Key points
- Diabetes is the modern pandemic. It represents a considerable global economic and social burden for the person with diabetes and the health system. The prevalence of both Type 1 and Type 2 is increasing.
- Primary prevention and early detection are essential to reduce the personal and community burden associated with the metabolic syndrome and Type 2 diabetes and its complications.
- Type 2 diabetes is a progressive disease and complications are often present at diagnosis. Thus, insulin will eventually be necessary in most people with Type 2 diabetes.
- The prevalence of obesity, the metabolic syndrome and Type 2 diabetes is increasing in children.
What is diabetes mellitus?
Diabetes mellitus is a metabolic disorder in which the body’s capacity to utilise glucose, fat and protein is disturbed due to insulin deficiency or insulin resistance. Both states lead to an elevated blood glucose concentration and glycosuria.
The body is unable to utilise glucose in the absence of insulin and draws on fats and proteins in an effort to supply fuel for energy. Insulin is necessary for the complete metabolism of fats, however, and when carbohydrate metabolism is disordered fat metabolism is incomplete and intermediate products (ketone bodies) can accumulate in the blood leading to ketosis, especially in Type 1 diabetes. Protein breakdown leads to weight loss and weakness and contributes to the development of hyperglycaemia and lethargy.
There are different types of diabetes that have different underlying causal mechanisms and clinical presentation. In general, young people are insulin-deficient (Type 1 diabetes), while older people may secrete sufficient insulin and plasma insulin levels may be high (hyperinsulinaemia) but demonstrate resistance to its action (Type 2 diabetes). However, Type 1 occurs in ~10% of older people (see latent autoimmune diabetes (LADA) later in this chapter) and Type 2 is becoming increasingly prevalent in children and adolescents as a result of the global obesity epidemic (Barr et al. 2005; Zimmet et al. 2007). Type 2 diabetes is the most common, accounting for ~85% of diagnosed cases; Type 1 accounts for ~15% of diagnosed cases.
Prevalence of diabetes
Diabetes affects approximately 0.5 to >10% of the population depending on the type of diabetes, age group, and ethnic group. The prevalence of diabetes is increasing, particularly in the older age group and in developing countries. The number of individuals with diabetes is projected to reach 215 million by 2010. In western countries the overall prevalence is 4−6% and up to 10−12% among 60−70 year olds. Most countries spend 6−12 of their annual health care budgets on diabetes and its consequences. Most of the morbidity and mortality is associated with Type 2, the prevalence rises to ~20% in developing countries (World Health Organisation (WHO) 2004).
In Australia, AusDiab data show 100 000 people develop diabetes annually (Cameron et al. 2003): 7.5% of people over 25 years and 16.8% of people over 65 have diabetes and a further 16.1% >65 have IGT. In addition, >200 000 progress from being overweight to obese, 3% of adults develop hypertension, and 1% reduced kidney function annually, and the average waist circumference increases by 2.1 cm, particularly in women. That is, a significant proportion of the population develop features of the metabolic syndrome with the associated increased risk of Type 2 diabetes and other associated conditions. This is associated with high health costs (Colaguiri et al. 2003; Australian Institute of Health and Welfare (AIHW) 2005).
In the UK, an estimated 1.4 million people have diabetes (Audit Commission 2000). In both countries Type 2 is the most common type, accounting for 80–90% of cases. There is wide variation in the incidence rates of newly diagnosed Type 1 diabetes in children in different populations. However, Type 1 in children and adolescents is increasing particularly in developed countries (EURODIAB 2000; The DIAMOND Project Group 2006; Soltesz et al. 2006).
Classification of diabetes
The American Diabetes Association (ADA) announced a revised diabetes classification system and diagnostic criteria in 1997. These revised data were a joint activity between the ADA and the WHO. As part of the new classification, the terms insulin-dependent diabetes (IDDM) and non-insulin-dependent diabetes (NIDDM) were replaced with Type 1 and Type 2 diabetes (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus 1997).
- Type 1 diabetes has two forms:
- Immune-mediated diabetes mellitus, which results from autoimmune destruction of the pancreatic beta cells leading to absolute insulin deficiency.
- Idiopathic diabetes mellitus refers to forms of the disease that have no known aetiologies.
- Immune-mediated diabetes mellitus, which results from autoimmune destruction of the pancreatic beta cells leading to absolute insulin deficiency.
- Type 2 diabetes mellitus refers to diseases associated with relative insulin deficiency as a result of progressive beta cell failure and insulin resistance.
- Impaired glucose homeostasis, which is an intermediate metabolic stage between normal glucose homeostasis and diabetes. It is a significant risk factor for cardiovascular disease and Type 2 diabetes. There are two forms:
(1) Impaired fasting glucose (IFG) where the fasting plasma glucose is higher than normal but lower than the diagnostic criteria.
(2) Impaired glucose tolerance (IGT) where the plasma glucose is higher than normal and lower than the diagnostic criteria after a 75 g glucose tolerance test. IFG and FPG often occur together and are associated with the metabolic syndrome.
- Impaired glucose homeostasis, which is an intermediate metabolic stage between normal glucose homeostasis and diabetes. It is a significant risk factor for cardiovascular disease and Type 2 diabetes. There are two forms:
- Gestational diabetes mellitus, which occurs during pregnancy.
- Other specific types, which include diabetes caused by other identifiable disease processes:
- Genetic defects of beta cell function such as MODY.
- Genetic defects of insulin action.
- Diseases of the exocrine pancreas such as cancer and pancreatitis.
- Endocrine disorders such as Cushing’s disease and acromegaly.
- Medicines, such as glucocorticoids and atypical antipsychotics have been associated with weight gain but the newest second-generation antipsychotic medications such as aripiprazole is weight neutral (Citrome et al. 2005). Possible causes of weight gain associated with medicines include food cravings and eating more, changed resting metabolic rate, changes in neurotransmitters and neuropeptides such as leptin, which regulate appetite, and weight loss before medicines are commenced (Zimmermann et al. 2003). Individuals with schizophrenia are generally more overweight than those without.
- Other specific types, which include diabetes caused by other identifiable disease processes:
- Chemical induced diabetes.
Overview of normal glucose homeostasis
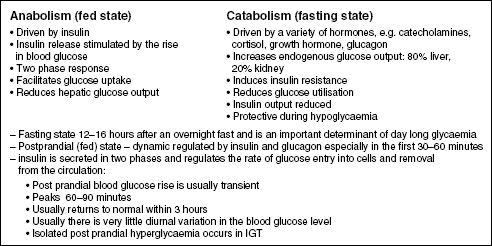
Blood glucose regulation (glucose homeostasis) relies on a delicate balance between the fed and fasting states and is dependent on several simultaneously operating variables including hormones, nutritional status, especially liver and muscle glucose stores, exercise, tissue sensitivity to insulin, and the type of food consumed. Figure 1.1 shows the key features of the fed and fasting states. Note that insulin release occurs in two phases. The first phase is important to control the post prandial blood glucose rise and is lost early in the progression to Type 2 diabetes. Insulin action is mediated via two protein pathways: Protein 13-kinase through insulin receptors and influences glucose uptake into the cells; and MAP-kinase, which stimulates growth and mitogenesis.
The metabolic syndrome
The metabolic syndrome consists of a cluster of risk factors for cardiovascular disease and Type 2 diabetes. Several researchers have explored the factors that predict diabetes risk including the Paris Prospective Study, Diabetes Epidemiology Collaborative Analysis of Diagnostic Criteria in Europe (DECODE), Epidemiology Study on the Insulin Resistance Syndrome (DESIR), and the European Group for the Study of Insulin
Figure 1.1 Overview of glucose homeostasis showing the key factors operating during the fed and fasting states. Usually the blood glucose is maintained within the normal range by the interplay of the anabolic and catabolic hormones, which are in turn influenced by other hormones and a number of factors such as nutritional status and intake.
The metabolic syndrome has been known as syndrome X, the deadly quartet, the awesome foursome, diabesity, and metabolic syndrome X. Currently, no agreed definition of the metabolic syndrome exists, particularly with respect to assessing risk or outcomes in children and adolescents. Four main definitions for adults have been described. They disagree about the predictive ability of the various metabolic features for cardiovascular disease and Type 2 diabetes due to ethnic, gender and age differences, which affect the syndrome components and may not identify groups at risk at lower waist circumference such as Asian people:
- World Health Organisation (1999).
- European Group for the Study of Insulin Resistance (2002).
- National Cholesterol Education Program – Adult Treatment Panel 111 (2001).
- International Diabetes Federation (IDF) (2005).
- The IDF Consensus Report on the Metabolic Syndrome in Children and Adolescents (2005).
Key features of the metabolic syndrome
- The metabolic syndrome appears to be a result of genetic predisposition and environmental factors, which include high saturated fat diets, inactivity, smoking, hormone imbalances contributing to metabolic stress, age and some medicines. These factors represent a cumulative risk and are largely modifiable.
- Central obesity – waist circumference: Europoids >94 cm in men and >80 cm in women. South Asian and South-East Asian men >90 cm, women >80 cm:(WHO 2004; Zimmet 2005) and childhood/adolescent Body Mass Index (BMI) 25–29 overweight, >30 obese.
- Raised serum triglycerides >1.7 mmol/L.
- Low serum HDL-c: <1.03 mmol/L males, <1.29 mmol/L women.
- Hypertension: systolic > 130 mmHg or diastolic >85 mmHg in women.
- IFG: >5.6 mmol/L or previously diagnosed diabetes (e.g. gestational diabetes (GDM). IFG is associated with a 20–30% chance of developing Type 2 diabetes within 5–10 years. The chance increases if FPG is also present.
Other key features include:
- Insulin resistance
- Hyperinsulinaemia, which occurs in the presence of insulin resistance and exaggerates the proliferative effects of the MAP-kinase pathway.
- Procoagulent state: elevated plasma fibrinogen and plasminogen activator inhibitor-1 (PAI-1).
- Vascular abnormalities: increased urinary albumin excretion and endothelial dysfunction, which affect vascular permeability and tone.
- Inflammatory markers such as cytokines, Interleukin, adhesion molecules, and TNF-alpha, which alter endothelial function. C-reactive protein is a significant predictor of cardiovascular disease and possibly depression, and there is an association among diabetes, cardiovascular diseases and depression. In fact some experts suggest depression could be an independent risk factor for Type 2 diabetes (Loyd et al. 1997) and accelerates the progression of coronary artery disease (Rubin 2002). Depression is associated with behaviours such as smoking, unhealthy eating, lack of exercise and high alcohol intake, which predisposes the individual to obesity and Type 2 diabetes. Peripheral cytokines induce cytokine production in the brain, which activates the hypothalamic–pituitary–adrenal axis and the stress response, which inhibits serotonin and leads to depression. Inflammation appears to be the common mediator among diabetes, cardiovascular disease and depression (Lesperance & Frasure-Smith 2007).
- Hyperuricaemia: More recently, liver enzymes such as sustained elevations of alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT), which are associated with non-alcoholc fatty liver disease and low adiponectin, have been associated with diabetes and cardiovascular disease. Therefore, the relationship is complex. Conversely, higher testosterone levels appear to be protective against diabetes in men but indicate greater risk in women and high oestradiol levels confer increased diabetes risk in both men and women (American Diabetes Association 2007 Preventing Diabetes).
Consequences of the metabolic syndrome include:
- A five-fold increased risk of Type 2 diabetes.
- A two to three fold increased risk of cardiovascular disease (myocardial events, stroke and peripheral vascular disease).
- Increased mortality, which is greater in men but women with Type 2 diabetes have a greater risk than non-diabetic women.
- Increased susceptibility to conditions such as:
- Gestational diabetes (GDM)
- Fetal malnutrition
- Polycystic Ovarian Syndrome
- Fatty liver
- Gallstones
- Asthma
- Sleep problems
- Some forms of cancer.
- Gestational diabetes (GDM)
The risk of developing cardiovascular disease and Type 2 diabetes increases significantly if three or more risk factors are present (Eckel et al. 2005).
The metabolic syndrome in children and adolescents
The prevalence of metabolic syndrome in children and adolescents is usually extrapolated from adult definitions and may not be accurate. However, it is vital that children and adolescents at risk of developing the metabolic syndrome be identified early. Future risk appears to be influenced in utero and early childhood by factors such as GDM, low birth weight, feeding habits in childhood, genetic predisposition and socio-economic factors (Burke et al. 2005). The IDF proposed that the metabolic syndrome should not be diagnosed before age 10 but children at risk should be closely monitored especially if there is a family history of metabolic syndrome, diabetes, dyslipidaemia, cardiovascular disease, hypertension and obesity, and preventative strategies implemented (Weiss et al. 2005; Zimmet et al. 2007).
In the 10–16-year-old age range diagnostic features are waist circumference >90th percentile, triglycerides >1.7 mmol/L, HDL-c >1.03 mmol/L, glucose >5.6 mmol/L (OGGT recommended), systolic blood pressure >130 mmHg and diastolic >85 mmHg. Adult criteria are recommended for adolescents over 16 years. The long-term impact on morbidity and mortality will emerge as young people with the metabolic syndrome become adults. However, heart disease may be apparent in children as young as 10 (Sinaiko 2006) and early onset of Type 2 diabetes in adolescents is associated with more rapid progression of complications than occurs in Type 1.
Management of the metabolic syndrome in children and adults consists of primary prevention through population-based strategies aimed at early detection, regular follow-up of at-risk individuals and personalised education. Secondary prevention concentrates on preventing the progression to diabetes and cardiovascular disease. Lasting effects demonstrating reduced cardiovascular and Type 2 diabetes risk has been demonstrated in studies such as the Diabetes Prevention Program (DPP), the Finnish Diabetes Prevention Study and the Da Quing IGT and Diabetes Study. These studies showed the importance of multidisciplinary team care, modifying lifestyle factors that contribute to obesity by improving diet and activity levels to reduce weight (10% body weight in the long term), and stop smoking. Some programmes include health coaching but the cost–benefit has not been demonstrated (Twigg et al. 2007). The transformational Model of Change is frequently used to implement preventative strategies.
Medicines might be required for secondary prevention for example, to control blood glucose and lipid lowering, antihypertensive, and weight management medicines in addition to lifestyle modification. Several medicines have been shown to reduce the incidence of diabetes in people with the metabolic syndrome. These include Metformin 850 mg BD, which showed a 31% risk reduction in the DPP; 100 mg of acarbose TDS by 25% after three years (STOP-NIDDM); and women with a history of GDM in the TRIPOD trial were less likely to develop diabetes when they were treated with troglitazone. Troglitazone was withdrawn from the market because of the tendency to cause liver disease. Other thiazolidinediones such as pioglitazone and rosiglitazone do not have the same adverse effects on the liver. Rosiglitazone reduced the risk of prediabetes progressing to diabetes by 60% over three years in the DREAM study. Orlistat, an intestinal lipase inhibitor taken TDS, reduced the risk of progression to diabetes in obese adults with metabolic syndrome by 37% over four years (XENDOS study). However, compliance with orlistat is low due to the side effects, see Chapter 5.
The macrovascular risk factors need to be managed proactively and screening programmer are imperative so abnormalities treated early, see Chapter 8. A 75 g OGGT may be performed initially to diagnose the metabolic syndrome and repeated after 12 months to determine whether glucose tolerance has changed, then the test interval can be increased to every two to three years (WHO 1999). However, if an individual demonstrates significant changes in weight gain, then OGGT may be performed earlier.
The Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes (2004) recommend monitoring people on antipsychotic medicines including:
- BMI at baseline and every visit for 6 months then quarterly and treat if weight increases by one BMI unit.
- Blood glucose and lipids at baseline and if weight increases by 7% and then annually; HbA1c 4 months after starting antipsychotic medicines and then annually in people with metabolic syndrome or diabetes risk factors.
Type 1 and Type 2 diabetes
Type 1 diabetes
Type 1 diabetes is a disease of absolute insulin deficiency that usually affects children and young adults but can occur in older people where it is known as latent autoimmune diabetes (LADA), see the following section.
The symptoms usually occur over a short space of time (two to three weeks) following a subclinical prodromal period of varying duration where the beta cells are destroyed. The precipitating event may have occurred many years prior to the development of the symptoms. Type 1 diabetes can be due to an autoimmune or idiopathic process. Various researchers have demonstrated that exogenous factors play a role in the development of Type 1 diabetes on the basis that <10% of susceptible people develop diabetes and <40% of monozygotic twins both develop diabetes, the >10-fold increase in the incidence of Type 1 diabetes in European Caucasians in the last 50 years, and migration studies that show the incidence of Type 1 has risen in people who migrated from low to high incidence regions (Knip et al. 2005). This is known as the trigger-bolster hypothesis. Seasonal variations in incidence of new diagnosis occur.
The EURODIAB substudy 2 Study Group researchers (1999) suggested low plasma 25-hydroxyvitamin D may be implicated in the development of Type 1 diabetes (1999). Later, Stene & Jones (2003) suggested there was no link between vitamin D supplementation and lower rates of Type 1 diabetes. More recently, a systematic review and meta-analysis of observational studies and a meta-analysis of cohort studies suggest vitamin D supplementation in early childhood might reduce the risk of Type 1 diabetes by 30% (Zipitis & Akoberng 2008). However, randomised controlled trials are required to clarify whether there is a causal link and the optimal dose, duration of treatment, and the best time to begin using vitamin D supplements.
A range of other environmental triggers has been implicated in the development of Type 1 such as potatoes, cow’s milk, and various viruses. Thus, the cause of Type 1 diabetes appears to be multifactorial due to a combination of genetic predisposition and a diabetogenic trigger that induces an immune response, which selectively destroys pancreatic beta cells. Islet cell antibodies (ICA), glutamic acid carboxylase (GAD), or tyrosine phosphatase (IA-2A) antibodies are present in 85% of cases.
Type 1 diabetes in children usually presents with the so-called classic symptoms of diabetes mellitus:
- Polyuria
- Polydipsia
- Lethargy
- Weight loss
- Hyperglycaemia
- Glycosuria
- Blood and urinary ketones.
In severe cases the patient will present with diabetic ketoacidosis (DKA) (see Chapter 7). Bed-wetting may be a consequence of hyperglycaemia in children. Classically, insulin secretion does not improve after treatment but tissue sensitivity to insulin usually does.
Figure 1.2 is a schematic representation of the progression of Type 1 diabetes. It shows the progressive relentless destruction of the beta cells from the time of the initial triggering event. Five to ten per cent of first-degree relatives of people with Type 1 diabetes have beta cell antibodies, usually with normal glucose tolerance, and some progress to diabetes.
Immunosuppression with Azathioprine or Cyclosporin, and immunomodulation using Nicotinamide to prevent further beta cell destruction has been used in newly diagnosed or pre-Type 1 diabetes but these treatments are uncommon. These medicines are potent immunosuppressive agents and their use cannot be warranted in the long term. Insulin has also been used in an attempt to stimulate immune tolerance, but not successfully.
Latent autoimmune diabetes (LADA)
LADA is a genetically linked autoimmune disorder that occurs in ~10% of people initially diagnosed with Type 2 diabetes. The prevalence varies among ethnic groups (www.actionlada.org). LADA has some features of both Types 1 and 2 diabetes. The UKPDS (1998) identified that one in 10 adults aged between 25 and 65 presumed to have Type 2 diabetes were GAD antibody positive, and these findings have been evident in other studies (Zinman et al. 2004). LADA often presents as Type 2 but has many of the genetic and immune features of Type 1 (see the previous section and Table 1.2).
Figure 1.2 Schematic representation of the slow progressive loss of beta cell mass following the initial trigger event in Type 1 diabetes.
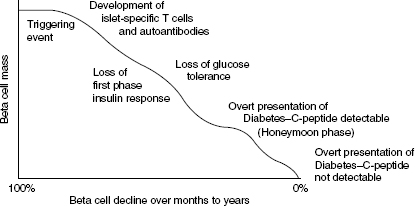
The progression to insulin dependency is usually rapid when GAD and IA-2A antibodies are present. The clinical features also resemble Type 1 in that people with LADA are not usually obese, are often symptomatic, and do not have a family history of Type 2 diabetes. A number of LADA trials are currently underway such as ACTIONLADA in Europe and the INIT 11 (LADA Trial) in Australia. LADA is also known as latent autoimmune diabetes of adulthood, late-onset autoimmune diabetes of adulthood, slow onset Type 1, and Type 1.5 (type one-and-a-half) diabetes.
Management includes:
- Testing non-obese people presenting with Type 2 diabetes for autoantibodies especially GAD, which has higher sensitivity to prevent episodes of ketoacidosis (Niskanen et al. 1995).
- Testing C-peptide levels in the blood to assess beta cell function. C-peptide is usually low in LADA indicating deficient insulin secretion, which predicts the need for insulin.
- Diet and exercise changes relevant to the individual.
- Avoiding metformin because of the potential risk of lactic acidosis in the presence of insulin deficiency.
- Introducing insulin early to support insulin secretion. Insulin is usually required within 4–6 years of diagnosis.
- Stress management and regular complication screening and mental health assessment (as per Types 1 and 2 diabetes).
- Appropriate education and support.
Type 2 diabetes
Type 2 diabetes is not ‘just a touch of sugar’ or ‘mild diabetes’. It is an insidious progressive disease that is often diagnosed late when complications are present. Therefore, population screening and education programs are essential. Type 2 diabetes often presents with an established long-term complication of diabetes such as neuropathy, cardiovascular disease, or retinopathy. Alternatively, diabetes may be diagnosed during another illness or on routine screening. The classic symptoms associated with Type 1 diabetes are often less obvious in Type 2 diabetes, however, once diabetes is diagnosed and treatment instituted, people often state they have more energy and are less thirsty. Other subtle signs of Type 2 diabetes especially in older people include recurrent candida infections, incontinence, constipation, symptoms of dehydration and cognitive changes. As indicated, insulin resistance often precedes Type 2 diabetes.
Insulin resistance is the term given to an impaired biological response to both endogenous and exogenous insulin that can be improved with weight loss and exercise. Insulin resistance is a stage in the development of impaired glucose tolerance. When insulin resistance is present, insulin production is increased (hyperinsulinaemia) to sustain normal glucose tolerance; however, the hepatic glucose output is not suppressed and fasting hyperglycaemia and decreased postprandial glucose utilisation results.
Insulin resistance is a result of a primary genetic defect and secondary environmental factors (Turner & Clapham 1998). When intracellular glucose is high, free fatty acids (FFAs) are stored. When it is low FFAs enter the circulation as substrates for glucose production. Insulin normally promotes tryglyceride synthesis and inhibits postprandial lipolysis. Glucose uptake into adipocytes is impaired in the metabolic syndrome and Type 2 diabetes and circulating FFAs as well as hyperglycaemia have a harmful effect on hepatic glucose production and insulin sensitivity. Eventually the beta cells do not respond to glucose and this is referred to as glucose toxicity. Loss of beta cell function is present in over 50% of people with Type 2 diabetes at diagnosis (United Kingdom Prospective Study (UKPDS) 1998) (Figure 1.2). Figure 1.3 depicts the consequences of insulin resistance.
Insulin is secreted in two phases: the first phase plays a role in limiting the post prandial rise in blood glucose. The first phase is diminished or lost in Type 2 diabetes leading to elevated post prandial blood glucose levels (Dornhorst 2001). Postprandial hyperglycaemia contributes to the development of atherosclerosis, hypertriglyceridaemia and coagulant activity, endothelial dysfunction, and hypertension, which add to the effects of chronic hyperglycaemia and contribute to long-term diabetes complications (Ceriello 2003).
Figure 1.3 Consequences of the insulin resistance syndrome.
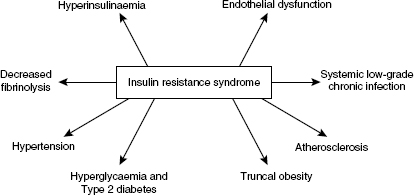
Interestingly, the beta cells do respond to other secretagogues, in particular sulphonylurea medicines.
The net effects of these abnormalities is sustained hyperglycaemia as a result of:
- impaired glucose utilisation (IGT)
- reduced glucose storage as glycogen
- impaired suppression of glucose-mediated hepatic glucose production
- high fasting glucose (FPG)
- reduced postprandial glucose utilisation.
People most at risk of developing Type 2 diabetes:
- have the metabolic syndrome,
- are overweight: abdominal obesity, increased body mass index (BMI), and high waist-hip ratio (>1.0 in men and >0.7 in women). The limitations of the waist circumference in some ethnic groups are outlined later in the chapter. Elevated FFAs inhibit insulin signalling and glucose transport (see Figure 1.4) and are a source of metabolic fuel for the heart and liver. Binge eating precedes Type 2 diabetes in many people and could be one of the causes of obesity; however, the prevalence of eating disorders is similar in Type 1 and Type 2 diabetes (Herpertz et al. 1998):
- are over 40 years of age, but note the increasing prevalence in younger people (see also Chapter 13)
- are closely related to people with diabetes,
- are women who had gestational diabetes or who had large babies in previous pregnancies.
Other associated risk factors have already been described. In addition, active and former smoking and acanthosis nigricans are associated with hyperinsulinaemia (Kong et al. 2007). Baseline and hypertension progression are independent predictors (Conen et al. 2007). Recent research suggests insulin lack might be partly due to the enzyme PK Cepsilon (PKCe), which is activated by fat and reduces the production of insulin. Future medicines may target this deficiency and restore normal insulin function (Biden 2007). The characteristics of Type 1 and Type 2 diabetes are shown in Table 1.1.
The majority of people with Type 2 diabetes require multiple therapies to achieve and maintain acceptable blood glucose and lipid targets over the first nine years after diagnosis (UKPDS 1998). Between 50% and 70% require insulin, which is often used in combination with OHAs. This means diabetes management progressively becomes more complicated for people with Type 2 diabetes, often coinciding with increasing age when their ability to manage may be compromised, which increases the likelihood of non-compliance and the costs of managing the disease for both the patient and the health system.
Gestational diabetes
Gestational diabetes is the most common medical complication of diabetes accounting for 0–95% (Menato et al. 2008). Diabetes occurring during pregnancy is referred to as gestational diabetes (GDM). GDM describes carbohydrate intolerance of varying degrees that first occurs or is first recognised during pregnancy, generally indicated by fasting blood glucose >6 mmol/L. GDM occurs in 1–14% of pregnancies. The exact cause of gestational diabetes is unknown, but several factors have been implicated including diet and lifestyle, smoking, some medicines, older age, genetic background, ethnicity, number of previous pregnancies and recently, short stature (Langer 2006). For more information on GDM refer to Chapter 14.
Figure 1.4 Diagrammatic representation of insulin binding, insulin signalling, translocation of GLUT-4 and glucose entry into the cell. GLUT-4 is a glucose transporter contained in vesicles in the cell cytoplasm. Once insulin binds to an insulin receptor GLUT-4 moves to the cell membrane and transports glucose into the cell. During fasting GLUT-4 is low and increases in response to the increase in insulin. Failure of GLUT-4 translocation could explain some of the insulin resistance associated with Type 2 diabetes. The effects of insulin are mediated by two protein pathways: P13-kinase through the insulin receptors (glucose uptake) and MAP-kinase, which stimulates growth and mitogenesis.
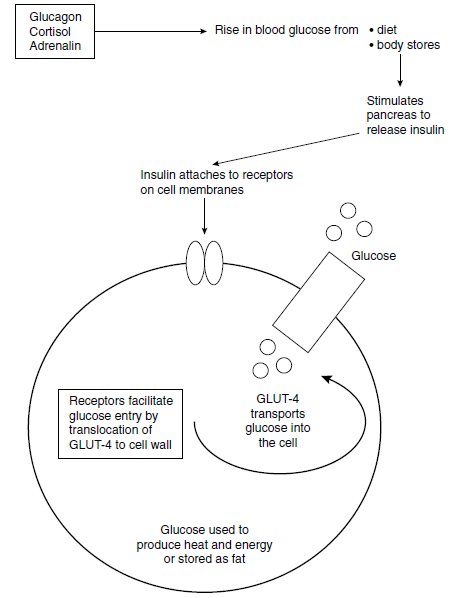
Table 1.1 Characteristics of Type 1 and Type 2 diabetes mellitus.
| Type 1 | Type 2 | |
| Age at onset | Usually <30 yearsa | Usually >40 yearsb |
| Speed of onset | Usually rapid | Usually gradual and insidious |
| Body weight | Normal or underweight; often recent weight loss | 80% are overweight |
| Heredity | Associated with specific human leukocyte antigen (HLA-DR3 or 4) | No HLA association Genetic predisposition |
| Autoimmune disease and environmental triggers | Environmental and lifestyle factors contribute | |
| Insulin | Early insulin secretion Impaired later; may be totally absent | Often preceded by the metabolic syndrome (see section on ‘The metabolic syndrome’). |
| Insulin resistance is reversible if appropriate diet and exercise regimens are instituted | Type 2 is associated with slow, progressive loss of beta cell function | |
| Ketosis | Common | Rare |
| Symptoms | Usually present | Often absent, especially in the early stages |
| Acanthosis nigricans is common | ||
| Frequency | ~15% of diagnosed cases | ~85% of diagnosed cases |
| Complications | Common but not usually present at diagnosis | Common, often present at diagnosis |
| Treatment | Insulin, diet, exercise, stress management, regular health and complication assessment | Diet, OHA, exercise, insulin, stress management, regular health and complication assessment |
aIncreasing incidence of the metabolic syndrome and Type 2 diabetes in children and adolescents.
bOccurs in older people; see LADA.
Malnutrition-related (tropical) diabetes
Childhood malnutrition, genetic predisposition and environmental factors are implicated in the development of diabetes in people living in tropical countries. It is not included as a specific category in the revised classification but could fit into the ‘other’ category. ‘Tropical diabetes’ or malnutrition-related diabetes differs from Type 1 diabetes because ketoacidosis is rare, and from Type 2 diabetes because it often occurs in young, thin people with no family history of diabetes. However, researchers have not yet agreed about the underlying causal mechanisms.
Maturity onset diabetes of the young (MODY)
Maturity onset diabetes of the young is a rare group of diabetes disorders, formerly also called Mason-type diabetes and non-insulin-dependent diabetes of the young (NIDDY). MODY can develop at any age up to 55 in 2–2% of people with non-Type 1 diabetes. It has a genetic basis and there are at least six varieties in many ethnic groups (Dean & McEntyre 2004). MODY can be distinguished from Type 1 diabetes by the absence of GAD antibodies and ketosis and from Type 2 because MODY is caused by a single gene and Type 2 is caused by minor problems in several genes that occur simultaneously (Froguel & Velho 1999). Likewise, MODY people often do not have insulin resistance. The age at onset in at least one genetic type depends on which parent carries the mutant gene: it occurs at a younger age if the mother passed it on especially if she had GDM. Women with MODY are often diagnosed during pregnancy. The varieties of MODY are shown in Table 1.2.
Treatment is with oral hypoglycaemic agents, diet and exercise, although insulin may eventually be required. Interestingly, Ehilishan et al. (2004) suggested diabetes diagnosed in children younger than six years is likely to be a genetic defect rather than Type 1 diabetes. If that is the case, there are implications for diabetes education and management.
Recognition can be difficult and the diagnosis is often missed (Appleton & Hattersley 1996). This can have implications for the individual and their family in commencing appropriate treatment for the specific type of MODY and genetic counselling.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree