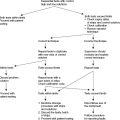
Introduction
The main ‘diabetes medicines’ listed below are discussed in this chapter. Other medicines are described in the relevant chapters:
The author prefers to use the term ‘medicines’ rather than ‘drugs’, which is reserved for describing illegal drugs. Medicines should be managed within a quality use of medicine framework (QUM), which is an holistic risk management approach that encompasses all types of medicines and recommends using non-medicine options first. People with diabetes also frequently use complementary medicines (CAM) and these are discussed throughout the book but principally in Chapter 19. Medicines are also mentioned in other chapters throughout the book where relevant.
Quality Use of Medicines (QUM)
The information in this section was adapted from The Quality Use of Medicines in Diabetes (2005) a paper developed by the Pharmaceutical Health and Rational use of Medicines (PHARM) Committee, a Committee of the Australian Commonwealth Department of Health and Ageing. It is reproduced with permission. QUM is a key aspect of the National Medicines Policy. QUM aims to help health professionals and consumers make the best possible use of medicines to improve their health outcomes. QUM recognises the central role of the consumer in medicines use and that many people maintain their health without using medicines, while for others medicines are important to their health and wellbeing. It also recognises that medicines may be needed for prevention as well as treatment.
‘QUM means:
- Selecting management options wisely.
- Choosing suitable medicines if a medicine is considered necessary.
- Using medicines safely and effectively’ (Commonwealth Department of Health and Ageing, 2002).
Quality Use of Medicines and diabetes
Medicines are central to effective diabetes management to control metabolic abnormalities and manage the complications of diabetes and other concomitant conditions. However, even when medicines are required, lifestyle factors, diet, exercise and smoking cessation, are necessary to achieve optimal outcomes. Prevention programmes such as SNAP have a central, primary and ongoing role (Commonwealth Department of Health and Aging 2001).
Medicines are used in four main areas in diabetes:
QUM is a useful framework for reducing polypharmacy and duplicate prescribing because it encompasses prevention using non-medicine options, regular medication reviews, and effective communication among health professionals and with people with diabetes (National Prescribing Service 2000).
Medicines are selected taking into account:
- The individual’s physical and mental health status.
- Whether there is a suitable non-medicine option.
- The risks and benefits of using medicines for the individual.
- Dosage, dose interval, and duration of treatment.
- Other medicines, CAM and therapies that the individual may be using.
- The process required to monitor the outcomes of medicine use including adverse events, medication self-management and other relevant self-care such as blood glucose monitoring, processes for communicating the medication plan among the relevant health professionals when the individual makes transitions among health providers and services, and strategies for regularly reviewing the continued benefits and risks of the medication regimen and the individual’s self-care capacity.
- The costs to the individual, the community, and the health system. A process for integrating QUM into existing care is shown in Figure 5.1.
Oral hypoglycaemic agents (OHA)
Different OHAs target the various underlying abnormalities of glucose homeostasis in Type 2 diabetes. They are not appropriate for Type 1 diabetes. Type 2 diabetes is a progressive disease of beta cell decline, thus the medication regimen needs to be constantly monitored and adjusted and medicines included as necessary. The UKPDS study (Turner et al.1999) showed monotherapy was ineffective in 75% of people with Type 2 diabetes. An appropriate diet and exercise regimen remain essential regardless of whether medicines are required.
Sulphonylureas were the first OHAs to become available in the 1940s. The Biguanides followed in the 1950s. The value of these medicines has been established and they have been consistently improved over time with new generations of the original sulphonylureas. In addition, new classes of OHAs have been introduced, which might extend the life of the beta cells and delay the need for insulin (Dornhorst 2001).
Figure 5.1 The Quality use of Medicines process applied to diabetes care reproduced from The Quality Use of Medicines in Diabetes Pharmaceutical Health And Rational use of Medicines (PHARM) Committee, Commonwealth Department of Health and Ageing (2005). The medication regimen should be reviewed each time a new medicine is required, if an adverse event occurs, and at least annually as part of routine diabetes complication procedures (September 2005 with permission). *HMR = home medicines reviews, †CMI = consumer medicines information.
OHAs target the different underlying abnormalities of glucose homeostasis associated with Type 2 diabetes. These OHAs are:
- Biguanides, which reduce insulin resistance and fasting glucose and are the medicine of first choice in overweight people with Type 2 diabetes (UKPDS 1998). Biguanides are insulin sensitisers.
- Sulphonylureas and Glitinides, which are secretagogues that stimulate insulin production. Therefore, the beta cells must be capable of responding by producing insulin. Sulphonylureas are insulin secretagogues.
- Thiazolidinediones (TZD), which reduce insulin resistance, reduce daytime preprandial hyperglycaemia, and have some effect on the fasting blood glucose.
- Alpha-glucosidase inhibitors, which slow carbohydrate digestion and glucose absorption and reduce postprandial glucose levels (Phillips 2000; Braddon 2001).
Each of these medicines can be used alone or in combination, which is common practice because they individually target different metabolic abnormalities. Biguanides and sulphonylureas reduce HbA1c by 1–2% (Kuritzky 2006). OHAs can also be effectively combined with insulin. The commonly available OHAs are listed in Table 5.1.
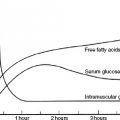
Blood glucose and HbA1c monitoring are essential to assess, whether, when, and which OHA should be commenced and when insulin is required. When OHAs are started it is necessary to monitor the blood glucose in order to appropriately adjust the dose and dose interval. Normal endogenous insulin secretion consists of two components:
The different OHAs and insulins target these components. Testing the blood glucose is a key to determining their effectiveness. HbA1c only provides an overall average blood glucose level (see Chapter 3). Thus both measures provide important information. Key testing times are:
Sometimes testing at both of these times will be required and sometimes overnight (2–3 AM) to detect nocturnal hypoglycaemia, see Chapter 6.
Biguanides
Biguanides are the medicine of first choice for overweight people with Type 2 diabetics when the HbA1c is >7%. Metformin effectively lowers all-cause mortality and diabetes complications among overweight people with diabetes (UKPDS 1998). It is also used to manage insulin resistance associated with PCOS, where it may delay the progression to Type 2 diabetes and is being trialed in the treatment of GDM (Hamilton et al. 2003), see Chapter 14. Metformin is the most commonly used Biguanide. It acts by:
- Impairing the absorption of glucose from the gut.
- Inhibiting hepatic glucose output.
- Increasing glucose uptake in peripheral tissues (muscle and fat).
- Increasing the effects of insulin at receptor sites.
- Suppressing the appetite (mild effect).
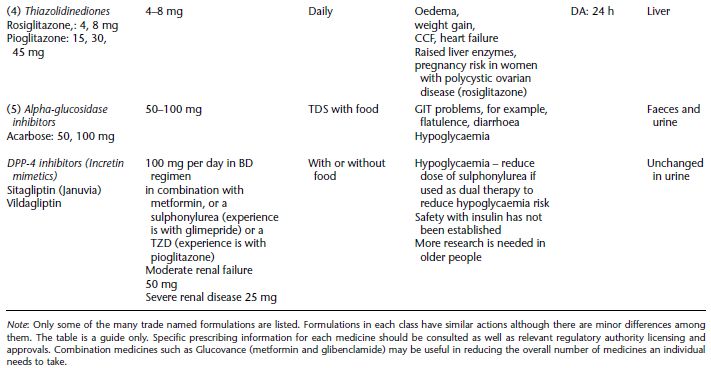
Table 5.1 Oral hypoglycaemic agents, dose range and dose frequency, possible side effects, the duration of action and main site of metabolism.
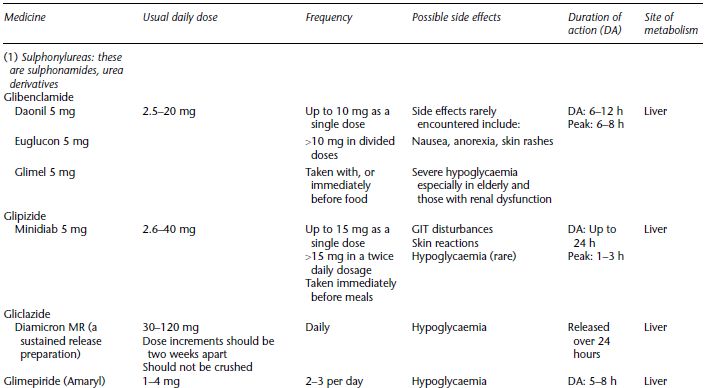

Possible side effects
- anorexia
- nausea and vomiting
- abdominal pain
- cramps
- weight loss
- lethargy
- respiratory distress.
- during pregnancy
- for patients with chronic renal failure
- Type 1 diabetes
- any disease likely to cause hypoxia such as severe respiratory diseases and hepatic or cardiovascular disease.
There is some evidence that at higher doses and longer duration of use metformin inhibits absorption of vitamin B12. The metformin dose is the strongest predictor of vitamin B12 deficiency (Ting et al. 2006). However, Ting et al. did not assess calcium intake or measure vitamin B12 metabolites (homocysteine and methylmalonic acid), which could have affected the results. Nevertheless, Vitamin B12 deficiency should be considered in people at high risk of malnourishment, for example, older people and those with eating disorders, people who have been on metformin for long periods of time especially at high doses, and those with malabsorption syndromes.
Biguanides should be ceased for two days before IVP, CAT scans and investigations that require IV-iodinated contrast media to be used (Calabrese et al. 2002).
Sulphonylureas
Sulphonylureas can be used alone or combined with metformin. They are usually well tolerated but there is a tendency for people to gain weight, especially with older OHAs. Weight gain occurs to a less extent with newer OHAs (Inzucchi 2002). They generally have a rapid onset of action except for long-acting formulations. Hypoglycaemia is a risk especially in older people on long-acting agents, although these are rarely used and are no longer available in some countries. However, glibenclamide is available in combination with metformin (Glucovance). People with renal impairment and those who are malnourished are also at risk of hypoglycaemia.
Sulphonylureas act by:
- Stimulating insulin secretion from the pancreatic beta cells.
- Increasing the effects of insulin at its receptor sites.
- Sensitising hepatic glucose production to inhibition by insulin.
Possible side effects
Note: (2)–(6) are very uncommon. Sulphonylureas are contraindicated in pregnancy although they are used during pregnancy in some countries. They are mostly metabolised in the liver and severe liver disease is a contraindication to their use. Caution should be taken in people who are allergic to ‘sulphur medicines’ because the sulphonylureas have a similar chemical makeup.
Glitinides
These medicines increase insulin secretion at meal times and they should only be taken with meals, usually 2–3 times per day. They are short acting and have a low hypoglycaemic risk.
They target early phase insulin secretion, which is essential for postprandial glucose and control the postprandial glucose load (Dornhorst 2001). In this way they initiate an insulin response pattern close to normal. They can be used in combination with Biguanides and possibly Thiazolidinediones (TZD). The two main formulations are repaglinide and nataglinide. These medicines are rarely used in Australia because they are not listed on the PBS. However, because of their short duration of action and the requirement to take them with meals they could be very useful in older people at high risk of hypoglycaemia.
Thiazolidinediones
The Thiazolidinediones (TZD) are also known as peroxisome-proliferator-activated receptor-γ (PPAR-γ). They are given as a daily dose. It takes several days before they show an effect. In Australia, authority is required to prescribe TZDs and they are not subsidised for use as monotherapy (Schedule of Pharmaceutical Benefits (PBS) 2006). There are two forms Pioglitazone and Rosiglitazone. Pioglitazone can be used as dual therapy in combination with metformin OR a sulphonylurea in Type 2 diabetes when the HbA1c is >7% when combining metformin and a sulphonylurea is contraindicated. It can be combined with insulin in Type 2 diabetes if the HbA1c is >7% despite treatment with OHAs and insulin, OR insulin alone OR if metformin is contraindicated. Rosiglitazone can be combined with metformin AND a sulphonylurea in Type 2 diabetes when the HbA1c is >7% despite maximum tolerated doses of these medicines.
TZDs reduce HbA1c by ∼1–2% (Ko et al. 2006) with similar improvements to adding insulin. However, long-term data is not yet available for TZDs. If adding a TZD does not adequately reduce HbA1c to <8 5% in 3 months, insulin should be commenced (Nathan et al. 2006).
TZDs lower fasting and postprandial blood glucose by increasing insulin sensitivity in muscle, fat, and liver cells. Some improve lipid profiles, enhance insulin sensitivity and may restore the beta cell mass.
An early form of the medicine was responsible for causing liver failure and was withdrawn from the market. This effect is unlikely with currently available TZDs but it is recommended that liver function be monitored and caution be exercised in people with liver damage or whose albumin excretion rate is elevated.
Hypoglycaemia is possible because TZDs reduce insulin resistance and enhance the effectiveness of endogenous insulin.
Side effects
- Localised oedema, which can be significant and may occur to a greater extent in people treated with TZD and insulin (product prescribing information: Actos 2004; Avandia 2005).
- Congestive cardiac failure and heart failure. People with diabetes are 2.5 times more likely to develop heart failure than non-diabetics (Nichols et al. 2004) and TZDs increase the risk of heart failure. Rosiglitazone doubles the risk among those with pre-existing cardiovascular disease (Home et al. 2007). TZDs are contraindicated in people with New York Heart Association Class III or IV heart failure. The person should be closely monitored for signs of heart failure such as oedema and rapid weight gain.
- Myocardial infarction and death associated with rosiglitazone (Nissen & Wolski 2007). This report caused significant debate among diabetes experts and stress for patients prescribed rosiglitazone. The risk appears to be small (DREAM 2006). The information should be used in the context of individual risk. For example, rosiglitazone might increase the risk of MI in people with ischaemic heart disease, those on insulin or nitrates, those with an atherogenic lipid profile, and those at high risk of MI (ADRAC 2007). Pioglitazone does not appear to carry the same risk because it has fewer adverse effects on lipids (Dormandy et al. 2005). Diabetes Australia recommended patients not to stop rosiglitazone without consulting their doctor. The Australian Diabetes Society (2007) issued a position statement recommending patient’s concerns be acknowledged, rosiglitazone continued to be used until further evidence arises, or discontinuing rosiglitazone and maintaining glycaemic control using other OHAs or commencing insulin. Regulatory authorities such as the FDA and NPS issued advisories but did not withdraw or limit the prescribing indications for rosiglitazone at the time,
- Reduced red and white cell counts.
- Weight gain, especially deposition of subcutaneous fat, while visceral obesity is reduced. Weight gain appears to continue as long as TZDs are continued. Gains between 2 and 5 kg are reported (Dormandy et al. 2005). However, insulin also causes weight gain (∼3 kg), as do sulphonylureas (∼4 kg). Weight gain is lower when patients are taking metformin before a TZD is added (Strowig 2004).
- Hypercholesterolaemia.
- Liver damage although it is uncommon. TZDs are contraindicated if liver disease is present or serum transiminase is >2.5 times the upper limit of the normal. Liver function tests should be performed before starting a TZD and then monitored regularly while the patient remains on TZDs. Signs of liver toxicity include nausea, vomiting, jaundice, dark urine, and right upper abdominal discomfort.
- Macular oedema (European Medicine Agency Press 2005). Macular oedema is a known complication of diabetes and there some reports the condition worsens with TZD.
- Fractures occurring in the arms and lower leg usually in women (Khan et al. 2006), however the risk is small. Fracture risk may be significant in older women at risk of osteoporosis and increase the risk of falls if the fractures occur in the feet or legs.
- Women with Polycystic Ovarian Disease should be counselled about contraception because TZDs may improve fertility in these women.
- They are contraindicated in pregnancy and during breast feeding.
- Care should be taken in lactose intolerant people because TZDs contain a small amount of lactose.
These data suggest a thorough assessment including a medication review is warranted before commencing TZDs. Health professionals and people with diabetes should be alert to the possibility of silent MI.
Alpha-glucosidase inhibitors
These medicines are usually taken in a TDS regimen.
They act by slowing glucose uptake of many carbohydrates by inhibiting alpha-glucosidase, which slows the breakdown of complex and simple carbohydrates in the brush border of the proximal small intestine so the glucose load is spread over a longer time frame. They reduce fasting and postprandial glucose.
Their major side effects are due to the arrival of undigested carbohydrate in the lower bowel – bloating, flatulence, and diarrhoea. These symptoms can be distressing and embarrassing and people often stop their medications because of these side effects.
Taking the medicines with meals, starting with a low dose and increasing slowly to tolerance levels, and careful explanation to the patient can reduce these problems.
Hypoglycaemia is possible if they are combined with other OHAs. They may be contraindicated or need to be used with caution in people with gastrointestinal disease such as gastroparesis, coeliac disease, and irritable bowel syndrome.
Oral glucose may not be an effective treatment for hypoglycaemia occurring in people on alpha-glucosidase inhibitors because absorption from the gut will be delayed. IM Glucagon is an alternative.
The incretin hormones
The incretins enhance glucose-mediated insulin secretion by the beta cells. Approximately 60% of insulin secreted in response to food is due to the activity of incretins (Eli Lilly 2005). The incretin effect is due to peptide hormones released by K and L cells in the intestine directly into the blood stream. The incretins include:
The incretin hormones are either incretin mimetics or incretin enhancers. GLP-1 is a mimetic (receptor agonist) injectable synthetic agent, for example, Exanatide (Byetta); sometimes referred to as ‘lizard spit’. It lowers HbA1c by 0.5–1% (Nathan et al. 2006). Incretin enhancers are oral agents known as DPP-1V inhibitors, for example, Sitagliptin (Januvia) and Vildagliptin, seeTable 5.1.
Medicine interactions
Possible interactions between OHAs and other commonly prescribed medicines are shown in Table 5.2.
Medicine and OHA interactions are possible and can lead to hypo- or hyperglycaemia. A number of mechanisms for the interactions are known and they include:
- Displacing the medicine from binding sites;
- Inhibiting or decreasing hepatic metabolism;
- Delaying excretion;
- Reducing insulin release;
- Antagonising insulin action.
Consideration should also be given to potential medicine and herb or herb/herb interactions, see Chapter 19.
Table 5.2 Potential medicine interactions between oral hypoglycaemic agents and other medicines (based on Shenfield 2001).
| Medicine | Possible mechanism |
| Medicines that increase blood glucose | |
| Clonidine | Adrenergic response |
| Clozapine | Impaired insulin secretion |
| Corticosteroids | Oppose insulin action |
| Diuretics, especially Thiazides | Oppose insulin action |
| Nicotinic acid | Unknown |
| Nifedipine | Delays insulin action |
| Oral contraceptives | Unknown |
| Phenytoin | Impairs insulin secretion |
| Sugar-containing medicines, for example, cough syrup | Increase blood glucose |
| Medicines that lower blood glucose | |
| ACE inhibitors | Enhance insulin action |
| Alcohol | Reduce hepatic glucose production |
| Fibrates | Unknown |
| MOA inhibitors | Unknown |
| Salicylates | Unknown |
- beta blockers can mask tachycardia and other signs of hypoglycaemia resulting in delayed recognition and treatment increasing the risk of hypoglycaemic coma.
- chronic alcohol consumption can stimulate the metabolism of sulphonylureas and delay their effectiveness, cause hypoglycaemia, mask signs of hypoglycaemia, and with metformin, predispose the individual to lactic acidosis. It may also increase the bioavailability of ACE-1.
Potential interactions have not yet emerged for TZIs and the glitinides. Medicines that alter hepatic enzymes have the potential to cause interactions with these OHAs because they are metabolised in the liver.
Medicines that interfere with access to the gut by alpha-glucosidase inhibitors can inhibit their action, for example, charcoal, digestive enzymes, cholestyramine, neomycin, and some CAM medicines such as slippery elm.
Combining OHAs and insulin
Any combination of currently available OHAs only lowers HbA1c by ∼3% (Endocrinologists Medical Guidelines for the Management of Type 2 Diabetes 2002), thus people with HbA1c >10% are unlikely to achieve management targets using OHA alone. Therefore, insulin is assuming an increasingly important role in Type 2 diabetes. As indicated, most people with Type 2 diabetes have progressive beta cell dysfunction and a decline in beta cell mass. Proposed mechanisms for these defects include the interplay among a range of factors that reduce beta cell mass and secretory function such as hyperglycaemia, elevated free fatty acids, and inflammatory processes associated with adipocyte-derived cytokines. Apoptosis appears to be a key underlying mechanism (Leiter 2006). In addition, lifestyle factors, concomitant diseases, and often medicines, compound the metabolic abnormalities. In some cases medication nonadherence may be a factor and should be assessed in a non-judgmental manner.
When should insulin be initiated in Type 2 diabetes?
Commencing insulin should be a proactive decision and should not be delayed. Some experts refer to ‘tablet failure’. The choice of words should be considered carefully when conveying the need for insulin to a patient: they may interpret ‘tablet failure’ to mean they have failed.
The time to commence insulin in Type 2 diabetes depends on the individual’s blood glucose pattern, HbA1c, adherence to medicines, and complication status especially cardiovascular and renal status and willingness to use insulin. Indications for insulin include:
- Women with Type 2 diabetes who become pregnant and sometimes women with gestational diabetes, see Chapter 14.
- When the person actually has LADA (see Chapter 1).
- As rescue therapy in DKA, HONK, during other acute illnesses, and surgical procedures, see Chapters 7 and 9.
- Persisting hyperglycaemia indicated by elevated fasting blood glucose and/or random postprandial blood glucose.
- Symptomatic especially polyuria, polydipsia and weight loss.
- OHA intolerance or contraindication, for example, metformin if creatinine is high.
- People on two OHAs and not achieving targets where insulin may be preferable to adding a third OHA given that most people with Type 2 diabetes eventually need insulin especially if the OHAs are at maximal doses. However, there is no consensus about whether the second agent added to metformin should be another OHA or insulin. Insulin might be preferable if the HbA1c is >8.5% or the person is very symptomatic (Nathan et al. 2006). In these cases it might be appropriate to consider LADA especially if the individual is thin. The National Prescribing Service advised health professionals to be ‘ . . . more aggressive in their management of patients with Type 2 diabetes’ (NPS media release, 26 March 2008).
- Recent research suggests insulin may have anti-inflammatory properties in addition to its other actions (Dandona et al. 2008).
The goals are to achieve optimal control without causing hypoglycaemia or excessive weight gain and with minimal impact on lifestyle. Thus, understanding the individual’s perspective is essential. For some peoples commencing insulin and ceasing OHAs may represent a simpler more manageable medication regime.
Insulin is often added to the OHA regimen at bedtime to reduce fasting glucose levels (Riddle et al. 2003; Janka et al. 2005). The dose is titrated according to the fasting blood glucose pattern including self-adjustment by the patient to achieve targets with minimal hypoglycaemia according to a simple algorithm (Yki-Jarvinen et al. 2007). Yki-Jarvinen et al. (2007) showed insulin could be successfully initiated in groups and achieve glycaemic targets. In addition, group insulin initiation was acceptable to patients.
The specific initiation process depends on the policies and guidelines of individual health services. Over recent years, research has demonstrated the efficacy of several processes for initiating and titrating insulin in Type 2 diabetes using various insulins and usually starting with small doses. They all used a stepwise approach and include:
- The Treat-To-Target study used basal Glargine at bedtime and titrated the dose weekly in 10 weeks (Riddle et al. 2003). This regimen is suitable for older people because it is associated with fewer hypoglycaemic events and is well tolerated (Janka et al. 2005). Isophane insulin can also be used as the basal insulin but has a higher risk of hypoglycaemia.
- The 1-2-3 study, which used daily bedtime doses of NovoMix 30 initially and subsequently added doses pre-breakfast then pre-lunch if necessary to achieve targets (Raskin et al. 2005).
- The INITIATE study that used BD doses of NovoMix 30 (Jain et al. 2005).
The advantage of using basal bolus insulin dose regimens is that they usually achieve better postprandial control but eating after injecting is important. BD Lispro/isophane mix and Metformin also improves pre and postprandial blood glucose with few episodes of nocturnal hypoglycaemia (Malone et al. 2005).
Many researchers have compared different brands of insulin and dose regimens. Overall, the findings suggest the pharmacokinetic differences among insulin brands may not be clinically relevant and there are likely to be variations in individual patient’s response to the different formulations (Zeolla 2007). In reality, the choice of insulin may actually be made according to prescriber preference and local availability. Despite the similarities, indiscriminate switching between different insulin brands or using them simultaneously is not generally recommended.
Except in specific circumstances, outpatient or community-based initiation is preferable.
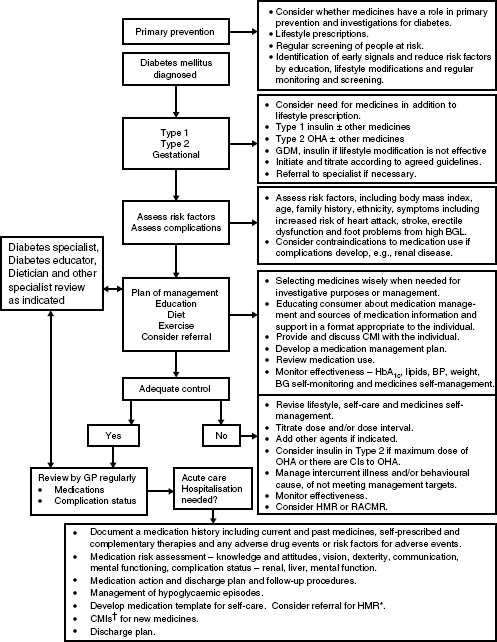
A proforma initiation process is outlined in this chapter that can be adapted as necessary and a simple algorithm for commencing insulin in Type 2 diabetes is shown in Figure 5.2. Usually, OHAs are continued with basal insulin regimens if there are no contraindications to their use. When bolus insulin doses are added secretagogue doses are usually reduced or the medicines discontinued.
Figure 5.2 Algorithm for achieving blood glucose targets in Type 2 diabetes that encompasses a quality use of medicines approach and adopts a proactive stepwise approach to initiating insulin.
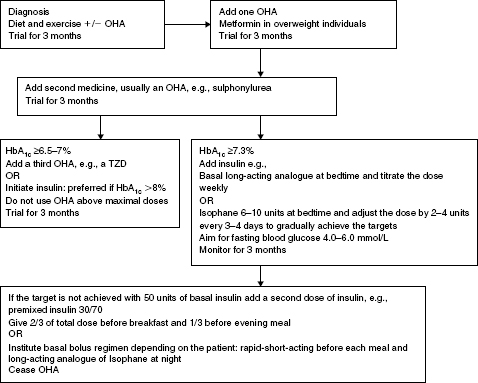
The algorithm is based on the premise that the individual undertakes blood glucose monitoring, relevant monitoring and assessments are undertaken, relevant education is provided and the response to therapy is monitored at each step. Consider LADA if the person is not overweight, loses weight, has significant hyperglycaemia, and is very symptomatic because insulin should not be delayed in these people, see Chapter 1.
Management aims:
OHA = oral hypoglycaemic agents; TZD = Thiazolidinediones.
Barriers to insulin therapy
There are many barriers to initiating insulin therapy in Type 2 diabetes: most relate to either the individual with diabetes or health professional factors. Although patients often view insulin negatively, early explanations about the nature of Type 2 diabetes (from diagnosis), with support and encouragement most people usually accept they need insulin. However, their fears and concerns must be acknowledged and respected. People often regard insulin as ‘the last resort’, fear hypoglycaemia, and weight gain (Dunning & Martin 1999). The DAWN study (Rutherford et al. 2004) showed people on insulin worried about hypoglycaemia more than non-insulin users and insulin was associated with worse quality of life in people with Type 2 diabetes.
Type 2 diabetes is a silent disease with few symptoms; thus, it is often difficult for people to accept they have a serious, progressive disease. They are often reluctant to test their blood glucose frequently and feel blood glucose monitoring and insulin interferes with their lifestyle. For some people, the stigma associated with needles is an issue.
Many doctors are reluctant to use insulin in Type 2 diabetes and often compound the patient’s concerns, albeit usually unintentionally, by delaying insulin initiation. This has been referred to as ‘clinical inertia’ (Riedel et al. 2006), and, like non-adherence is a complex, multifactorial issue. Other health professional-related barriers include inadequate knowledge, lack of time, support and resources, worry about causing hypoglycaemia and complicating the management regimen. For example, UK practice nurses felt commencing insulin in primary care was beneficial for patients but lack of time, support, and confidence, and concerns about medico-legal implications and personal accountability made it difficult to achieve (Greaves et al. 2003).
Some strategies to overcome the barriers
The therapeutic relationship between the patient and the health professional has a significant effect on health outcomes and patient behaviours including adherence to medicines and improved safety (Worthington 2003). Thus, a first step is to establish a non-judgmental, trusting relationship. Specific approaches depend on the individual and the circumstances in which insulin is required. Health professionals need to acknowledge that:
- Managing insulin is a complex process and is only one self-care and life task the person is expected to fulfil.
- ‘Things might get worse before they get better’. For example vision often deteriorates temporarily.
- The ability to self-care changes over time due to physical and mental functional changes often associated with diabetes complications.
- There are usually added costs involved.
The transformational model of change can be a useful framework for addressing the need to commence insulin, especially if it is integrated with aspects of the health belief model and holistic care, where insulin is discussed as one aspect to be addressed not an isolated event in a person’s life. Research suggests timing is important when trying to initiate change. That is it needs to be linked to an individual’s stage in the change process (Prochaska & Velicer 1997) and significant life transitions, which are often also accompanied by changes in identity and behaviour (George 1993).
Providing personalised and customised advice and written information is most useful when it is delivered in a caring relationship where the individual’s concerns are acknowledged and they are invited to suggest ways they could address the issues they identify. Thus, listening, clarifying, and following up by asking about progress are essential. However, despite the focus on patient-centred care, the patient’s views are often not sought and they are inadvertently placed in a passive rather than an active role particularly when communication barriers such as language, culture, or disabilities are present.
Identifying and discussing the person’s concerns is essential. For example, ‘needle phobia’, weight gain, and hypoglycaemia. Demonstrating the various insulin delivery devices often helps reduce some concerns about needles. People on insulin gain more weight than those on diet over 10 years (Mayfield & White 2004) due to better glycaemic control and losing less glucose in the urine, the fact that insulin is an anabolic hormone, lower metabolic rate and people may eat more to prevent hypoglycaemia (Birkeland et al. 1994). Weight gain appears to be less on Levemir in both Type 1 and Type 2 diabetes (Dornhurst et al. 2007).
Strategies to avoid weight gain include selecting insulin formulations and other medicines least likely to contribute to weight gain if possible, regular exercise perhaps using a pedometer, and individualised nutrition advice. Once symptoms are controlled people often feel more active and exercise helps control weight and blood glucose.
Understanding and exploring the person’s concerns about hypoglycaemia and helping them identify strategies to reduce the incidence. Explain that the frequency and severity of hypoglycaemia is lower in people with Type 2 diabetes than Type 1 diabetes (UKPDS 1999). The risk increases when the HbA1c is <7.4%. Carefully explaining the likely risk factors and helping the person develop strategies to minimise their individual risk is important.
Vision can deteriorate when insulin is commenced. Sight is not usually threatened but it is very distressing for the person with diabetes and they need a careful explanation and reassurance to help them understand. Activities of daily living such as reading and driving can be affected. Visual changes occur because the lens absorbs excess glucose in much the same way as sponge soaks up water. Changes in the amount of glucose in the lens can lead to blurred vision and can occur with high and low blood glucose levels. This phenomenon is quite different from diabetic retinopathy, which can threaten the sight.
The starting dose may not be the ‘right’ dose and the ‘right’ dose changes according to specific circumstances and individual need.
Insulin therapy
Overview of insulin action
Insulin is a hormone secreted by the beta cells of the pancreas. Normal requirements are between 0.5 and 1.0 units/kg/day. Insulin synthesis and secretion are stimulated by an increase in the blood glucose level after meals. Insulin attaches to insulin receptors on cell membranes to facilitate the passage of glucose into the cell for utilisation as fuel or storage, and reduces hepatic glucose production. Insulin also stimulates the storage of fatty acids and amino acids, facilitates glycogen formation and storage in the liver and skeletal muscle, and limits lipolysis and proteolysis. Therefore, insulin deficiency results in altered protein, fat and carbohydrate metabolism (also refer to Chapter 1).
Insulin is vital to survival for people with Type 1 diabetes and is eventually required by most people with Type 2 diabetes.
Objectives of insulin therapy
Types of insulin available
Insulin cannot be given orally at this stage. It is a polypeptide. Polypeptides are digested by gastric enzymes and do not reach the circulation. Research is currently underway to coat insulin in a substance that can withstand gastric juices and enable it to pass unchanged into the intestine before breaking down. Inhaled insulin is also the subject of research but is not generally used in clinical practice. Likewise, some trials have been abandoned.
A number of different brands of insulins are available, for example, Novo Nordisk, Eli Lilly, and SanofiAventis.
Animal insulins (bovine and porcine) are rarely used nowadays but still available in some countries often under special access schemes. ‘Human’ insulin (HM) is manufactured by recombinant DNA technology . The amino acid sequence of HM insulin is the same as that of insulin secreted by the beta cells of the human pancreas. In the past few years rapid acting insulin analogues have been developed that give a more physiologic response after injection and improve the blood glucose profile, for example, Humalog, Aspart, and Apidra. The advantages of these insulins are reduced postprandial hyperglycaemia and reduced risk of hypoglycaemia.
Long-acting analogues are Glargine and Levemir. Glargine has a slower onset than Isophane insulins and a smooth peakless action profile for up to 24 hours (Buse 2001). Levimir may be shorter acting than Glargine and has its maximal effect between 3 and 14 hours. It was only registered for use in Type 1 diabetes in Australia at the time of writing. These insulins enable greater management flexibility and lower risk of hypoglycaemia.
Rapid acting insulin
Rapid acting insulin should be clear and colourless. Examples are:
- Lispro (Humalog)
- Novorapid (Aspart)
- Glulisine (Apidra)
They have a rapid onset of action, within 10–15 minutes, peak at 60 minutes and act for 2–4 hours.
They need to be given immediately before meals and are used in basal bolus regimes, in combination with intermediate acting insulin and long–acting analogues, or used in combination with OHAs or in insulin pumps.
Combining them with alpha-glucosidase inhibitors, which reduce glucose absorption from the gut, can increase the risk of hypoglycaemia.
The first hour after injection and 2–3 hours after exercise are other peak times for hypoglycaemia.
Short-acting insulin
This should be clear and colourless. Examples are:
- Actrapid
- Humulin R
- Hypurin neutral (beef)
They begin to take effect in 20–30 minutes, and act between 4 and 8 hours. They can be used:
- Alone two to four times per day.
- In combination with intermediate or long-acting insulins.
- As IV insulin infusions
Intermediate-acting insulin
This must be mixed gently before use and should be milky after mixing. Examples are:
- Protophane
- Humulin NPH
- Hypurin (isophane)
They begin to act in 2–3 hours. The duration of action is between 12 and 18 hours. They can be used:
- In combination with short-acting insulin – this is the usual method.
- Alone for patients who are sensitive to short-acting insulin, or in combination with oral hypoglycaemic agents.
Long-acting insulin
- Glargine (Lantus)
- Detemir (Levemir) as described in the preceding section.
Biphasic insulins
Biphasic insulins are often prescribed for people with Type 2 diabetes.
These contain both short- and intermediate-acting insulins in various combinations. They must be mixed before using. They do not enable independent adjustment of the short or intermediate components. Examples are:
- Mixtard 30/70 Humalin 30/70
- Mixtard 50/50 Humulin Mix 50
- NovoMix 30 Humalog Mix 25
The range of Lilly insulins is shown in Figure 5.3 and the Novo Nordisk range in Figure 5.4. They also depict the insulin delivery devices available for each product.Apidra is dispensed in a prefilled disposable injection device (Apidra SoloStar) and is blue to distinguish it from the Lantus SoloStar, which is white and mauve and is also disposable. Lantus is also dispensed in 3 mL cartridges and 10 mL glass vials. Each insulin administration device has advantages and disadvantages and patient preference should be considered (see Table 5.3). The diabetes educator can help individuals decide which device (and therefore, to some extent which insulin) suits them best.
Storing insulin
The temperature at which insulin is stored is important to maintaining its efficacy. Insulin should be stored according to the manufacturer’s directions. Unopened vials should be stored in the refrigerator at 2–8°C. Insulin vials in use can be stored out of the refrigerator, for example, in the patient’s medication drawer, provided they are not stored near a source of heat or light (Campbell et al. 1993; individual product prescribing information).
People with diabetes need to be educated about correct storage and handling of insulin as well as sharps disposal as part of their education about insulin therapy.
Figure 5.3 Range of Lilly insulins, their presentation and schematic action profiles. Reproduced with permission from Eli Lilly Australia Pty Ltd. PBS information refers to Australian prescribing information.
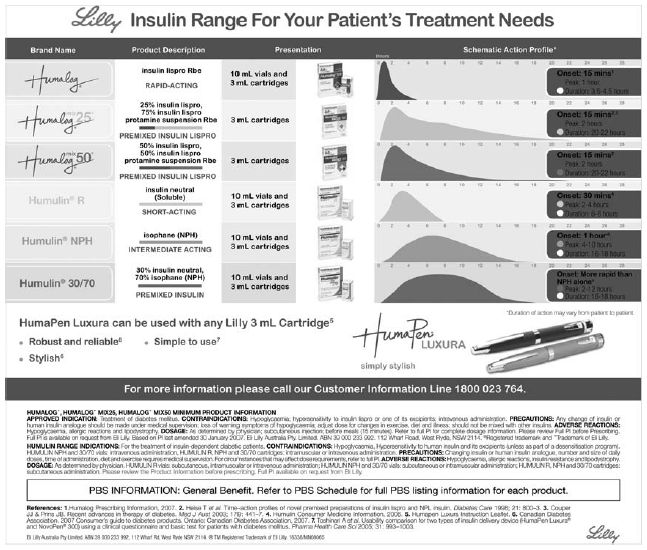
Figure 5.4 (a) Range of NovoNordisk insulins with their time action characteristics and insulin profiles and (b) the delivery systems available with each insulin. Reproduced with permission from NovoNordisk (Australia). PBS information refers to Australian prescribing information.
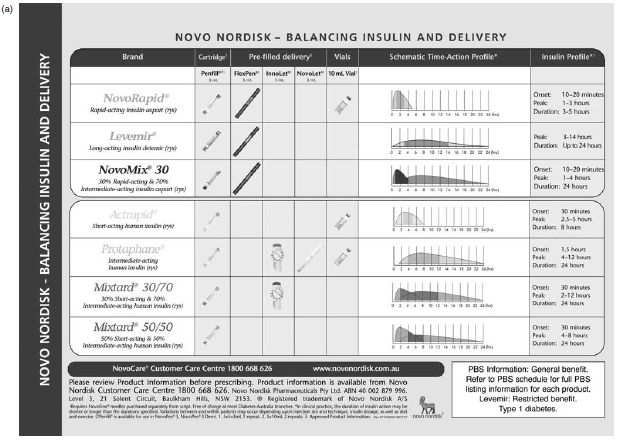

Table 5.3 Some examples of commonly used insulin delivery devices and some of the issues to consider when helping the individual decide which device to use.
| Device | Issues to be aware of |
| (a) Insulin syringes | • There is still a place for syringes |
| 30, 50, and 100 unit sizes | • Patients need to be able to recognise different dose increments on different sized syringes |
| • Select size appropriate for dose | |
| • Needles are usually longer than other devices | |
| • Can be used with all available insulins but it is not advisable to withdraw insulin from penfills with a syringe | |
| • May lead to dose errors in hospital settings and in some hospitals only one routine stock size is available | |
| • A retractable syringe is available, which was designed to reduce needle stick injuries. | |
| (b) Insulin ‘pens’ | • Insulin ‘pens’ are not suitable for people who need to mix insulins |
| • They make injecting simpler because there is no need to draw up the dose | |
| • They can sometimes be difficult to manage for people with manual dexterity problems | |
| SanofiAventis devices | |
| Apidra SolorStar | Prefilled disposable devices |
| Lantus SoloStar | |
| Novo Devices | |
| NovoPen 3 | • Dose range 2–70 units |
| A new device, NovoPen, | • Accurate dosing |
| 4 is under development | • Uses 3 mL insulin cartridges |
| • Small, fine needles | |
| • Replacing the insulin cartridge can be difficult for some people | |
| • Has a function to check the accuracy of the device | |
| • Pen is reusable | |
| NovoPen Demi | • Similar to NovoPen 3, but 1/2 unit dose increments are possible |
| • Useful for children and insulin-sensitive patients who require very small doses | |
| INNOLET | • Dose range 1-50 units |
| • Accurate dosing | |
| • Small, fine needles | |
| • Device is preloaded and disposable | |
| • Limited range of insulins available | |
| • Contains 3 mL of insulin | |
| • Clear, easy to see numbers on the dose dial, which is an advantage for vision impaired people | |
| • Is easier for older people to manage | |
| • Larger than other devices – takes up less storage space in fridge | |
| PenMate 3 | • Automatic needle insertion device |
| • Used with NovoPen 3 | |
| • Hides needle and injects insulin quickly and automatically | |
| • May benefit people with needle phobia and children | |
| FlexPen | • Contains 3 mL of insulin |
| • Disposable, preloaded device | |
| • Small, fine needles | |
| • A range of insulin is available | |
| Eli Lilly devices | |
| Humapen Luxura | • Dose range 1-60 units |
| • Accurate dosing | |
| • Easy to correct if an incorrect dose is dialed up without wasting insulin | |
| • Uses 3 mL insulin cartridges | |
| • Small, fine needles | |
| • Replacing the insulin cartridge can be difficult for some people | |
| • Has a function to check the accuracy of the device | |
| • Pen is reusable | |
| • Pen has audible clicks representing each unit dialled | |
| Insulin pumps | • Provide continuous basal insulin with a facility for giving bolus doses with meals |
| • Uses only short-acting insulin | |
| • Uses small, fine needles | |
| • Expensive | |
| • Requires considerable expertise and time to be used effectively |
Note: Other devices may be available. The devices should only be used with the insulin specified by the manufacturer. Needles and syringes should only be used once and then discarded into an appropriate sharps container.
Injection sites and administration
Administer at the appropriate time before the meal. The abdomen is the preferred site; but upper arms, thighs, and buttocks can also be used. Injection sites must be rotated to avoid lipoatrophy and lipodystrophy and should be checked on a regular basis.
How to inject
The insulin injection technique can influence insulin absorption and, therefore, its action. Insulin should be administered subcutaneously. IM injections lead to unstable blood glucose levels (Vaag et al. 1990).
- Pinch up a fold of skin (dermis and subcutaneous tissue) between the thumb and index finger.
- Inject at 90-degree angle. If the needle is long, pinch up may not be needed and a 45-degree angle can be used to avoid giving an IM injection.
- Release the skin, remove the needle, and apply gentle pressure to the site.
- Document dose and time of the injection.
- Injection sites should be regularly checked for swelling, lumps, pain or leakage of insulin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree