Abstract
Due to advancements in diagnostic and multimodality therapy, patients with stage IV breast cancer can achieve long-term survival. For women with asymptomatic primary tumors, the mainstay of therapy remains systemic and has been quite effective in controlling disease. However, the concept of elective resection of the intact primary has become a topic of interest in the past few decades. Biological explanations such as the role of cancer stem cells and immunosuppression in the presence of a primary tumor suggest a possible role for locoregional therapy. Retrospective data from multiple large population databases and single institutions suggest a benefit for a subset of patients with favorable features and low metastatic burden. However, the only published randomized trial from Tata Memorial shows local therapy for the primary does not improve overall survival, although there is a benefit to local progression-free survival. There is some conflict in the results illustrated in the retrospective studies and the Tata trial, hence the remaining ongoing trials will provide clarification on the role, extent, and timing of locoregional therapy for stage IV breast cancer. The present indication for locoregional therapy for the primary tumor in the setting of metastases continues to be the presence of a symptomatic tumor and should only be offered to patients with the explanation that there is no evidence of improvement in survival.
Keywords
local therapy, metastatic breast cancer, Tata Memorial Trial, surgical management of breast primary, overall survival, cancer stem cells
Approximately 6% of all breast cancer patients present with an intact primary and synchronous distant disease. For these patients, overall survival is dictated by the systemic disease burden rather than the status of the primary tumor. Consequently, systemic therapy is first-line treatment, and resection of the intact breast tumor is generally not recommended because the expectation is that most patients succumb to their disease before they develop uncontrolled local disease (ULD). If surgical extirpation of an intact primary is undertaken, it is performed in an effort to avoid future complications of ULD or to palliate chest wall progression once it has occurred.
However, the clinical course of metastatic breast cancer is changing. Overall, patients with stage IV disease are living longer. In an analysis of temporal trends in survival for metastatic breast cancer patients, 724 patients who presented to three French centers with de novo stage IV breast cancer were divided by time periods. Those diagnosed in an earlier time period (1987–1993) had a 27% 3-year survival rate, whereas those diagnosed in a later period (1994–2000) had a 44% 3-year survival rate. Similar trends are seen in data from the Surveillance, Epidemiology and End Results (SEER) registry. Some of this difference may be attributable to lead-time bias from earlier diagnosis of metastases in the later time period, but much of it is related to improved therapy with targeted agents, and continued improvements in survival can be expected in the future as additional molecular targets are identified and manipulated for therapeutic gain. Several trials have shown that between 3% and 30% of patients with distant metastases treated with multimodality therapy can achieve long-term survival. Thus improved systemic therapy and more sensitive imaging modalities both contribute to the fact that women live longer with known stage IV disease in the 21st century, many with a relatively small disease burden.
As a result of these trends, recent analyses have examined the value of surgical approaches such as metastasectomy (lung, liver) and resection of the intact primary in the management of patients with stage IV breast cancer. Metastasectomy has been studied in breast cancer patients with oligometastases and patients with multiple localized metastases that are amenable to surgical resection. Nieto and colleagues reported on a highly selected series of 60 patients with oligometastases who were treated with surgical excision, with or without radiation, and adjuvant chemotherapy. At a median of 62 months, 51.6% of patients were alive and disease-free. These data and others are reviewed by Salama and Chmura. The value of local therapy for limited metastatic disease, in addition to systemic therapy, is currently being evaluated in a randomized trial ( clinicaltrials.gov identifier NCT02364557; NRG Oncology trial NRG-BR002).
The concept of elective (rather than palliative) resection of the intact primary in the setting of metastatic disease has not been widely discussed in the breast cancer arena until recently, but there is precedent in other malignancies, such as gastric, colon, and ovarian carcinoma. For example, in metastatic renal cell carcinoma, two prospective, randomized trials (Southwest Oncology Group [SWOG] and European Organization for Research and Treatment of Cancer) compared radical nephrectomy with nonoperative management of the primary tumor in patients treated with systemic therapy (interferon alfa-2β). Both trials demonstrated a statistically significant survival advantage for patients treated with surgery (11.1 vs. 8.1 months, p = .05; 17 vs. 7 months, p = .03, respectively). A meta-analysis of patients with stage III and IV ovarian cancer found that maximal cytoreduction surgery was associated with improved survival. There are several studies supporting a role for palliative gastrectomy in the setting of metastatic or advanced gastric cancer. SEER analysis of over 8200 patients undergoing treatment for stage IV gastric cancer showed that those who had surgery had higher 3-year cancer-specific survival rates than nonsurgery patients (2.1% vs. 9.4% p < .001). In a retrospective review of 105 patients with stage IV gastric cancer who were treated without surgery, with bypass surgery, or with surgical resection, there was a statistically significant difference in overall survival in the patients who had resection versus patients who did not (5.5 vs. 13.2 months, p = .0006).
Along with clinical data, there have been new biological insights that suggest a unique role for the primary tumor in the continued dissemination of metastases. The identification of cancer stem cells and the recognition of their role in the metastatic process lead to the suggestion that the intact primary is a particularly efficient source of these cells, and the continued presence of the primary tumor may facilitate the development of new metastases. Additionally, an increasing body of evidence suggests that there is molecular communication between the primary tumor and the premetastatic niche. A specific role for mesenchymal stem cells that endow primary tumor cells with enhanced metastatic capacity provides a possible explanation for a beneficial role for resection of the primary tumor even in the setting of established distant disease. Other hypotheses relating the presence of the primary tumor to the metastatic process implicate immune suppression caused by the primary tumor. Conversely, laboratory data suggest that the presence of the primary tumor may restrain the growth of metastatic lesions, although this has never been demonstrated in humans.
Prompted by the data from trials of metastatic renal cell carcinoma, the paradigm that surgical resection of an intact breast primary in the setting of metastatic disease has only palliative value has been questioned, and multiple retrospective studies have evaluated the possibility of a survival benefit of primary site local therapy for stage IV breast cancer. These have shown a consistent association of the use of surgical resection of the intact primary with improved survival, leading to several randomized prospective trials. Two of these have been completed but thus far reported results have been null, showing no survival benefit for primary site local therapy (PSLT). These data are discussed at greater length next.
Retrospective Analyses of Primary Site Local Therapy
Surgical Resection of the Primary Tumor and Survival
Prompted by the results of SWOG 8949, which showed a survival advantage for stage IV renal cell carcinoma patients undergoing nephrectomy, Khan and associates analyzed survival data on patients reported to the National Cancer Database (NCDB) of the American College of Surgeons. Among 16,023 patients presenting with stage IV breast cancer in the NCDB from 1990 to 1993, surgical resection of the primary tumor was performed in 57%. The great majority of patients were treated with systemic therapy, but data on primary site radiation therapy was not available in the NCDB at that time. Surgical resection of the primary tumor was associated with a 39% reduction in the hazard of death from any cause. Other characteristics associated with overall survival in multivariate analysis included the use of systemic therapy, the number of organ sites involved, and the presence of visceral disease. In the NCDB report, the 3-year survival was 35 months in the surgically resected patients with free margins, compared with 26 months in women undergoing resection with involved margins and 17 months in the nonsurgical group.
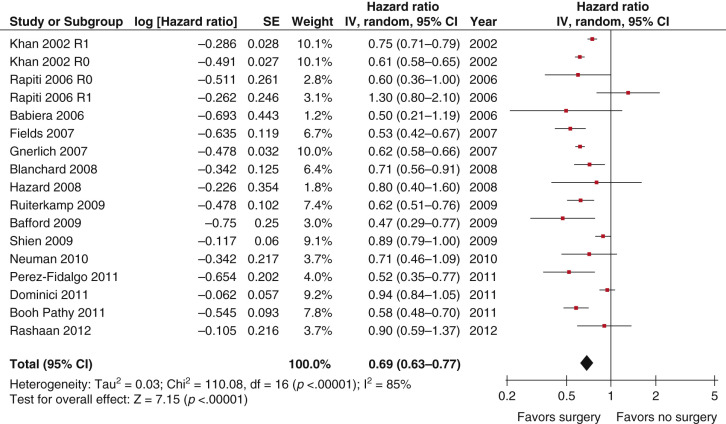
Subsequently, 18 retrospective analyses have evaluated the possible role of local therapy for the primary tumor, with the majority showing improved overall survival in women receiving treatment. The local therapy has consisted largely of surgery, although some studies have also addressed primary radiotherapy (RT). Six multiinstitutional studies (see Table 67.1 ) include a total of 27,000 patients, 14,443 of whom underwent surgical resection of the primary tumor. These analyses come from NCDB, the Geneva Cancer Registry, and the SEER database of the National Cancer Institute, the South Netherlands Eindhoven Cancer Registry, Massachusetts General Hospital and Boston Women’s Hospital registries, and NCCN Breast outcomes database. Thirteen additional studies come from single institutions, six from large academic institutions in the United States and seven from Europe and Asia, all of which are structured similarly and include data on 4000 patients, 1670 of whom had surgery ( Table 67.2 ). These single-institution studies provide detailed medical records so that questions regarding rates of positive margins, use of axillary surgery, RT, metastatic burden, and local control can be studied. More than half of these studies demonstrated a similar association between surgery and improved survival. Analyses of SEER data shows that surgically treated patients lived 11 to 15 months longer than those not receiving surgery ( p < .001). On the basis of this literature, Petrelli and Barni performed a meta-analysis that included 15 of these retrospective studies, with the goal of evaluating the relationship between survival and surgery or RT for the primary tumor and showed a hazard ratio (HR) of 0.69 (95% confidence interval [CI] 0.63–0.77, Fig. 67.1 ) associated with the use of surgery. Survival benefit was independent of multiple factors such as age, tumor burden, type of surgery, margin status, site of metastases, hormone receptor status, and HER2 status.
| Author and Setting | Year | N (% Surgery/RT) | Outcome Measured | Difference (Surgery vs. No Surgery) | p Value for Main Effect |
|---|---|---|---|---|---|
| Khan, NCDB | 2002 | 16,023 (57%) | 3-Year Survival | 32% vs. 17% | .0001 |
| Rapiti, Geneva Cancer Registry | 2006 | 300 (42%) | Disease-specific survival | HR 0.6 (CI 0.4–1) | .049 |
| Gnerlich, SEER | 2007 | 9734 (47%) | Median survival | 36 mo vs. 21 mo | .001 |
| Ruiterkamp, Southern Netherlands Registry | 2009 | 728 (40%) | 5-year survival | 24.5% vs. 13.1% | <.0001 |
| Cady, two-hospital database, Boston | 2008 | 622 (38%) | Median survival | 36 mo vs. 24 mo | .0001 a |
| Dominici, NCCN | 2011 | 551 (10%) | Median survival | 42 mo vs. 36 mo | .29 |
a Attrition of significance was seen in some subsets with matched pair analyses.
| First Author | Year | N (% Surgery/RT) | Outcome Measured | Difference (Surgery vs. No Surgery) | p Value for Main Effect |
|---|---|---|---|---|---|
| Babiera | 2006 | 244 (37) | Progression-free survival | RR .54 (CI .38-.77) | .0007 |
| Blanchard | 2008 | 395 (61) | Median survival | 27 mo vs. 17 mo | .0001 |
| Hazard | 2008 | 111 (42) | Median survival | 26 mo vs. 29 mo | NS |
| Fields | 2007 | 409 (46) | Median survival | 32 mo vs. 15 mo | .0001 |
| Bafford | 2009 | 147 (41) | Overall survival | HR 0.47 a | .003 a |
| Le Scodan b , | 2009 | 581 (55) | 3-year survival | 43.4% vs. 26.7% | .00002 |
| Shien | 2009 | 344 (47) | Median survival | 27 mo vs. 22 mo | .049 |
| Leung | 2010 | 157 (33) | Median survival | 25 mo vs. 13 mo | .06 |
| Nguyen | 2012 | 733 (51.6) | 5-year survival | 21 vs. 14 mo | <.001 |
| Perez Hidalgo | 2011 | 208 (59.1) | Overall survival | 40.1 vs. 24.3 mo | <.001 |
| Rashaan | 2012 | 171 (34.5) | Overall survival | HR 0.9 (0.59–1.37) | NS |
| Pathy | 2011 | 375 (37.1) | 2-year survival | 46.3% vs. 21.3 | <.001 |
| Neuman | 2010 | 186 (37) | Overall survival | RR .71 (CI 0.47–1.06) | NS |
a On subset analysis, those diagnosed with metastases postoperatively showed no survival advantage.
The role of axillary surgery on overall survival has been difficult to evaluate in the published retrospective analyses. A meta-analysis by Hartmann and colleagues considered six retrospective studies that gave information about whether an axillary surgical procedure was performed in case of surgery. Of the patients reviewed, 42% had surgery. In the surgery group, 527 patients (69%) had axillary procedures. Only three studies investigated the impact of axillary surgery on survival, and did not find a benefit. Given current concepts regarding the role and value of axillary dissection in nonmetastatic breast cancer, this procedure cannot be recommended in patients with metastatic disease.
Radiotherapy for the Primary Tumor and Survival
The use of primary RT for the primary tumor appears to show a survival benefit similar to that seen with surgical resection, although it is difficult to decipher individually because it is typically combined with surgery. Some patients in these series were treated with both surgery and RT, and the data suggest that the surgical group had higher rates of RT use. The larger RT studies have come mainly from single institutions in France and Canada. In 2009, Le Scodan and associates identified 581 patients with de novo stage IV breast cancer treated between 1984 and 2004; RT included both nodal fields and a boost to the tumor site for most patients. Of these patients, 320 received locoregional RT, 30 received only surgery, and 41 women received both surgery and RT. The 3-year overall survival rate was 43% versus 27% in the group receiving locoregional RT (LRT) versus those who did not, with an adjusted HR of 0.7 (95% CI 0.58–0.85). Nguyen and colleagues have reported a series from British Columbia, evaluating the effect of LRT on survival in 733 patients presenting between 1996 and 2005. Of these, 378 patients had PSLT that consisted of surgery alone in 67% of patients, RT alone in 22%, and both in 11%. 355 patients had no local therapy. The 5-year overall survival rates were 21% in those who had PSLT compared with 14% in those who did not ( p < .001). The rates of locoregional progression-free survival were higher in those who had PSLT (72% vs. 46%; p < .001). Patients who had both surgery and RT had a better 5-year overall survival of 32.5% compared with those who had surgery or RT alone (21 and 17%, respectively). A second French study of 239 patients comparing those who had surgery or RT alone showed a trend toward increased survival. However, no advantage was noted between either group after adjustment for prognostic factors, although RT alone did improve local control.
Effect of Primary Site Local Therapy on Locoregional Control
The rationale for treatment of the primary tumor may relate to a need for palliation of a symptomatic tumor, or a fear of ULD. Clearly, a woman living with metastatic breast cancer will be further distressed if the primary site is uncontrolled. Chest wall outcomes are therefore important, but data regarding these is scant. The largest data set comes from a single-institution retrospective review, where chest wall control was related to use of surgery and to survival in patients with metastatic breast cancer and an intact primary tumor. Between 1995 and 2005, 111 patients were identified at Northwestern Memorial Hospital, 42% of whom underwent surgical resection of the primary tumor within 6 months of diagnosis, in the absence of symptoms caused by the primary tumor. The nonoperative arm included patients who had delayed surgery secondary to symptomatic local progression or did not have surgery at all. Both groups were well matched, and all received systemic therapy. Local control was more often maintained in patients treated surgically (82% vs. 34%; p = .002). Surgical resection was associated with longer time to first progression (adjusted HR 0.5, 95% CI 0.298–0.838), but there was no statistically significant difference in terms of overall survival. However, when survival was examined as a function of chest wall control, women who maintained a controlled chest wall survived significantly longer than those who developed symptomatic chest wall disease (i.e., skin nodules or ulceration) with better overall survival (HR 0.42, 95% CI 0.26–0.66; p = .0002).
Similar questions regarding management of the primary site pertain to the scenario where metachronous distant recurrence is accompanied by an in-breast recurrence. Data regarding this come from a retrospective review of 5502 patients within the Danish Breast Cancer Group database, who had breast conserving surgery for stage I–II breast cancer from 1976 to 1998. Three hundred and seven patients were identified within breast tumor recurrence, and the role of surgical resection for recurrence was evaluated. Resection of the in-breast recurrence was shown to protect against both ULD and death. After in-breast recurrence, patients treated nonoperatively had the highest rate of ULD (32%), followed by those treated with repeat breast conserving therapy (16%). Patients treated with salvage mastectomy had the lowest rate of ULD (10%; p = .004). The maintenance of local control was associated with longer survival duration compared with patients who developed ULD (5-year survival, 78% vs. 21%). Patients with the highest risk of ULD were patients with disseminated disease (odds ratio [OR] 12.7; p < 0) and patients treated nonsurgically (OR 5.6; p = .003).
Retrospective Studies Questioning the Benefit of Primary Site Local Therapy in De Novo Stage IV Breast Cancer
Contrary to the positive findings already discussed, a few studies have demonstrated only a trend toward improvement in survival or no improvement in survival. The largest study showing a trend was a review by Cady and associates in an analysis of 622 patients with de novo stage IV breast cancer, where surgically treated stage IV patients were matched to controls who did not receive PSLT. Matched-pair analysis lessened but did not eliminate the survival benefit associated with PSLT in all subsets. However, among women with visceral metastases only (100 patients), the matched analysis showed no significant survival benefit. A detailed review of medical records for 100 women within the study showed that tumor stage and surgical procedure were categorized inaccurately, which may have influenced results. The authors also noted benefits for patients who received surgery after systemic therapy, indicating that patients who had more favorable responses to systemic therapy were more likely to benefit from surgery.
Selection Biases in the Retrospective Analyses
Although these retrospective studies showed significant benefit and no worse outcome for patients undergoing surgical intervention, they suffer from substantial biases that lead to the question of whether the apparent survival benefit from PSLT is a cause-and-effect relationship or whether this is explained entirely by selection bias. These trends are demonstrated in the 15 trials included in the meta-analysis by Petrelli and Barni, where a consistently better prognostic profile is seen in women receiving PSLT. The factors that significantly favor the PSLT groups range from younger age (in 10 of 15 trials), to smaller tumors (greater fraction of T1–T2 tumors in 10 of 11 trials where this is reported), greater frequency of single organ system metastases in 11 of 15 trials, significantly less frequent visceral disease in 11 of 15 trials, fewer comorbidities, and better access to care. Although these are recognizable biases that were adjusted for in statistical analyses, the possibility of unrecognized biases or incomplete adjustment remained. One bias that was recognized early on and particularly affects the data derived from tumor registries, is that of surgical timing. This relates to the fact that women who present with clinically nonmetastatic disease, do not undergo staging scans before resection of the primary tumor, then may be staged postoperatively and found to have metastases. Their metastatic burden is likely lower than that of women who present with symptomatic metastases; in both instances, however, they would be reported to the tumor registry as stage IV patients because this reporting typically occurs many weeks after surgery. This particular bias has been examined in several studies with mixed results. Rapiti and colleagues performed an analysis in which women diagnosed with metastases postoperatively were excluded and found a persistent association of the use of surgery with improved survival, although the effect was smaller. In an analysis from MD Anderson, the optimal timing of surgery appeared to be 3 to 6 months after diagnosis, suggesting that these women were known to have metastases preoperatively, although this aspect is not specifically addressed. In an analysis from Boston, the benefit of surgery appeared to be confined to the group who underwent resection before metastatic diagnosis. In a second analysis, the same group used a case-control set from the NCCN with similar results, although the design of the study was difficult to interpret because women who underwent surgery before systemic therapy were compared with those who received no surgery, and women with known metastases who received systemic therapy before surgery were excluded. The nonsurgery group comprised 236 patients versus 54 patients in the surgical group. Results showed that before matching, those in the surgery group were more likely to be younger, less likely to have more than one site of metastatic disease, and more likely to have received endocrine therapy. However, when matched for prognostic factors, there was no survival benefit to surgery when performed before systemic therapy. Survival was similar as well after adjusting for nonmatched factors such as presence of lung metastases, year of diagnosis, and use of trastuzumab.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








