Medical Nutrition Therapy
Karen Hanson Chalmers
A BRIEF HISTORY OF NUTRITION AND DIABETES
More than 50 years ago, Elliott Joslin stated:
The diet in health is made up chiefly of carbohydrate; the diet in diabetes before the discovery of insulin was made up chiefly of fat. Insulin has changed all this. The task of the modern diabetic is not so much to learn how to live comfortably upon less carbohydrate and more fat, but rather to balance the carbohydrate in his diet with insulin so that he can utilize it and thus keep his urine sugar-free. (1)
Little did Dr. Joslin realize that his guidelines would still hold true at the beginning of the 21st century.
Patients have identified “diet” as one of the most challenging aspects of their diabetes regimen. However, countless events have resulted in positive changes in nutrition science as it relates to diabetes. From the rigidly controlled, semistarvation diets in ancient times to the present “all-foods-can-fit,” we have arrived at nutrition science as we know it today—medical nutrition therapy.
One of the earliest references to a so-called diabetic diet was noted in a medical writing as far back as 1550 B.C., although for the first 3,500 years of diabetes history, no clear distinction was
made between type 1 and type 2 diabetes. The writings recommended a diet high in carbohydrates, which included fruit, wheat grain, and sweet beer to stop the passing of too much urine. Aretaeus, a follower of Hippocrates, first used the name diabetes in the first century A.D. to describe the “melting down of flesh and limbs into the urine” (2). He concluded that diabetes was a disease of the stomach and should be treated with milk, gruel, cereals, fruits, and sweet wines. Milk, water, wine, and beer were used as the main fluids to relieve excessive thirst until the second century A.D., when diabetes was thought to be a disease of the kidneys. At this time, restriction of fluids was recommended. By the sixth century, diabetes was thought to be directly caused by overeating and thought to be a disease of “sweet urine” (3).
made between type 1 and type 2 diabetes. The writings recommended a diet high in carbohydrates, which included fruit, wheat grain, and sweet beer to stop the passing of too much urine. Aretaeus, a follower of Hippocrates, first used the name diabetes in the first century A.D. to describe the “melting down of flesh and limbs into the urine” (2). He concluded that diabetes was a disease of the stomach and should be treated with milk, gruel, cereals, fruits, and sweet wines. Milk, water, wine, and beer were used as the main fluids to relieve excessive thirst until the second century A.D., when diabetes was thought to be a disease of the kidneys. At this time, restriction of fluids was recommended. By the sixth century, diabetes was thought to be directly caused by overeating and thought to be a disease of “sweet urine” (3).
Little information about diabetes and food is mentioned again until the late 1600s, when the suggested diabetic diet returned to foods higher in carbohydrates, such as milk, bread, and barley water. Also, at this time, opium was introduced as a staple of the diet to decrease appetite, as physicians recognized that those people with diabetes who ate the least food lived the longest.
In the late 1700s, a French physician began prescribing undernutrition or semistarvation diets, interspersed with frequent periods of fasting, for those with diabetes. Finally, a physician from England related carbohydrates to high glucose levels and excessive urination. Again, the diet reverted back to low carbohydrates and included more fat and protein in the form of milk, butter, suet, and rancid meats. Low-carbohydrate vegetables were added only when the urine became completely sugar free. Although fat in the diabetic diet was further liberalized to add enough calories for survival, most people with diabetes could not adhere to the extremely limited quantity of food. Those people with diabetes who were “thin” did not survive, whereas those people with diabetes who were “fat” improved.
In the early 1900s, Dr. Frederick M. Allen, in the United States, developed his starvation diet and was one of the first to tailor foods in diets to his patients’ preferences while still providing only 1,000 calories a day. Although many of his patients were malnourished, he is credited with helping many survive before the introduction of insulin therapy in 1921 (3).
The starvation diet disappeared with the discovery of insulin. The prescribed diabetic diet was still 4% carbohydrate, 21% protein, and 75% fat—all measured and weighed to exact amounts. Although no consistent food lists were available at the time, the carbohydrate foods were categorized according to the amount of carbohydrate each food contained, e.g., 5%, 10%, and 20% vegetables. Vegetables were cooked three times, the water changed each time to remove as much carbohydrate as possible. Even after insulin was initiated, Dr. Elliott P. Joslin continued to use a high-fat, carbohydrate-restricted diet for his “severe diabetics” and noted in his first preinsulin Diabetic Manual that “olive oil forms an excellent lunch for a diabetic” (1). Dr. Joslin gradually increased his diet for “mild diabetics” to 23% carbohydrate, 15% protein, and 62% fat.
In the 1940s, it became evident that there was a great need to develop consistent and standardized food values and to design a simple method for planning the diabetic diet. The American Dietetic Association, the American Diabetes Association, and the diabetes branch of the U.S. Public Health Service developed a method that became what we know today as the exchange system. Six “convenient” sample meal plans were developed that detailed the grams of carbohydrate, protein, and fat for various calorie amounts. These plans were developed to be used by the health professional, who would modify the plan to suit the particular needs of the patient. Although the meal plans were not meant to be distributed to patients, they soon became widely available.
In the early 1970s, the sample meal plans were being used extensively. These preplanned diets did not focus on nutrition education or on follow-up by a registered dietitian. Patients quickly lost their motivation to adhere to these diets, and their interest in nutrition quickly diminished. A 1973 publication by Kelly West, Diet Therapy of Diabetes: An Analysis of Failure, examined deterrents to successful diet therapy, which included lack of physician support in promoting adequate nutrition care and of third-party reimbursement for dietary instruction for outpatients (4).
In the 1970s, the high-carbohydrate diet was rediscovered (5,6), and the officially recommended diet was higher in complex carbohydrate and lower in fat and protein than most of the earlier diets. The attitude toward sucrose and other sugars became more liberal. The American Diabetes Association began emphasizing the need for nutrition education about the exchange system. This eventually led to the publication in 1971 of the Association’s Principles of Nutrition and Dietary Recommendations (7). The exchange lists for meal planning were revised in 1976, 1986, and again in 1995; each revision placed an increasing emphasis on carbohydrates. These changes reflect a move from the main focus on the lethal effects of short-term complications (ketoacidosis and hypoglycemia) to a new focus: concern about long-term complications.
The exchange system continued to be taught and used and was perceived as the diabetic diet until the 1980s. However, surveys from dietitians who provided nutrition education for patients with diabetes identified a need for a variety of meal-planning methods to be used in addition to the exchange system. In 1987, the Diabetes Care and Education Practice Group of the American Dietetic Association promoted the concept of the individualization of the meal planning approach to diabetes nutritional care with new nutrition resources for diabetes meal planning (8). More and more people with diabetes were seeking a dietitian’s care and counseling for updating their methods of meal planning.
Current medical nutrition therapy recommendations for overall health of persons with diabetes are the same as those for all healthy Americans, as seen in the U.S. Dietary Guidelines 2000 (9). These guidelines include the following:
Aim for a healthy weight
Choose a variety of grains daily, especially whole grains
Choose a variety of fruits and vegetables daily
Choose a diet that is low in saturated fat and cholesterol and moderate in total fat
Choose beverages and foods to moderate your intake of sugars
Choose and prepare food with less salt
If you drink alcoholic beverages, do so in moderation.
The volume of research on issues of nutrition and diabetes and the number of changes in nutrition recommendations over recent years are now greater than ever. The evolution of new knowledge is rapidly affecting the management of diet for the patient with diabetes. Therefore, the 2000 Dietary Guidelines, which are published every 5 years by the Department of Health and Human Services (HHS) and the Department of Agriculture (USDA), are currently in the process of review in light of recent scientific evidence to determine if revision is needed in the 2005 sixth edition. The USDA’s Food Guide Pyramid, which was introduced in 1992, is also under review. Prominent experts in nutrition and health are now proposing a revised food guide pyramid that clearly emphasizes the benefits of healthy fats, daily exercise, and avoidance of excessive intake of calories. Recommendations promote high fiber, healthy and unprocessed carbohydrates, as well as adequate consumption of fresh fruits
and vegetables. Healthy sources of protein are encouraged, such as nuts, legumes, fish, poultry, and eggs, whereas red meat, butter, refined grains, potatoes, and sugar are minimized.
and vegetables. Healthy sources of protein are encouraged, such as nuts, legumes, fish, poultry, and eggs, whereas red meat, butter, refined grains, potatoes, and sugar are minimized.
The availability of a greater variety in the types of oral antidiabetes agents and insulins, together with the availability of technology from self-monitoring of blood glucose to continuous blood glucose sensors and insulin pump therapy, has allowed increased flexibility in meal planning. A continued research focus on the glycemic response of the nutrients—carbohydrate, protein, and fat—is gradually changing how we think about diet in diabetes.
The dietary management of type 2 diabetes is now recognized to be quite different from that of type 1 diabetes. Our knowledge about the treatment of obesity in diabetes also has increased. In addition, attention is being given to the special considerations required among different subgroups of people with diabetes, specifically the needs of ethnic minority groups, pregnant women, growing children and adolescents, and the elderly. Emphasis is placed on providing individualized, flexible meal plans that people are willing and able to follow. Dramatic changes in our methods of diabetes education have also been instituted in recent years. New strategies, knowledge, and techniques for teaching and improving the overall management of diabetes, as well as dietary management, were clearly demonstrated in the 1993 published results of the Diabetes Control and Complications Trial (DCCT) for those with type 1 diabetes (10) and the smaller Stockholm Diabetes Intervention Study (11) of similar design. These studies recognized the importance of a coordinated team approach to the achievement of nutrition goals. The diabetes team used in the DCCT consisted of the patient and family as the primary participants, a diabetes nurse educator, a registered dietitian, a behaviorist, and the diabetologist. Today, exercise physiologists also have been included as important members of the diabetes team. The DCCT also provided specific information about important nutrition intervention strategies, based firmly on scientific evidence. In 1994, shortly after publication of the DCCT clinical findings, the American Diabetes Association published a revised set of nutrition guidelines, refocusing on an “individualized approach to nutrition self-management that is appropriate for the personal life-style and diabetes management goals of the individual with diabetes” (12).
The results of another 20-year landmark study, the United Kingdom Prospective Diabetes Study (UKPDS) (13), for those with type 2 diabetes, were published in 1998 and further confirmed that the intensive use of pharmacologic therapy, together with diet and exercise, would have clinical benefits. In 1996, recruitment began for a more recent randomized clinical trial, The Diabetes Prevention Program (DPP) (14). The DPP was designed to test strategies to prevent or delay glucose concentrations and impaired glucose tolerance (IGT). This major clinical trial compared intensive lifestyle intervention (diet and exercise) with metformin treatment in 3,234 people with impaired glucose tolerance. Lifestyle intervention worked as well in men and women and in all ethnic groups, reducing the risk of getting type 2 diabetes by 58%. It also worked well in people age 60 and older, reducing their development of diabetes by 71%. Metformin was also effective in men and women and in all ethnic groups, reducing their risk of getting type 2 diabetes by 31%, but not as effective in the older volunteers and in those who were less overweight. The trial ended a year early because the data had clearly answered the main research questions. This is the first major trial to show that diet and exercise can prevent or delay diabetes in a diverse American population of overweight people with IGT and a major step toward reversing the epidemic of type 2 diabetes in the United States. A recently launched trial, the Look AHEAD (Action for Health in Diabetes) study, will examine how diet and exercise affect heart attack, stroke, and cardiovascular-disease-related death in people with type 2 diabetes.
Diet still remains the cornerstone of diabetes self-management, but additional research is needed to improve the contribution that diet can make for effective diabetes self-management.
GOALS OF MEDICAL NUTRITION THERAPY
Today, there is no one “diabetic diet.” The current nutrition recommendations can be defined simply as “a nutrition prescription based on assessment and treatment goals and outcomes” (15). The 1994 American Diabetes Association nutrition recommendations redistributed the calories provided from the various macronutrients (12), and these recommendations still hold true today. Table 36.1 gives a historical perspective of nutrition recommendations. The American Diabetes Association recommends that the distribution of calories from carbohydrate and fat be based on nutritional assessment and on blood glucose, weight, and lipid goals, with continued emphasis on a diet with fewer than 10% of calories from saturated fats and with 10% to 20% of calories from protein (lean).
TABLE 36.1. Historical Perspective of Nutrition Recommendations for People with Diabetes | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
The goals of medical nutrition therapy are summarized in Table 36.2, which outlines the most recent recommendations of the American Diabetes Association (15) and are discussed in more detail below.
TABLE 36.2. Goals of Medical Nutrition Therapy | ||||||
|---|---|---|---|---|---|---|
|
Goals in Type 1 Diabetes
The primary goals of therapy for persons with type 1 diabetes are as follows:
Provision of an individualized meal plan based on usual food intake and lifestyle. This plan is used as the basis for integrating insulin therapy into the usual eating and exercise patterns.
Consistency of carbohydrate intake to allow the synchronization of mealtimes with times of insulin action for persons receiving fixed insulin regimens.
Determination of premeal insulin dose and postprandial blood glucose response by monitoring of blood glucose levels and adjusting insulin doses for the amount of total carbohydrate consumed for persons receiving intensive insulin therapy.
Prevention of weight gain is desirable, with attention therefore paid to total caloric intake from carbohydrate, protein, and fat, for persons with improved glycemic control.
Adjustment of rapid- or short-acting insulin for deviations from usual eating and exercise habits is the preferred choice for prevention of hypoglycemia. Additional carbohydrate may be needed for unplanned exercise.
Goals in Type 2 Diabetes
The primary goals of therapy for persons with type 2 diabetes depend on body weight and level of glucose control.
Emphasis on lifestyle changes that result in reduced caloric intake and increased energy expenditure through physical activity for those who are overweight and insulin resistant
Achievement and maintenance of glucose, lipid, and blood pressure goals by reduction in dietary intake of carbohydrate, saturated fat, cholesterol, and sodium when necessary
Maintenance of moderate caloric restriction and a nutritionally adequate meal plan with a reduction of carbohydrate and total fat—especially saturated fat—together with an increase in exercise for those with excessive weight
Increase of activity and exercise to improve glycemia, decrease insulin resistance, and reduce cardiovascular risk factors
CALORIES
The diet of the diabetic patient should contain the minimum number of calories which the normal individual would require under similar conditions. If the patient is allowed more than the minimum amount of food there is far more likelihood that a portion will be lost, unassimilated, and appear as sugar in the urine. (1)
Prescribing enough calories (kilocalories, or kcals) to achieve and maintain a desirable weight should be carefully considered. Caloric requirements for persons with diabetes are not different from those for persons without diabetes, assuming the person with diabetes is not losing calories through glycosuria. Caloric needs vary with a patient’s weight, age, gender, activity level, and genetic background. The recommended calorie level is based on the weight that the patient and his or her healthcare provider acknowledge as one that can be achieved and maintained, for both the short term and the long term. This may not be the same as ideal body weight.
Persons with type 1 diabetes are often thin when the diagnosis is first made, and the diet should include enough calories to ensure normal growth and development and to sustain the usual level of physical activity. For infants, children, and adolescents, caloric intake should maintain consistent growth curves based on energy needs during periods of growth and
sexual maturation. The prescribed calories must be adjusted on a regular basis. If diabetes is not properly controlled, growth may be retarded and the height potential may not be reached. Any abnormal or unexplained deviation in growth and weight demands an assessment of diabetes control, eating patterns, and caloric intake, as well as insulin dosage.
sexual maturation. The prescribed calories must be adjusted on a regular basis. If diabetes is not properly controlled, growth may be retarded and the height potential may not be reached. Any abnormal or unexplained deviation in growth and weight demands an assessment of diabetes control, eating patterns, and caloric intake, as well as insulin dosage.
Persons with type 2 diabetes are often overweight when the diagnosis is first made. A moderate weight loss (5 to 9 kg), irrespective of the patient’s starting weight, is perhaps the most important aspect of medical nutrition therapy and is associated with an improvement in lipids, blood glucose, and blood pressure (15,16,17,18,19). Weight loss leads to a reduction in insulin resistance and has long-term effects on the maintenance of reduced blood glucose levels. Overweight and obese patients with type 2 diabetes should be encouraged to attain a reasonable weight rather than attempting to achieve the traditionally defined desirable or ideal body weight. Setting intermediate weight goals may be useful when a patient becomes overwhelmed by the magnitude of his or her necessary weight loss. Weight management also should include behavioral modification to encourage healthy eating behaviors, together with increased physical activity. A moderate reduction in calories of approximately 250 to 500 kcal per day less than the average daily intake (calculated from a food history) can result in losses of 2 to 4 kg per month, a rate that is considered excellent.
Several different means of estimating desirable body weight and caloric needs are included in Table 36.3. The recommended calorie level need not be absolutely precise but should be considered a starting point for fine-tuning during follow-up until a level has been established that will help the patient achieve his or her goals for weight and blood glucose levels.
TABLE 36.3. Estimating Caloric Intake and Desirable BodyWeight for Adults | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
There is evidence to support the use of body mass index (BMI) (Table 36.4) in risk assessment for the diagnosis of diabetes or response to weight loss. The BMI provides a more accurate
measure of total body fat than does assessment of body weight alone. Measurement of waist circumference (20) is particularly helpful when patients are categorized as normal or overweight. Men whose waist circumference is greater than 40 inches and women whose waist circumference is greater than 35 inches are at high risk of diabetes, dyslipidemia, hypertension, and cardiovascular disease because of excess abdominal fat. The relationship between BMI and waist circumference for defining risk is shown in Table 36.5.
measure of total body fat than does assessment of body weight alone. Measurement of waist circumference (20) is particularly helpful when patients are categorized as normal or overweight. Men whose waist circumference is greater than 40 inches and women whose waist circumference is greater than 35 inches are at high risk of diabetes, dyslipidemia, hypertension, and cardiovascular disease because of excess abdominal fat. The relationship between BMI and waist circumference for defining risk is shown in Table 36.5.
TABLE 36.4. Body Mass Index Table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 36.5. Classification of Overweight and Obesity by Body Mass Index, Waist Circumference, and Associated Disease Risk | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CARBOHYDRATE
The diet of the normal and diabetic individuals differs very little these days, chiefly because of the discovery of insulin. At one time my hospital patients did not have over 30 grams of carbohydrates per day, or the equivalent of about one ounce or two tablespoons of sugar. Today no patient has less than 150 grams of carbohydrate or the equivalent of 10 tablespoonfuls of sugar or 10 slices of bread. (1)
Calories from carbohydrate are variable and should be individualized on the basis of nutritional assessment, diabetes treatment goals, other medical issues, and the patient’s eating habits and response of blood glucose to carbohydrate intake.
If protein contributes 10% to 20% of calories, carbohydrate and fat can be distributed between the remaining 80% and 90% of calories, after the blood glucose and lipid levels are taken into account (21). Although carbohydrate foods vary in the ability to promote good health, the total amount of carbohydrate consumed is more important than the source or the type of carbohydrate. Even sucrose-containing foods may be substituted for other carbohydrate grams and are acceptable as long as these foods are within the context of a healthy meal plan and metabolic control and desirable body weight are maintained.
A nutrition prescription (based on nutritional assessment and treatment goals) may result in a reduction in carbohydrate and dietary fat—particularly saturated fat—which in turn reduces cardiovascular risk. Although there are supportive studies indicating that high-carbohydrate meal plans improve glucose tolerance and insulin sensitivity (21,22,23,24), there is still some disagreement about what constitutes the optimal percentage of carbohydrate for persons with diabetes (25,26). Although some researchers are concerned about high-carbohydrate diets and their potential for influencing glucose and lipid metabolism and blood pressure (27,28,29,30,31,32), others have demonstrated that the limited elevation in lipids can be prevented if carbohydrate and fiber in the diet are increased in parallel (33,34,35). Joslin Diabetes Center has recently updated nutrition recommendations for people with type 2 diabetes who are overweight and obese. Although additional research will be required to define the maximum nutrients that promote both blood glucose control and weight loss success, we currently assess each patient individually to determine an appropriate meal plan composition based on reducing calories to achieve a negative caloric balance and using the guidelines below:
Carbohydrates
No more than 40% of total daily calorie intake should come from carbohydrates, which should be mainly low-glycemic-index foods such as vegetables, fruits, and whole and minimally processed grains. Refined carbohydrates or processed grains and starchy food such as pasta, bread, cereal, and white potatoes should be avoided or consumed in limited quantities.
Protein
To maintain muscle mass and energy expenditure, 30% of total daily calorie intake should come from protein. Preferable protein sources include fish, particularly cold water fish such as salmon, tuna, or sardines, and chicken, turkey, and other poultry and soy, rather than red or processed meat. Furthermore, data suggest that protein aids in the sensation of fullness and that low-protein meal plans are associated with increased hunger. Thus, protein may serve to reduce appetite and assist one in achieving and maintaining the desired lower calorie level.
Fat
Of total daily calorie intake 30% should come from fat, which should be mainly derived from monounsaturated and polyunsaturated fat (e.g., nuts, olive oil, canola oil) and fish, particularly those high in omega-3 fatty acids. Meats high in saturated fat, including beef, pork, lamb, and high-fat dairy products, should be consumed only in small amounts. Similarly, foods high in trans-fatty acids should be avoided.
The above distribution of nutrients for overweight and obese people with type 2 diabetes will
Promote long-term weight loss in those with type 2 diabetes. The combination of restricting calories while increasing the intake of protein and low-glycemic-index foods may diminish the sensation of hunger.
Improve blood glucose control because of the relatively lower carbohydrate content and the lower glycemic index of those carbohydrates that are consumed.
Improve the body’s response to insulin (insulin sensitivity), whether or not weight loss is achieved.
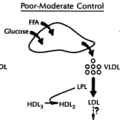
Improve blood lipid profile, particularly triglycerides and high-density lipoprotein (HDL) cholesterol, whether or not weight loss is achieved.
Reduce the stress on the insulin-producing cells in the pancreas by reducing the need for as much insulin.
It is also important to recognize that a meal plan prescription alone is not sufficient to maximize significant and sustained weight loss. Physical activity, behavior modification, and good support systems are extremely important adjuncts to the dietary prescription described above.
Glycemic Index
All complex carbohydrates and all simple carbohydrates (or sugars) traditionally were thought to generate different blood glucose responses based on molecular structure. However, an inconsistent relationship of glucose response demonstrated from so-called simple and complex carbohydrates suggests this terminology may be misleading and restrictive in meal planning. The position of the American Diabetes Association is that priority should be given to the total amount rather than the source of carbohydrate. Controversy is ongoing about whether the ingestion of similar amounts of carbohydrate foods produces different blood glucose and insulin responses and whether this information will have useful clinical applications.
In 1981, Jenkins et al. (36) suggested that the blood glucose and insulin response to a food could be expressed as a glycemic index, which quantifies the postprandial glucose response to a particular food in comparison to the response to a standard amount of glucose, or an attempt to classify foods by the extent to which they raise the blood glucose level. Glucose alone produces the largest increase in blood glucose level and is assigned a glycemic index of 100. Fiber-rich foods, acidic foods, and high-fat foods often have low glycemic indexes. The glycemic index of sucrose, a disaccharide made up of glucose and fructose, is lower than that of some starches, such as potatoes, because sucrose contains less pure glucose.
Those in favor of replacing foods having a high glycemic index with foods having a low glycemic index refer to studies demonstrating the value of the glycemic index in the dietary management of diabetes (37,38,39,40,41,42,43,44,45,46). However, others find that the study results are not consistent or predictable and not clinically useful (47,48,49,50). Another controversy exists over the impact of meals with a high glycemic index versus meals with a low glycemic index on weight loss and satiety. Ludwig (41) suggests that the ingestion of low-glycemic-index foods typically induces higher satiety than does the ingestion of high-glycemic-index foods and is followed by the intake of fewer calories at subsequent meals. It is theorized that slower rates of digestion and absorption of low-glycemic-index foods stimulate nutrient receptors in the gastrointestinal tract for a longer period, resulting in a longer period of feedback to the satiety center in the brain. Brand-Miller et al. (42) suggest that high-glycemic-index meals dictate differences in satiety and caloric intake because of the faster digestion and absorption and high insulin responses, and that they expand fat stores. Pi-Sunyer (49) takes the position that the data are not yet sufficient and that most of the data relating high-glycemic-index meals to increased food intake were collected in single-meal experimental designs. Although the weight-loss benefit of a low-glycemic-index diet compared with a high-glycemic-index diet is still only a hypothesis, it may be helpful to use the glycemic index as an additional weight-loss tool. A low-glycemic-index diet consisting of vegetables, fruits, and legumes, a moderate amount of protein and unsaturated fats, and fewer refined carbohydrate foods—along with overall decreased calorie intake and increased physical activity—will assist patients in their weight-loss efforts (40). As more long-term research trials on glycemic response to single foods and complete meals are conducted, the usefulness of this concept as a teaching tool is increasing (Table 36.6). However, the glycemic response to food depends not only on the amount and type of carbohydrate but also on other variables (Table 36.7), which could impact the glycemic effect of the carbohydrate food eaten. To further determine the impact of carbohydrate foods on blood glucose levels, researchers have come up with a way to describe the extent to which the blood glucose rises (GI) and remains high. This is called the glycemic load (GL). The GL provides a measure of the level of glucose in the blood, but also
the insulin demand produced by a normal serving of the food. The GL considers a food’s GI as well as the amount of carbohydrate per serving and gives a more detailed picture.
the insulin demand produced by a normal serving of the food. The GL considers a food’s GI as well as the amount of carbohydrate per serving and gives a more detailed picture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree