combination versus the sequential use of the single agents, and few studies reported the rate of “crossover” to an additional therapy following progression in the monotherapy arm. Therefore, the studies included evaluated the value of the use of two agents versus a single agent and do not address whether a simultaneous combination or a sequential monotherapy strategy should be pursued.
In the absence of strong evidence to guide the decision, and in agreement with different oncologic societies (53), we believe that use of single-agent therapy is preferable in the absence of rapid clinical progression, life-threatening visceral metastases, or the need for rapid symptom or disease control. Ultimately the choice of the use of sequential versus combination chemotherapy depends on a careful evaluation of risks and benefits for individual patients. The other major indication for combination therapy, as in the adjuvant setting, is the treatment of oligometastases.
The optimal duration of chemotherapy for MBC is controversial. Several studies have compared continuous (maintenance) chemotherapy with intermittent therapy. Several studies found that continuous therapy was associated with a longer time to relapse (54,55,56,57,58) but with worse side effects (57). None of the individual studies comparing continuous and intermittent therapy showed prolongation of life with continuous therapy. However, a recent meta-analysis of these data showed a statistically significant improvement in survival for patients receiving chemotherapy for a longer versus a shorter time (58). Some regimens, such as anthracycline-containing treatments, have inherent dose-limiting toxic effects that prohibit prolonged use. Other agents, such as trastuzumab, capecitabine, and, possibly taxanes given weekly, lend themselves to prolonged continued therapy. In an unplanned interim analysis, the recently presented Italian MANTA trial found no PFS or OS benefits for maintenance treatment with paclitaxel (175 mg/m2 every 3 weeks for eight cycles) after first-line chemotherapy for MBC with an anthracycline/taxane-containing regimen (six to eight cycles) (59). Many trials are designed to treat patients until they have progression of disease or for two to three cycles after maximum benefits. Currently, the optimal treatment duration is unknown. The practice at MDACC is to treat patients with MBC with continuous chemotherapy unless unacceptable toxicity arises, at least until a third-line or fourth-line regimen comes into play and/or Eastern Cooperative Oncology Group (ECOG) performance status is ≥3 in patients with MBC.
The introduction of anthracyclines (doxorubicin and epirubicin) in the 1970s represented a significant advance in the treatment of advanced breast cancer. In patients with MBC, response rates to single-agent doxorubicin (25-75 mg/m2 every 3 weeks) ranged from 25% to 60% and were heavily influenced by patient characteristics such as prior chemotherapy exposure, performance status, and extent and sites of disease (60,61,62,63,64). At MDACC, doxorubicin-containing regimens have historically been the initial treatment of choice for MBC treated previously with non–anthracycline-containing chemotherapy. Patients who received anthracyclines and had a prolonged DFI before the development of metastatic disease occasionally benefit from repeat administration of doxorubicin. However, given the increasing number of active agents available to treat MBC, repeat management with anthracyclines should be reserved for patients in whom other treatments have failed, given the potential risk of heart failure.
Epirubicin is a doxorubicin analog that has been shown to have similar efficacy and somewhat less toxicity than doxorubicin at equimolar doses. Although not designed to perform a head-to-head comparison, results from a randomized trial suggested that epirubicin might be as efficacious as doxorubicin. A formal comparison of two different anthracyclines in combination (5-fluorouracil, doxorubicin, and cyclophosphamide [FAC] vs 5-fluorouracil, epirubicin, and cyclophosphamide [FEC]) at equimolar doses found both regimens to be equally effective in terms of response rate, TTP, and survival. The FEC regimen was associated with less gastrointestinal, hematologic, and cardiac toxicity (65).
Efforts to improve the safety profile of doxorubicin while preserving efficacy have resulted in liposomal formulations of doxorubicin. Response rates with these products appear comparable to those seen in other multicenter trials using conventional single-agent doxorubicin. In a phase III clinical trial, O’Brien et al compared the efficacy and safety of pegylated liposomal doxorubicin with those of conventional doxorubicin as first-line therapy in patients with MBC (66). A total of 509 women received a 1-hour infusion of pegylated liposomal doxorubicin (50 mg/m2 once every 4 weeks) or conventional doxorubicin (60 mg/m2 once every 3 weeks). The median PFS and OS were similar in both treatment groups (6.9 vs 7.8 months and 20.1 vs 22.0 months, respectively). The rates of alopecia, myelosuppression, nausea, and vomiting were lower with pegylated liposomal doxorubicin than with conventional doxorubicin. Perhaps most notably, pegylated liposomal doxorubicin was associated with a significantly lower incidence of cardiotoxicity, even at higher cumulative doses (P < .001) (66). The most important dose-limiting toxicity of pegylated liposomal doxorubicin is palmar-plantar erythrodysesthesia, which is both dose and duration related. The polyethylene glycol coating results in preferential concentration of the drug in the skin; this explains why small amounts of the drug can leak from capillaries in the palms and soles, resulting in redness, tenderness, and peeling of the skin that can be uncomfortable and even painful. As with all liposomal drug delivery systems, there is a low incidence of hypersensitivity reactions.
Taxanes (paclitaxel, docetaxel, and the nanoparticle albumin-bound [nab]-paclitaxel) are among of the most active classes of cytotoxic drugs available today for the treatment of breast cancer. They rival the anthracyclines in terms of response rates and positive impact on TTP. Taxanes are frequently used as first-line chemotherapy for treatment-naive MBC and also in MBC treated with anthracyclines or if anthracyclines are contraindicated. Response rates with paclitaxel range from 21% to 62%; in anthracycline-resistant breast cancer, response rates are 40%.
Two trials have directly compared doxorubicin and paclitaxel, using different dosing and administration schedules. In the Intergroup E1193 study (67), similar response rates and TTP were demonstrated with doxorubicin administered at 60 mg/m2 and paclitaxel at 175 mg/m2 over 24 hours. Thus, paclitaxel may be as effective as doxorubicin when administered as a single agent. The dose and administration schedule may influence the response to paclitaxel. The use of weekly paclitaxel has recently become very popular due to the improvement in the toxicity profile and the ability to deliver a more dose-intensive regimen. At MDACC, the most popular taxane regimen is weekly paclitaxel 80 mg/m2, commonly in a “3 weeks on, 1 week off” or “2 weeks on, 1 week off” schedule.
Docetaxel is a semisynthetic taxane with several preclinical, pharmacokinetic, biological, and clinical differences in comparison to paclitaxel. It has demonstrated a 37% to 57% response rate in patients with anthracycline-resistant tumors and was initially FDA approved for this indication at a dose of 60 to 100 mg/m2 every 3 weeks. At this dose, hematologic toxicity is the greatest and the rates of neutropenia are similar to those seen when paclitaxel is given every 3 weeks. In a large clinical trial, 527 patients were randomized to receive docetaxel 60, 75, or 100 mg/m2 every 3 weeks. A relationship between increasing dose of docetaxel and increased tumor response was observed, but toxicities were also related to increasing doses (68). Docetaxel every 3 weeks at doses between 75 and 100 mg/m2 are appropriate choices as first-line therapy for MBC; in most cases at MDACC, we use 75 mg/m2. Compared to every-3-week paclitaxel, docetaxel 100 mg/m2 was associated with longer TTP (HR, 1.64; 95% CI, 1.33-2.02) and improved OS (HR, 1.64; 95% CI, 1.33-2.02), but also greater incidence of treatment-related toxicities (69). To place these data in context, it is important to remember that paclitaxel has greater activity when given on a weekly schedule, yet it is not clear whether docetaxel or paclitaxel provides superior outcomes when each agent is administered at its optimal dose and schedule. In patients who had received paclitaxel previously, docetaxel administration was associated with response rates of 18% to 21%, demonstrating a lack of cross-resistance between the two agents (70).
Moderate nail changes and fatigue are commonly seen with weekly paclitaxel and docetaxel; excessive tearing due to partial or complete canalicular stenosis is seen with weekly docetaxel. Diarrhea, stomatitis, and neutropenia and its complications are uncommon with weekly taxane administration. Fluid retention is seen in patients who receive a docetaxel cumulative dose greater than 300 mg/m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree