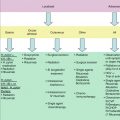
Better prognosis
Poorer prognosis
MIPIa score (Geisler et al. 2010)
Low
Intermediate or high
MIPI-bb score (Schaffel et al. 2010)
Low
Intermediate or high
Posttreatment MRDc (Pott et al. 2010a)
Negative
Positive
Ki-67 index (Determann et al. 2008)
<10 %
>30 %
Proliferation signature (Rosenwald et al. 2003)
Low
High
Negative
Positive
p53
Wild type (WT)
Mutated
As is true for most cancers, the natural history and time course from initial transforming event to clinical presentation is unknown. A series of MCL patients have been shown retrospectively to have harbored occult MCL as much as 7–15 years prior to a clinical diagnosis of MCL (Racke et al. 2010). This and other studies of incidentally identified in situ MCL support the notion that MCL has a long preclinical latency (Adam et al. 2012).
15.3 Pathology
15.3.1 Definition and Clinical Presentation
Mantle cell lymphoma represents approximately 6 % of non-Hodgkin lymphomas (Anonymous 1997). It presents in adults with a median age of 63 years and a male predominance of approximately 2.3:1 (Argatoff et al. 1997). Patients usually present with clinical stage IV disease (70 % with stage IV), with generalized adenopathy and bone marrow involvement. Hepatosplenomegaly is also frequent (30–60 %). B symptoms may occur in up to 50 % of cases (Camp et al. 2011). Blood involvement is seen in the majority of patients and occurs in up to 75 % of patients at diagnosis by conventional morphology. When sensitive flow cytometric methods are used, this number increases to over 90 % (Ferrer et al. 2007).
In addition to this common presentation, some clinical variants are also recognized. Gastrointestinal involvement with presentation as multiple lymphoid polyps in the small and large intestine is termed multiple lymphomatous polyposis (MLP). These patients often present with abdominal pain (O’Briain et al. 1989). It should be noted that other lymphomas such as low-grade follicular lymphoma may have similar presentation. Thus, pathologic confirmation of the diagnosis is required when a clinical picture of MLP is encountered.
A non-nodal, leukemic variant has been described that appears to have an indolent clinical course and should be distinguished from the typical mantle cell lymphoma with blood involvement (Royo et al. 2012; Ondrejka et al. 2011a; Orchard et al. 2003). These patients generally present with a variable degree of lymphocytosis with or without splenomegaly and appear to have an indolent course, some not requiring therapy for a prolonged period of time. The clinical, pathologic, and genetic features of this variant are the subject of ongoing investigation.
15.3.2 Histopathology
Lymph node involvement may manifest in diffuse, nodular, or mantle zone patterns. Mixtures of patterns can be seen. In a large series, 80.5 % of cases showed a diffuse growth pattern, 18.1 % had a nodular pattern, and a prominent mantle zone pattern was seen in only 1.4 % of cases (Tiemann et al. 2005). The infiltrate is typically very monotonous, without admixed large, transformed cells or paraimmunoblasts. However, scattered individual epithelioid histiocytes, which do not form granulomas, are characteristically distributed throughout (Figs. 15.1 and 15.2) the infiltrate. Hyalinized vessels are also sometimes seen. Follicular dendritic cell (FDC) networks may present but may be disrupted and poorly formed. In some examples, well-formed residual FDC networks are seen in the center of lymphomatous nodules or in the mantle zone pattern. These are best seen with immunostains for FDCs such as CD21 or CD23 (Tiemann et al. 2005; Schrader et al. 2006; Weisenburger et al. 1987; Jaffe et al. 1987; Lardelli et al. 1990). In contrast to other mature B-cell lymphomas of small lymphocytes in which mitotic figures are quite rare, mitotic figures are often readily seen in mantle cell lymphoma. While the pattern and cytologic features tend to be stable in an individual patient, progression from mantle zone to nodular and nodular to diffuse patterns may be seen.
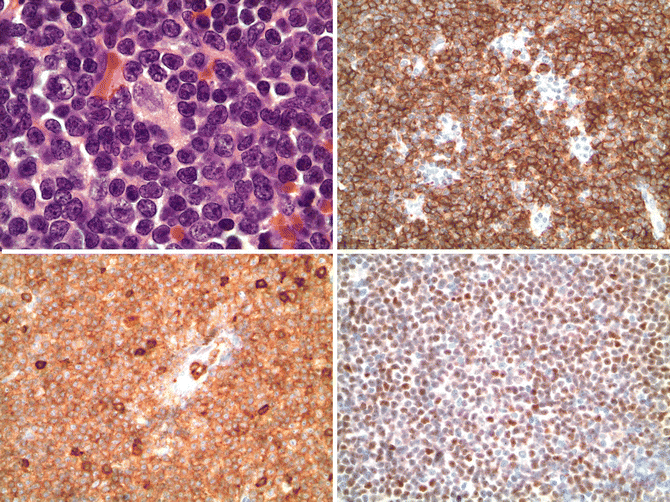

Fig. 15.1
Mantle cell lymphoma. A diffuse infiltrate of monotonous small lymphocytes (hematoxylin and eosin, 100x)

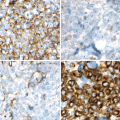
Fig. 15.2
Mantle cell lymphoma. The upper left panel shows the cytologic features of the common type of mantle cell lymphoma. The cells are small with mature, condensed chromatin with slight nuclear irregularities. An epithelioid histiocyte is present in the middle of the field (hematoxylin and eosin, 1000x). Immunostains show the cells express CD20 (upper right, 400x), CD5 (lower left, 400x), and cyclin D1 (lower right, 400x)
Several cytologic variants are recognized. In the common classical type, the cells are small with mature, condensed chromatin and slightly irregular nuclear borders, resembling centrocytic cells. A small cell variant also exists (<5 % of cases) in which the cells are round and more closely resemble small lymphocytic lymphoma; however, paraimmunoblasts or prolymphocytes are not present (Tiemann et al. 2005). The blastoid variant has been divided into the pleomorphic type that resembles diffuse large B-cell lymphoma and a lymphoblastoid type that resembles lymphoblastic lymphoma (Fig. 15.3). In these types, mitotic figures are numerous (over 50/10 high-power fields) compared to the common type (usually <20/10 high-power fields) (Ott et al. 1997). The blastoid variants are typically seen at diagnosis rather than progression or “transformation” events, but such events have been reported and appear clonally related (Laszlo and Matolcsy 1999). Rare cases of mantle cell lymphoma may have plasmacytic differentiation that appears to be clonally related or a monocytoid B-cell appearance (Visco et al. 2013; Swerdlow et al. 1996).


Fig. 15.3
Blastoid variant of mantle cell lymphoma. The left panel shows the lymphoblastoid type and the right panel illustrates the pleomorphic variant
Recently, a so-called “in situ” form of mantle cell lymphoma has been recognized in which the lymphoma cells are confined to non-expanded mantle zones with overall intact and non-altered lymph node architecture (Nodit et al. 2003). It is often an incidental finding or found when prior biopsy tissue (often years prior) is examined in a patient subsequently diagnosed with mantle cell lymphoma. These cases deserve further study but many appear indolent and may not progress to overt lymphoma (Carbone and Santoro 2011; Carvajal-Cuenca et al. 2012). A leukemic counterpart may also exist in which circulating mantle cell lymphoma cells can be found in the absence of lymphadenopathy, with our without splenomegaly. Such cases show sparse interstitial bone marrow infiltration, simple karyotype, and propensity for kappa light chain expression by flow cytometry (Ondrejka et al. 2011a).
Bone marrow involvement can be seen histopathologically in over 90 % of cases and can take the form of nodular, interstitial, paratrabecular, and diffuse infiltrates, in order of decreasing frequency (Cohen et al. 1998). Blood involvement, as noted above, is quite common, and blastoid/pleomorphic variants also can be seen in blood as well. Cases previously diagnosed as B-prolymphocytic leukemia with t(11;14) are now considered leukemic variants of mantle cell leukemia (Schlette et al. 2001; Wong et al. 2002).
15.3.3 Immunohistochemistry
Mantle cell lymphoma has the distinctive immunophenotype of a CD5+ mature B cell expressing CD19, CD20, CD22, CD79a, PAX5, and monotypic surface immunoglobulin light chain with a lambda predominance. IgM and IgD are expressed. CD10 and CD23, typically strongly expressed in follicular lymphoma and chronic lymphocytic leukemia, respectively, are usually not expressed in mantle cell lymphoma, but some cases may variably be positive, thus limiting their utility unless one is familiar with detailed expression patterns (Asplund et al. 2005; Gong et al. 2001). Cyclin D1 is aberrantly expressed as a result of the t(11;14)(q13;q32) translocation and is a sensitive and specific marker for the diagnosis of mantle cell lymphoma, particularly with newer paraffin-reactive rabbit monoclonal antibodies to cyclin D1 (Fig. 15.2) are used (de Leon et al. 1998; Cheuk et al. 2004). Ki-67(MIB1) staining shows a variable proliferative index, and increased proliferative fraction (>30–35 %) has been associated with poor prognosis in patients treated with modern therapy (Determann et al. 2008; Hoster et al. 2008a; Hsi et al. 2008; Klapper et al. 2009). This correlates with the importance of the proliferative gene signature in mantle cell lymphoma.
Rare cases of cyclin D1-negative mantle cell lymphoma exist, comprising less than 5 % of mantle cell lymphoma cases (Fu et al. 2005). SOX11, a gene recently found to be upregulated in mantle cell lymphoma, is a useful immunohistochemical marker to identify such cases, but its widespread use has been hampered by lack of reliable reagents (Dictor et al. 2009; Mozos et al. 2009). However, a new commercially available monoclonal antibody has been developed that is promising (ED Hsi, personal observation).
15.3.4 Molecular Genetics
The hallmark of mantle cell lymphoma is the t(11;14)(11q13;q32) involving CCND1 and IGH@ and resulting in overexpression of cyclin D1 (Williams et al. 1992, 1993b). This is the primary genomic alteration, present in almost all (>95 %) cases. Diagnostically, this can be detected by in situ hybridization in paraffin tissues, or the gene product can be seen by immunohistochemistry. Rare cases of variant immunoglobulin light chain partner genes exist. The existence of cyclin D1-negative mantle cell lymphoma has been proven, as noted above, but it is a rare occurrence and appears to be associated with abnormalities/overexpression of cyclin D2 or D3 (Fu et al. 2005; Dictor et al. 2009; Mozos et al. 2009).
Extensive work has been done to understand the detailed molecular genetic diversity of mantle cell lymphoma. Evaluation of the IGH@ gene and somatic hypermutational analysis have shown that approximately 70 % of cases show at least some degree of somatic hypermutation of the IGHV gene segments with biased usage IGHV3–21, IGHV4–34, IGHV1–8, and IGHV3–23 genes accounting for 46.3 % of cases, suggesting that the cell or origin may, at least in some cases, be a post-germinal center, antigen-driven cell as opposed to a naive B cell (Agathangelidis et al. 2011; Hadzidimitriou et al. 2011). Gene expression profiling of mantle cell lymphoma has demonstrated key signatures such as a cell proliferation signature, which was shown to have prognostic significance, and high proliferation has been incorporated into the Mantle Cell Lymphoma International Prognostic Index (MIPI) (Hoster et al. 2008a; Rosenwald et al. 2003).
The genetic complexity of mantle cell lymphoma has been addressed by multiple methods, and numerous secondary abnormalities have been identified. Although detailed enumeration of these findings are beyond the scope of this chapter, some of the more common alterations (potential genes of interest) include loss of 1p, 6q, 9p (CDKN2A), 11q (ATM), 13q (miR-17-92 cluster), and 17p (TP53) and gains of 3q, 8q (MYC), 10p (BMI1), 12q (CDK4), 15q, and 18q (BCL2) (Halldorsdottir et al. 2011; Jarosova et al. 2004; Schraders et al. 2005; Bea et al. 1999; Monni et al. 1998; Bentz et al. 2000; Kohlhammer et al. 2004; Martinez-Climent et al. 2001; Allen et al. 2002; Salaverria et al. 2007; Royo et al. 2011). Pathways associated with genes in these regions may present important in the pathogenesis and progression of mantle cell lymphoma. These include cell cycle (INK4A/CDK4/RB1, ARF/MDM2/TP53, BMI1, CDKN2B, CDKN2C), DNA damage response (ATM, CHK1, CHK2), cell survival (BCL2, NFkB pathway), Hippo pathway signaling (MOBKL2B, MOBKL2A, LATS1, LATS2), and microtubule-associated proteins (MAP6) (Royo et al. 2011).
Application of next generation sequencing has identified recurrent NOTCH1 mutations in approximately 12 % of cases of MCL, clustered near the PEST domain and is similar to the PEST domain mutation seen in T-acute lymphoblastic leukemia. In keeping with a presumed tumor-promoting role, inhibition of NOTCH1 in mutated MCL cell lines decreased proliferation, and in clinical samples, NOTCH1 mutation appears to be associated with shorter progression-free and overall survival (Kridel et al. 2012).
15.3.5 Differential Diagnosis
The differential diagnosis of mantle cell lymphoma includes other small B-cell lymphomas. Immunophenotyping is critical (Table 15.2) since the profiles are different. The most difficult differential is with small lymphocytic lymphoma (SLL), particularly when only small biopsies are available such as needle core biopsies or endoscopic biopsy. In particular, the small cell variant of mantle cell lymphoma may closely mimic SLL because of the predominance of round nuclei. However, SLL will show paraimmunoblasts/prolymphocytes that often cluster to form proliferation centers, which are not seen in mantle cell lymphoma. Additionally, the epithelioid histiocytes seen in mantle cell lymphoma are absent in SLL. The presence of CD5 in both lymphomas further complicates the distinction, but expression of cyclin D1 and absence of LEF1 (Table 15.2) make accurate diagnosis possible (Tandon et al. 2011).
Table 15.2
Immunophenotypic profile of small B-cell lymphomas/leukemias
CD19 | CD20 | CD5 | CD10 | CD23 | BCL6 | Cyclin D1 | Sox11 | LEF1 | pERK | |
|---|---|---|---|---|---|---|---|---|---|---|
CLL/SLL | + | + | + | – | + | – | – | – | + | – |
MCL | + | + | + | – | −/+weak | – | + | + | – | – |
FL | + | + | – | + | – | + | – | – | – | – |
MZL | + | + | – | – | – | – | – | – | – | – |
LPL | + | + | – | – | – | – | – | – | – | – |
HCL | + | + | – | −/+weak | – | – | +weak | – | – | + |
Follicular lymphoma can enter the differential diagnosis in nodular variants of MCL, but the cytologic features of true centrocytes with highly convoluted, angulated nuclei in follicular lymphoma along with admixed centroblasts usually allow distinction between the two. Furthermore, expression of cyclin D1 and lack of the IGH@/BCL2 fusion as well as differences in immunophenotype help consolidate the diagnosis.
Marginal zone lymphoma and lymphoplasmacytic lymphoma are both usually CD5-negative and CD10-negative. The cytologic features of marginal zone cells differ from most cases of mantle cell lymphoma, although rare cases of mantle cell lymphoma may have marginal zone cytology with more abundant cytoplasm than normally seen. Again, expression of cyclin D1 in mantle cell lymphoma and lack of CD5 (usually) in marginal zone lymphomas will allow correct diagnosis. Lymphoplasmacytic lymphoma (LPL) shows plasmacytoid differentiation that can be also be seen by cytoplasmic immunoglobulin light chain restriction using immunohistochemistry. These latter features are distinctly unusual in mantle cell lymphoma. Again, LPL is also negative for cyclin D1. Hairy cell leukemia (HCL) usually does not enter the differential diagnosis due to distinctive morphology of HCL and the bone marrow/blood-based presentation; however, it is included for completeness due to the weak cyclin D1 expression that is common in HCL (Table 15.2). Phospho-ERKTHR202/TYR204 is expressed in essentially all cases of HCL in bone marrow sections but is not seen in other small B-cell lymphomas lacking BRAF V600E, likely as a consequence of this kinase-activating mutation (Tiacci et al. 2011; Warden et al. 2012).
15.4 Molecular Pathogenesis
MCL has proven to be a useful model of neoplastic pathogenesis, especially as relates to alterations in cell cycle machinery and the response to DNA damage. The overexpression of cyclin D1 dysregulates the G1/S-phase transition of the cell cycle. Cyclin D1 complexes with cyclin-dependent kinase-4 (CDK4) and −6 (CDK6) which in turn phosphorylate the retinoblastoma protein (Rb), leading to cell cycle progression (Zhao et al. 2010). Cyclin D1/CDK complexes also sequester the CDK inhibitors p27kip1 and p21 to further promote G1 to S-phase progression (Quintanilla-Martinez et al. 2003).
The DNA damage response pathway is altered in MCL via loss-of-function mutations. Examples include hemizygous deletion of the chromosomal region 11q22-23 affecting the ataxia-telangiectasia mutated (ATM) gene, often in association with mutation of the remaining ATM allele (Fang et al. 2003). ATM encodes a kinase that belongs to the PI3 kinase-related superfamily and plays a pivotal role in the cellular response to DNA damage. The tumor suppressor gene p53 is inactivated in approximately 30 % of MCL cases with blastoid morphology and with high proliferation rates. Loss-of-function mutations affecting the 17p13/p53 or 9p21/CDKN2A/Hippo signaling loci, as well as 3q gain or deletion 13q14, have been associated with poorer survival in MCL.
The role of microRNA aberrations in MCL pathogenesis has also been recognized. Examples include the loss of expression of miR-29 family members and overexpression of the miR-17-29 cluster, which have been associated with a more aggressive clinical courses and poorer outcome (Zhao et al. 2010). Of interest, truncation of the 3′ untranslated region of cyclin D1 mRNA, itself a marker of poorer prognosis in MCL, leads to loss of miR-16-1 binding sites which in turn impairs normal cell cycle regulation.
The transcription factor SOX11 is constitutively expressed in most MCL, including cyclin D1-negative variants. Recent investigation has shown that its expression blocks normal B-cell differentiation via PAX5 modulation and serves an oncogenic function (Vegliante et al. 2013). Additionally, identification of recurring mutations in the NOTCH1 and Hippo pathways is shedding light on MCL pathogenesis (Hartmann et al. 2010; Kridel et al. 2012).
15.5 Staging
The majority of patients present with advanced stage, symptomatic disease. All patients should have a thorough history and physical examination with attention to potential extranodal disease common to MCL including the GI tract and soft tissue sites. Routine blood counts and chemistry profiles plus LDH are necessary, as are CT and/or PET/CT scans and bone marrow aspirate and biopsy (Brepoels et al. 2008). Bone marrow analysis should include flow cytometry and cytogenetics with fluorescent in situ hybridization (FISH) for the t(11;14). Colonoscopy and esophagogastroduodenoscopy should be considered in the staging evaluation if there is evidence of gastrointestinal bleeding or abdominal symptoms. Leukemic involvement can be confirmed in nearly all patients at diagnosis using flow cytometric and molecular detection assays, although only about 25 % of patients have overt lymphocytosis. In the latter event, peripheral blood flow cytometry and FISH analysis may replace the need for marrow biopsy.
15.6 Prognostic Factors
While the majority of MCL patients ultimately follow a steadily progressive clinical course and require therapy at diagnosis or shortly thereafter, up to 25 % of patients have a slow pace of disease typical of indolent B-cell lymphomas and may defer anti-lymphoma therapy for 6–12 months or more (Ondrejka et al. 2011b). As we better understand the molecular and cellular pathogenesis of this heterogeneous disease, we are able to approach each patient with individualized prognosis and risk-adapted treatment (Table 15.2).
15.6.1 Phenotypic and Molecular Markers
The proliferation rate was identified as the most important prognostic factor in studies supporting a two-step model with initial inhibition of apoptosis pathways and secondary cell cycle alteration. These findings are extended by gene expression profiling of MCL by Rosenwald and colleagues that provided a quantitative measurement of tumor cell proliferation, termed “proliferation signature,” allowing for the definition of prognostic subgroups that differ in their median survival by more than 5 years (see above Rosenwald et al. 2003). Accordingly, the clinical application of Ki-67 immunostaining has been confirmed as a major prognostic factor in the vast majorities of studies (Determann et al. 2008). Immunohistochemical staining of paraffin-embedded MCL samples for Ki-67 expression, a marker of cellular proliferation, correlates inversely with survival (Determann et al. 2008). Significant differences in overall survival were shown for MCL patients treated with CHOP or R-CHOP stratified by fewer than 10, 10–29, and 30 % or more Ki-67-positive tumor cells, suggesting that the Ki-67 index may be a useful surrogate for the molecular profile proliferation index (Fig. 15.2).
15.6.2 Morphologic Subtype
Most patients show a diffuse nodal effacement, although nodular or mantle zone patterns are well recognized and often correlate with a more indolent pace of disease. A blastoid cell type may be present at diagnosis or may develop with disease progression and usually portends a survival averaging 1–2 years as compared with the non-blastoid MCL survival of more than 5 years.
Markers of “indolent MCL” have been characterized in a cohort of MCL patients for whom therapy was not required for months or years (Espinet et al. 2010). These include the presence of mutated immunoglobulin heavy variable chain genes (IgVH), a lack of p53 mutations, limited secondary genetic aberrations, lack of SOX11 expression, and a unique 13-gene molecular expression array signature (Navarro et al. 2009; Wang et al. 2008).
15.6.3 Clinical Prognostic Factors
Patients with predominantly peripheral blood, bone marrow, and splenic involvement without significant lymphadenopathy at presentation often experience an indolent clinical course; these cases may be misdiagnosed as chronic lymphocytic leukemia if FISH for the t(11;14) and CLL markers (e.g., del 13q, trisomy 12) are not determined.
The Mantle Cell Lymphoma International Prognostic Index (MIPI) has been validated in the context of several therapeutic regimens as a highly useful tool that incorporates clinical and laboratory parameters: patient age, ECOG (Eastern Cooperative Oncology Group) performance status, total leukocyte count, and serum lactate dehydrogenase (LDH) (Geisler et al. 2010). A biologic MIPI (MIPIb) further incorporates data for Ki-67 staining, described above, to provide an index with improved predictive power – patients with low-risk scores had median overall survival rates following induction chemotherapy of more than 6 years, whereas high-risk patients had median survivals of only 3 years (Fig. 15.4) (Geisler et al. 2010; Schaffel et al. 2010).
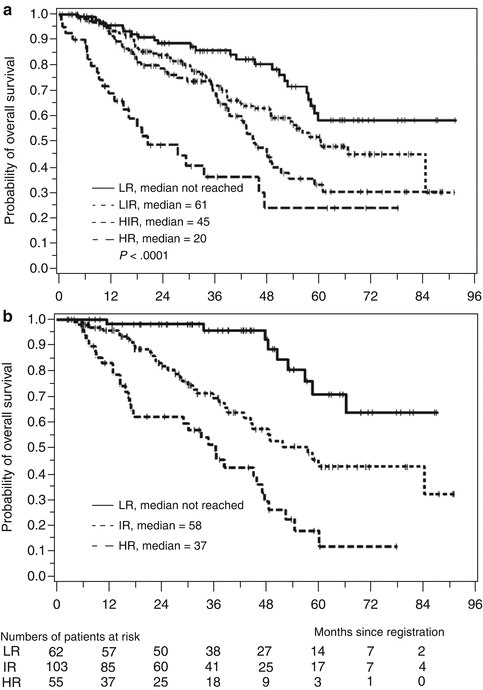

Fig. 15.4
Mantle Cell Lymphoma International Prognostic Index (MIPI) (a) and combined biologic index (MIPIb) (b) predicts overall survival (Hoster et al. 2008a)
15.6.4 Minimal Residual Disease
Using sensitive RQ-PCR assays for clonal IgVH and t(11;14) breakpoints using patient-specific probes, one can determine the presence of low-level minimal residual disease (MRD) in the peripheral blood and bone marrow (Pott et al. 2010a). Achieving an MRD-negative molecular remission has been associated with prolonged clinical remission both in younger patients undergoing intensive induction chemotherapy with autologous stem cell transplant consolidation as well as in older patients receiving lower-intensity immunochemotherapy (Liu et al. 2012). In these trials, 90 % of patients had a detectable molecular marker, and MRD could be assayed serially during and after therapy. The achievement of MRD negativity correlated with significantly longer response duration.
15.7 Initial Therapy
Increasing numbers of effective agents and regimens have evolved for MCL therapy in recent years, improving the outcomes for most patients. However, durable remissions remain a challenge for most patients. Given its clinical and biologic heterogeneity, MCL is one of the most difficult to manage, with a median survival of only 5–6 years and a high incidence of chemotherapy refractoriness (Anonymous 1997). Most cases of MCL present with symptomatic, advanced stage disease. Nonetheless, about 20 % of patients present with slow-paced and low-volume disease and may be considered for cautious “watchful waiting” (Martin et al. 2009), as frequently employed for follicular and other indolent NHL subtypes (Table 15.1).
The following sections will focus on emerging phase III data and on promising novel agents that are rapidly changing treatment approaches.
15.8 Limited Stage
A rare patient will present with limited stage disease. In a retrospective study of 17 patients with stage I–II MCL, 5-year progression-free and overall survival was 68 and 71 % after involved field radiotherapy, respectively, either alone or in combination with conventional chemotherapy (Leitch et al. 2003). On the other hand, median PFS in such patients was below 1 year in a phase III trial of the German lymphoma study group after radiation only. Optimal management of these patients, and those found incidentally to have MCL in situ, is not established.
15.9 Aggressive Upfront Therapy
15.9.1 Monoclonal Antibody and Combined Immunochemotherapy
In the modern era of MCL treatment, combined immunochemotherapy forms the backbone for first-line treatment (Table 15.3). A randomized phase III trial confirmed earlier studies showing that the addition of rituximab resulted in a superior overall response rate of 94 vs. 75 % with CHOP alone; CR rates were also improved (34 vs. 7 %), with a doubling of PFS from 14 to 28 months (Hoster et al. 2008b). Another randomized trial compared combined rituximab immunochemotherapy to chemotherapy only with the MCP regimen (mitoxantrone, chlorambucil, prednisone) and also showed a trend towards higher complete and overall response rates in the experimental arm, but the study was underpowered to detect a significant difference (Herold et al. 2004). A meta-analysis of R-chemotherapy in MCL demonstrated a benefit in overall survival, although the studies were statistically heterogeneous (Schulz et al. 2007). The observation of constant relapses in MCL patients after induction immunochemotherapy has led to the use of maintenance or consolidation strategies to translate high initial response rates into improved long-term survival.
Table 15.3
Selected frontline therapies in MCL
Phase | n | Disease status
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|