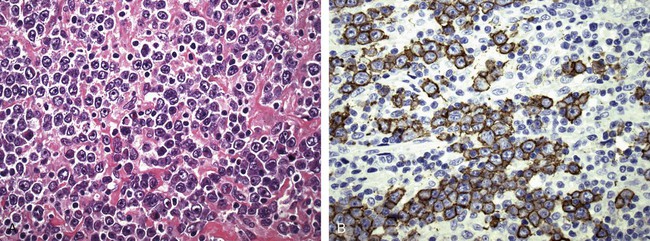
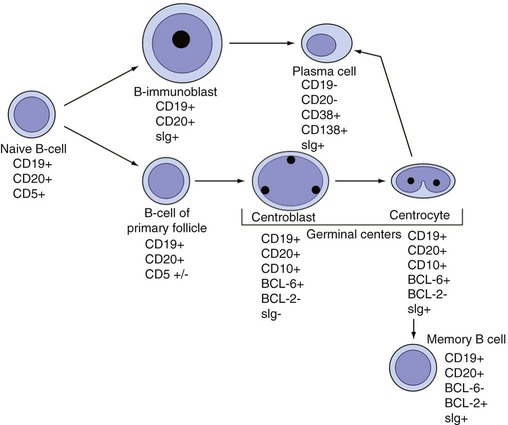
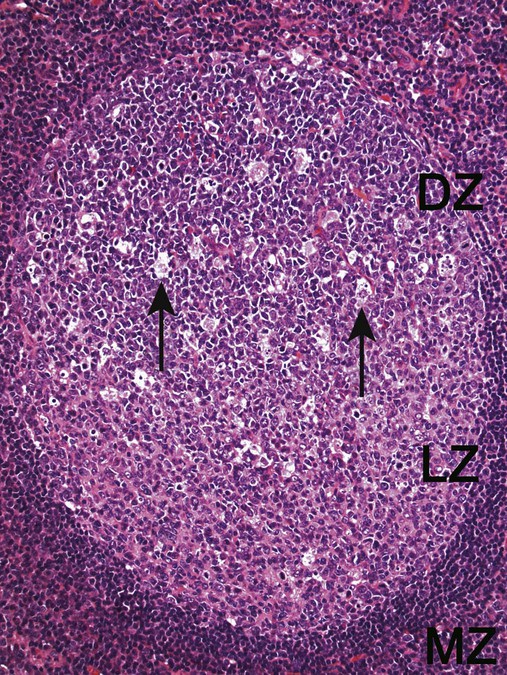
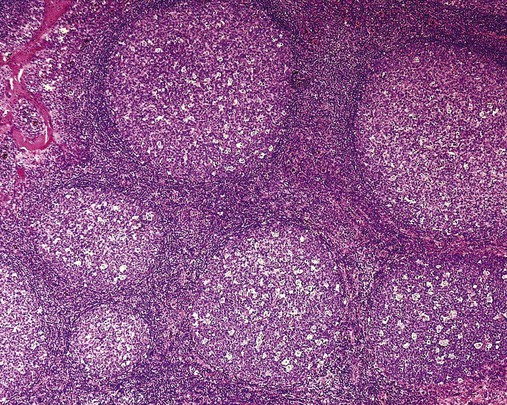
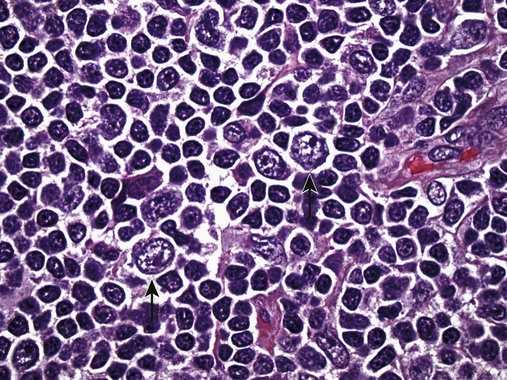
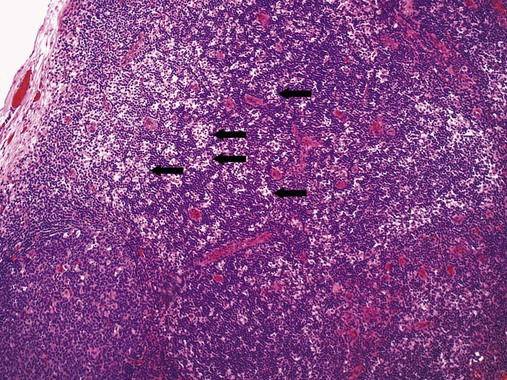
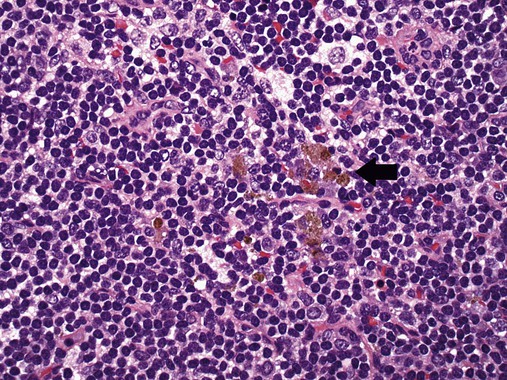
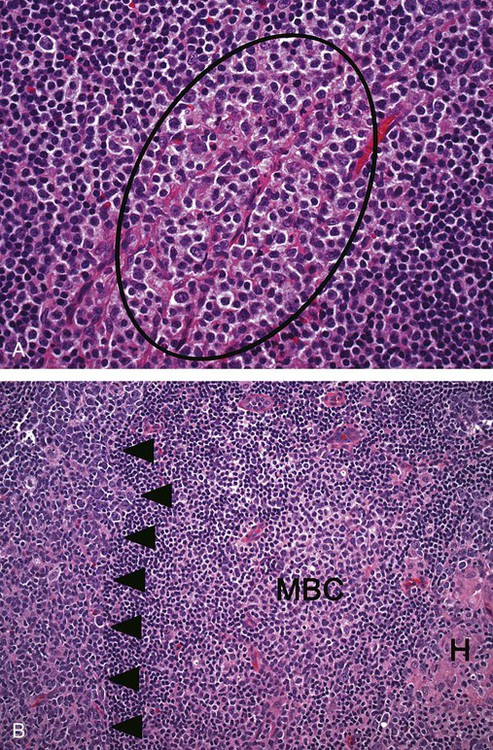
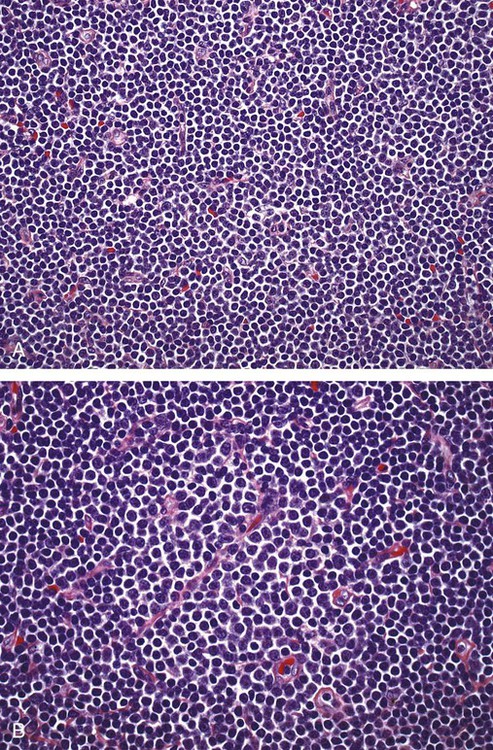
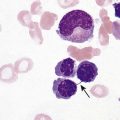
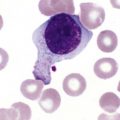
After completion of this chapter, the reader will be able to: 1. Describe normal lymph node morphology and discuss the function of various compartments and constituent cells. 2. Outline the most common histologic patterns of reactive lymphadenopathies. 3. Describe the peripheral blood findings in chronic lymphocytic leukemia and hairy cell leukemia. 4. Describe the approach to the diagnosis of lymphomas as outlined by the World Health Organization classification. 5. Discuss the most commonly occurring mature B- and T-cell neoplasms, including epidemiology, clinical presentation, pathophysiology, lymph node histologic features, peripheral blood or bone marrow findings, and diagnostic test results. 6. Interpret diagnostic test results to identify lymphoproliferative disorders. A 46-year-old, previously healthy man came for evaluation of an enlarged left cervical lymph node. The patient had discovered this isolated lymphadenopathy 2 weeks previously and did not complain of any other symptoms. The lymph node measured approximately 2 cm. The findings of his physical examination were otherwise unremarkable. The lymph node was excised, and microscopic examination showed the histologic features presented in Figure 37-1, A. Immunohistochemical stains showed CD20 (see Figure 37-1, B), CD10, and BCL-6 positivity, and focal CD30 antigen expression. 1. What is your diagnosis based on the histologic and immunophenotypic features? 2. What additional immunophenotypic features that confirm the diagnosis could be seen using flow cytometry? 3. Is it likely that this patient would show disseminated disease, including bone marrow involvement? Lymphomas are neoplastic lesions of the lymphoid system. Original microscopic observations and, more recently, immunophenotypic and molecular studies have confirmed that these neoplasms recapitulate specific stages of differentiation of normal lymphoid cells. The diagnosis is based on the combination of biologic features such as morphology, immunophenotype and molecular genetic characteristics, and clinical information.1 Thus, during initial sample processing the appropriate steps must be taken to ensure tissue preservation and availability for microscopic examination and immunophenotypic and molecular studies. Histologic components of the lymph node include the cortex, paracortex, medullary cords, and sinuses (Figure 37-2). These are not only structural but also functional compartments serving as sites of immunologic reactions for specific antigenic stimuli. The lymph node is surrounded by a capsule of fibrous tissue. Immediately below the capsule is the cortex, the most superficial portion of the lymph node consisting of primary and secondary follicles. Primary follicles are microscopic aggregates of small, round naive B lymphocytes. These lymphocytes express pan–B-cell markers, including CD19 and CD20, and are frequently CD5+ (Figure 37-3). The formation of secondary follicles, including germinal centers, starts with antigen presentation by follicular dendritic cells.2,3 On antigen encounter, naive B lymphocytes undergo transformation, proliferation, and differentiation into precursors of antibody-producing plasma cells and memory B cells (see Figure 37-3). The remaining naive B cells are displaced into the periphery of the germinal center and form the mantle zone. Germinal center B cells have a specific immunophenotype. In addition to pan–B-cell markers, they express germinal center cell antigens CD10 and BCL-6, and, in contrast to circulating B cells, they lack antiapoptotic BCL-2 protein. The functional compartments of the germinal center include the dark zone occupied by centroblasts, large B cells with round vesicular nuclei, small nucleoli adjacent to nuclear membrane, and basophilic cytoplasm (Figure 37-4). The dark zone is a site of high proliferative activity and somatic mutations of B-cell immunoglobulin variable regions. The latter process allows for the production of immunoglobulins with the best affinity for a particular antigen. After completing somatic mutations, centroblasts differentiate into centrocytes, smaller cells with dense chromatin and irregular nuclear outlines, which form the light zone (see Figure 37-4). Subsequently, centrocytes with low-affinity (“unfit”) surface immunoglobulins undergo apoptosis and are phagocytized by germinal center macrophages (tingible body macrophages). The presence of numerous macrophages with apoptotic debris contributes to the characteristic “starry sky” pattern of the germinal center. Centrocytes with immunoglobulins with high affinity for a particular antigen lose their germinal center antigens (CD10 and BCL-6) and differentiate into memory B cells that form a marginal zone at the periphery of the mantle zone. Marginal zone lymphocytes are medium-sized with abundant clear cytoplasm and indented nuclei. The filtration of lymphatic fluid through the lymph node is accomplished through afferent lymphatics communicating with the subcapsular sinus, which is situated immediately beneath the capsule (see Figure 37-2). The subcapsular sinus drains to cortical sinuses, which run through the cortex and empty to medullary sinuses. The latter converge into the efferent lymphatic vessel at the hilum. The sinuses are filled with macrophages or sinus histiocytes. These cells play an important role in antigen capture and processing. Several thin sections of a lymph node are placed in 10% buffered formalin for paraffin embedding. Some pathology laboratories fix additional tissue samples in a variety of fixatives with protein-precipitating properties (B5 fixative, zinc chloride formalin) for better preservation of cytologic detail.4 Regardless of the fixative used, thin sectioning of the fresh lymph node is crucial for the proper permeation of the tissue. A portion of the lymph node is placed in culture medium (Roswell Park Memorial Institute medium) and transported to the flow cytometry laboratory for immunophenotyping. The remaining fresh tissue can be stored at –70° C for further studies. Follicular hyperplasia is the most common of the reactive lymphadenopathies. It is seen frequently in lymph nodes and tonsils of children and adolescents as a reaction to infections. In adults, it occurs in association with autoimmune disorders (rheumatoid arthritis, systemic lupus erythematosus), syphilis, and early human immunodeficiency virus (HIV) infection. Microscopically, the expansion of reactive follicles can be prominent and extend beyond the cortex into the medulla (Figure 37-5). The follicles retain all the hallmarks of reactive germinal centers, including distinct polarization, presence of tingible body macrophages, abundant mitotic figures, and a preserved mantle zone (see Figure 37-4). Paracortical expansion is associated with viral infections (e.g., infectious mononucleosis) and drug reactions and is also seen in patients with chronic skin diseases (dermatopathic lymphadenopathy). In addition to small lymphocytes, the paracortex shows numerous immunoblasts, increased mitotic activity, and vascular proliferation (Figure 37-6). Focal areas of necrosis may also be seen. In dermatopathic lymphadenopathy, the paracortex has a characteristic mottled appearance as a result of an increased number of large cells with abundant clear cytoplasm scattered among small lymphoid cells (Figure 37-7). These cells include histiocytes, often carrying melanin pigment, and Langerhans cells (Figure 37-8). Scattered immunoblasts, plasma cells, eosinophils, and vascular proliferation are also encountered. Expanded subcapsular, cortical, and medullary sinuses are often seen in lymph nodes draining limbs, abdominal organs, various inflammatory lesions, and malignancies. In advanced cases, the prominent sinuses compress the nodal parenchyma. They may be completely filled with histiocytes showing abundant cytoplasm, a small oval nucleus with inconspicuous nucleolus, and delicate chromatin. Monocytoid B cells with abundant cytoplasm and oval indented nuclei that may mimic histiocytes are seen in nodal sinuses in HIV-associated lymphadenopathy and Toxoplasma lymphadenitis (Figure 37-9, A). Numerous malignant lesions show a predilection for sinuses, such as Langerhans cell histiocytosis, B-cell and T-cell lymphomas, and carcinomas; a high-power microscopic evaluation of expanded sinuses is always necessary. A classic example of mixed-pattern hyperplasia is seen in Toxoplasma gondii infection, a common protozoal infection typically seen after ingestion of raw meat or contamination by cat feces. Histologically, the expansion of all lymph node compartments is seen (see Figure 37-9). Florid follicular hyperplasia is accompanied by paracortical expansion, aggregates of histiocytes encroaching on germinal centers, and expanded sinuses. Sinuses are focally filled with a specific subset of B cells, so-called monocytoid B cells. Approximately 86,000 new cases of lymphoma are diagnosed annually in the United States.5 Most lymphomas develop in previously healthy individuals. The strongest risk factor for development of lymphoproliferative disorder is altered immune function as seen in immunocompromised patients or individuals with autoimmune disease.6,7 Similarly, certain viral and bacterial infections are associated with a higher risk for the development of lymphoma.8 Accumulating evidence indicates that exposure to chemicals and herbicides may predispose to lymphoid neoplasms. Most lymphomas present in lymph nodes. Certain types show a predilection for extranodal sites. The frequency of bone marrow and peripheral blood (leukemic phase) involvement varies depending on the lymphoma subtype. Over the years, numerous classifications have been proposed based mainly on the morphology and clinical characteristics (e.g., Rappaport classification, Kiel system, Working Formulation). With increased understanding of the development and function of the immune system, however, it became clear that lymphomas, like myeloid neoplasms, recapitulate normal stages of lymphoid differentiation. In addition, the elucidation of specific molecular events occurring in lymphomagenesis helped in devising a clinically relevant subclassification, especially for morphologically heterogeneous entities. Currently, numerous subtypes of lymphoma are distinguished based on morphology, immunophenotype, molecular genetics, and clinical characteristics. The integration of these features is mandatory for comprehensive lymphoma diagnosis. On the basis of cellular origin, lymphomas can be categorized into lesions of lymphoid precursors and neoplasms of mature lymphoid cells (Table 37-1). In this chapter, only mature B-cell and T-cell neoplasms are discussed; the precursor malignancies are covered in Chapter 36. TABLE 37-1 2008 World Health Organization Classification of Mature Lymphoid Neoplasms Mature B-cell lymphomas are neoplasms derived from various stages of B-cell differentiation. Although they show significant morphologic and immunophenotypic heterogeneity, all B-cell lymphomas produce monoclonal light chain immunoglobulins, clonal immunoglobulin gene rearrangements, or both. Follicular lymphoma and diffuse large B-cell lymphoma (DLBCL) are the most common subtypes of B-cell lymphoma.9 Most cases are lymph node based and occur in elderly individuals. However, leukemic involvement (peripheral blood and bone marrow) can occur with any lymphoma subtype. The most common mature B-cell neoplasms are discussed in the following paragraphs and are summarized in Table 37-2. TABLE 37-2 Morphologic and Immunophenotypic Features of Mature B-Cell Lymphomas Bone marrow and peripheral blood films shows small lymphoid cells with a coarse chromatin pattern, inconspicuous nucleoli, and scant cytoplasm10 (Figure 37-10). Larger lymphoid cells with less condensed chromatin and distinct nucleoli (prolymphocytes) are rare. Smudge cells, representing disintegrated lymphoid cells, are present on the peripheral blood film. These cells are helpful in the diagnosis because they are not often seen in other subtypes of malignant lymphoma. The bone marrow biopsy specimen shows nodular, diffuse, or interstitial infiltrates of small lymphoid cells (Figure 37-11). Lymph nodes involved by SLL show an effacement of normal nodal architecture (Figure 37-12, A) by a diffuse proliferation of small round lymphoid cells with coarse chromatin, indistinct nucleoli, and scant cytoplasm. In addition, scattered nodules (so-called pseudofollicles, growth centers, or proliferation centers) composed of medium-sized and large lymphoid cells with dispersed chromatin and distinct nucleoli are observed (see Figure 37-12, B). The diffuse proliferation of small lymphoid cells with pseudofollicles is pathognomonic for SLL. As noted earlier, CLL/SLL is derived from circulating CD5+IgM+IgD+ B cells. Currently, two groups of CLL/SLL are recognized.11 The first corresponds to the pre–germinal center phenotype with naive B cells showing no mutations in the variable region of the immunoglobulin heavy chain (VH) gene. The second form is derived from memory B cells (the post–germinal center stage), with mutated VH genes. Both types are positive for pan–B-cell antigens, including CD19 and weakly expressed CD20. In addition, the expression of CD5, CD23, and weak surface monoclonal κ or λ light chains is seen. The presence of CD23 and absence of FMC7 and cyclin D1 distinguish CLL/SLL from mantle cell lymphoma. The presence of monoclonal B cells with an immunophenotype similar to that of CLL/SLL has been described in a small proportion of healthy individuals; thus the demonstration of these cells by flow cytometry must always to be interpreted in the context of other clinical and laboratory features.12 Overall, CLL/SLL is regarded as an indolent lymphoproliferative disorder of the elderly (median age at diagnosis is 65 years). Although this disease is incurable by current therapeutic approaches, the median survival is 10 years.13
Mature Lymphoid Neoplasms
Case Study
Morphologic and Immunophenotypic Features of Normal Lymph Nodes
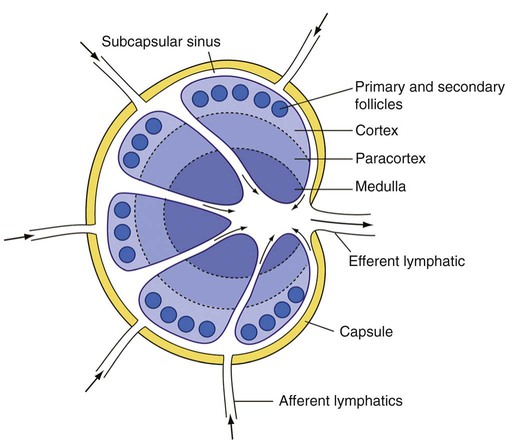
Cortex
Sinuses
Lymph Node Processing
Reactive Lymphadenopathies
Follicular Pattern
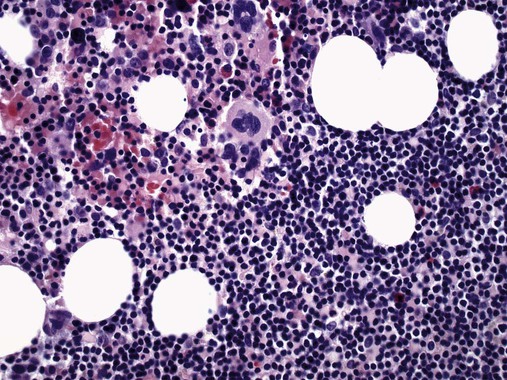
Paracortical Pattern
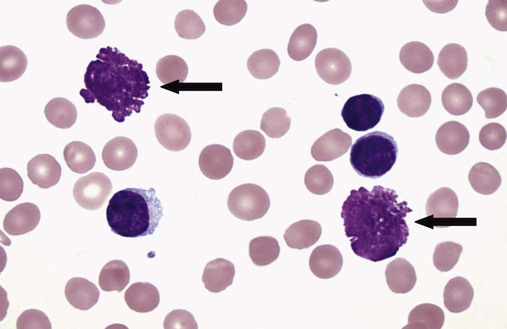
Sinusoidal Pattern
Mixed Pattern
Lymphomas
Type of Lymphoma
Examples
Mature B-cell lymphomas
Chronic lymphocytic leukemia/small lymphocytic lymphoma
B-cell prolymphocytic leukemia
Splenic B-cell marginal zone lymphoma
Hairy cell leukemia
Splenic B-cell lymphoma/leukemia, unclassifiable
Splenic diffuse red pulp small B-cell lymphoma
Hairy cell leukemia-variant
Lymphoplasmacytic lymphoma
Heavy chain diseases
Gamma Heavy chain disease
Mu Heavy chain disease
Alpha Heavy chain disease
Plasma cell neoplasms
Monoclonal gammopathy of undetermined significance (MGUS)
Plasma cell myeloma
Solitary plasmacytoma of bone
Extraosseous plasmacytoma
Monoclonal immunoglobulin deposition diseases
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma)
Nodal marginal zone lymphoma
Follicular lymphoma
Primary cutaneous follicle center lymphoma
Mantle cell lymphoma
Diffuse large B-cell lymphoma (DLBCL), not otherwise specified
T-cell/histiocyte-rich large B-cell lymphoma
Primary DLBCL of the central nervous system
Primary cutaneous DLBCL, leg type
Epstein-Barr virus (EBV)–positive DLBCL of the elderly
DLBCL associated with chronic inflammation
Lymphomatoid granulomatosis
Primary mediastinal (thymic) large B-cell lymphoma
Intravascular large B-cell lymphoma
ALK-positive large B-cell lymphoma
Plasmablastic lymphoma
Large B-cell lymphoma arising in human herpesvirus 8–associated multicentric Castleman disease
Primary effusion lymphoma
Burkitt lymphoma
B-cell lymphoma, unclassifiable, with features intermediate between those of DLBCL and Burkitt lymphoma
B-cell lymphoma, unclassifiable, with features intermediate between those of DLBCL and classical Hodgkin lymphoma
Mature T-cell lymphomas
T-cell prolymphocytic leukemia
T-cell large granular lymphocytic leukemia
Chronic lymphoproliferative disorder of natural killer (NK) cells
Aggressive NK cell leukemia
EBV-positive T-cell lymphoproliferative diseases of childhood
Systemic EBV-positive T-cell lymphoproliferative disease of childhood
Hydroa vacciniforme–like lymphoma
Adult T-cell leukemia/lymphoma
Extranodal NK/T-cell lymphoma, nasal type
Enteropathy-associated T-cell lymphoma
Hepatosplenic T-cell lymphoma
Subcutaneous panniculitis-like T-cell lymphoma
Mycosis fungoides
Sézary syndrome
Primary cutaneous CD30+ T-cell lymphoproliferative disorders
Primary cutaneous peripheral T-cell lymphomas, rare subtypes
Primary cutaneous gamma-delta T-cell lymphoma
Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma
Primary cutaneous CD4+ small/medium T-cell lymphoma
Peripheral T-cell lymphoma, not otherwise specified
Angioimmunoblastic T-cell lymphoma
Anaplastic large cell lymphoma, ALK positive
Anaplastic large cell lymphoma, ALK negative
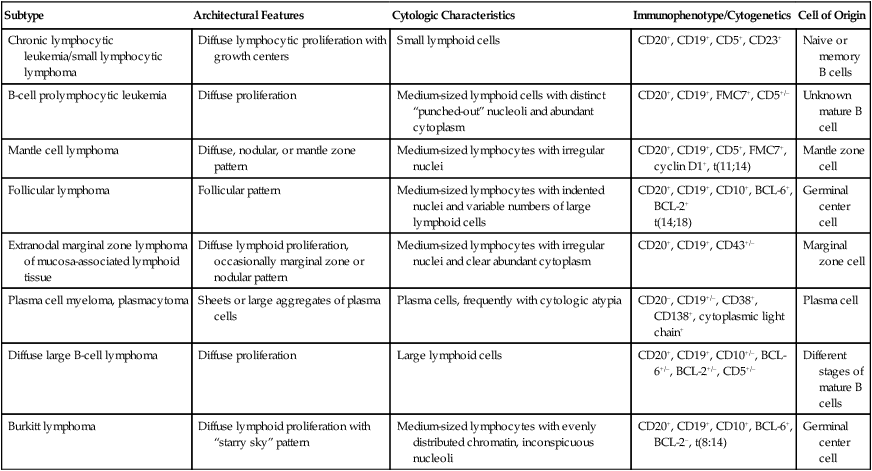
Mature B-Cell Lymphomas
Subtype
Architectural Features
Cytologic Characteristics
Immunophenotype/Cytogenetics
Cell of Origin
Chronic lymphocytic leukemia/small lymphocytic lymphoma
Diffuse lymphocytic proliferation with growth centers
Small lymphoid cells
CD20+, CD19+, CD5+, CD23+
Naive or memory B cells
B-cell prolymphocytic leukemia
Diffuse proliferation
Medium-sized lymphoid cells with distinct “punched-out” nucleoli and abundant cytoplasm
CD20+, CD19+, FMC7+, CD5+/–
Unknown mature B cell
Mantle cell lymphoma
Diffuse, nodular, or mantle zone pattern
Medium-sized lymphocytes with irregular nuclei
CD20+, CD19+, CD5+, FMC7+, cyclin D1+, t(11;14)
Mantle zone cell
Follicular lymphoma
Follicular pattern
Medium-sized lymphocytes with indented nuclei and variable numbers of large lymphoid cells
CD20+, CD19+, CD10+, BCL-6+, BCL-2+
t(14;18)
Germinal center cell
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue
Diffuse lymphoid proliferation, occasionally marginal zone or nodular pattern
Medium-sized lymphocytes with irregular nuclei and clear abundant cytoplasm
CD20+, CD19+, CD43+/–
Marginal zone cell
Plasma cell myeloma, plasmacytoma
Sheets or large aggregates of plasma cells
Plasma cells, frequently with cytologic atypia
CD20–, CD19+/–, CD38+, CD138+, cytoplasmic light chain+
Plasma cell
Diffuse large B-cell lymphoma
Diffuse proliferation
Large lymphoid cells
CD20+, CD19+, CD10+/–, BCL-6+/–, BCL-2+/–, CD5+/–
Different stages of mature B cells
Burkitt lymphoma
Diffuse lymphoid proliferation with “starry sky” pattern
Medium-sized lymphocytes with evenly distributed chromatin, inconspicuous nucleoli
CD20+, CD19+, CD10+, BCL-6+, BCL-2–, t(8:14)
Germinal center cell
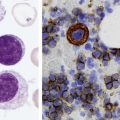
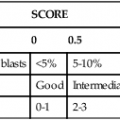
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
Morphology and Immunophenotype
Clinical Features and Prognosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree