Fig. 13.1
Lymphatic flow of the rectum. The rectal lymphatic flow is categorized into three categories including an upward lymphatic flow, which moves upward in line with the inferior mesenteric artery and collects in the para-aortic lymph node; a lateral lymphatic flow, which passes through the pelvic plexus in line with the middle rectal artery before reaching the lymph node below the aortic bifurcation after passing through the internal iliac lymph node and common iliac lymph node; and a downward lymphatic flow, which moves in line with the lower rectal artery, from the proctodeum, subcutaneously via the peritoneum, toward the superficial inguinal lymph node
The American Joint Committee on Cancer (AJCC) Cancer Staging Manual categorizes the internal iliac lymph node and external iliac lymph node as rectal cancer region lymph nodes, but does not give a clear definition of LPLN [20]. In Japan, the LPLN is defined as in Fig. 13.2 in the Japanese Classification of Colorectal Carcinoma, based on the experience of treatment utilizing LPLN dissection [21]. In other words, the internal iliac lymph node (#263), the lymph nodes that exist on the outside of the rectal ligament and pelvic plexus, in line with the internal iliac artery; the obturator lymph node (#283) which exists within the obturator lumen held between the external iliac artery and the internal iliac artery; the external iliac lymph node (#293), which is positioned in line with the external iliac artery; and the common iliac lymph node (#273), which is positioned in line with the common iliac artery are defined as LPLN.
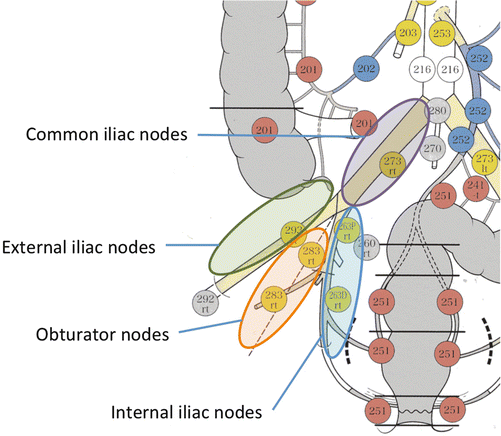

Fig. 13.2
Lateral pelvic lymph nodes according to the Japanese classification. The Japanese Classification of Colorectal Carcinoma defines the internal iliac lymph node (#263), the lymph nodes that exist in line with the internal iliac artery; the obturator lymph node (#283) which exists within the obturator lumen held between the external iliac artery and the internal iliac artery; the external iliac lymph node (#293), which is positioned in line with the external the external iliac artery; and the common iliac lymph node (#273), which is positioned in line with the common iliac artery, as lateral pelvic lymph nodes
The Status of Lateral Pelvic Lymph Node Metastasis
Sugihara et al. retrospectively gathered data from rectal cancer cases at multiple facilities in Japan and reported on the status of LPLN metastasis [17]. Of 1977 cases in which curative surgery had been performed, lateral dissection was performed in 930 (47%), with LPLN metastasis confirmed in 129 (13.9%) of the cases undergoing LPLN dissection. The frequency of LPLN metastasis has been reported elsewhere as between 10.6% and 25.5%, with most reports suggesting it is somewhere around 15% [22–28] (Table 13.1). Of these, the proportion of cases testing positive for LPLN metastasis among cases of lymph node metastasis within the mesorectum (upward) was high, at 23.5%. When the tumor location was studied, LPLN metastasis was found in 8.2% of cases when the tumor was intraperitoneal (proximal to the peritoneal reflection) (the upper rectum, defined as Ra in the Japanese classification), but that in 14.9% of cases—a significantly higher rate—when the tumor was extraperitoneal (between the peritoneal reflection and the anus) (the lower rectum, defined as Rb in the Japanese classification). Kanemitsu et al. studied the tumor location in terms of its distance from the anal verge, demonstrating that in cases where distance from the anal verge was over 9 cm, the frequency of LPLN metastasis was low, at 1.4%, while in cases where it was 9 cm or smaller, the rate of metastasis suddenly increased (to 9.1% between 8.1 and 9.0 cm, 12.5% between 6.1 and 8.0 cm, 20.3% between 4.1 and 6.0 cm, 18.8% between 2.1 and 4.0 cm, and 23.3% between 0.0 and 2.0 cm). An analysis of the depth of tumor invasion by Sugihara et al. showed that the frequency of LPLN metastasis was 7.1% in cases T2 and shallower, but it was significantly higher (16.6%) in cases T3 or deeper [17]. Based on this analysis of metastasis frequency , the indication criteria for LPLN dissection according to Japan’s colorectal cancer treatment guidelines were set as “cases in which the lower border of the tumor is located distal to the peritoneal reflection and the tumor has invaded beyond the muscularis propria” [29]. Other risk factors in LPLN metastasis are reported to include being female, histologic types other than well-/moderately differentiated adenocarcinoma , etc. [17, 18, 28]. It is assumed that the reason being female is an independent risk factor in LPLN metastasis is due to some sort of oncological or anatomical difference between men and women; however, this has not been clarified.
Study | Year | Positive lateral nodes (%) | 5-year overall survival (%)a |
|---|---|---|---|
Moriya et al. [23] | 1997 | 14.0 | 49.3 |
Mori et al. [24] | 1998 | 25.5 | 43.0 |
Ueno et al. [25] | 2001 | 15.5 | 39.0 |
Shirouzu et al. [26] | 2001 | 15.5 | <3 positive nodes: 60 ≥3 positive nodes: 16.7 |
Shimoyama et al. [27] | 2003 | 13.6 | 38.9 |
Ueno et al. [28] | 2005 | 17.3 | 42.0 |
Sugihara et al. [17] | 2006 | 13.9 | 47.7 |
Frequency of metastasis by location within LPLN has been examined and reported. As above, the Japanese Classification of Colorectal Carcinoma divides the LPLN broadly into four defined areas [21] (Fig. 13.2). According to Kobayashi et al., a study of 117 cases of lower rectal cancer (Rb) cases testing positive for LPLN metastasis showed the highest rate of metastasis in the internal iliac lymph node [18]. The frequency of metastasis in these lymph nodes differs significantly on either side of the superior vesical artery , which is the branch of the internal iliac artery, occurring in 26% among lymph nodes on the proximal side of the superior vesical artery (#263P), but 47% on the distal side (#263D). Obturator lymph node cases (#283) had the next highest rate of metastasis frequency (38%). The frequency of metastasis in other areas was much lower than those in the internal iliac lymph node and obturator lymph node (external iliac lymph node (#293), 6%; common iliac lymph node (#273), 3%; aortic bifurcation lymph node (#280)/midline sacrum lymph node (#270), 6%). Based on analysis of these metastasis frequencies, lymph nodes defined in the Japanese Classification of Colorectal Carcinoma as LPLNs include the internal iliac lymph node and obturator lymph node within the scope of D3 dissection, with the common iliac lymph node and external iliac lymph node not included in D3 dissection.
Image Diagnosis of Lateral Pelvic Lymph Node Metastasis
Image diagnosis of rectal cancer lymph node metastasis is performed using endoscopic ultrasonography (EUS) , CT, or MRI. According to meta-analysis, the sensitivity and specificity of each method is 67% and 78% with EUS, 55% and 74% with CT, and 66% and 76% with MRI, respectively, revealing no significant difference between the methods [30]. EUS is considered useful for diagnosing lymph nodes within the mesorectum, but is not suitable for the diagnosis of LPLN metastasis for anatomical reasons. MRI gives better contrast resolution for soft tissue than CT and is a more specific method of diagnosis. The European Society for Medical Oncology (ESMO) guidelines stress the importance of correctly diagnosing lymph node metastasis both within and outside the mesorectum for rectal cancer staging, and as a result, MRI is considered the first choice [31].
Cross-sectional images using CT or MRI depict the LPLN in the deep part of the pelvis, surrounded by the external iliac artery as the ventral border, the internal iliac artery as the dorsal border, the psoas major muscle and internal obturator muscle as the outer (pelvic wall side) border, and the proper rectal fascia as the inside (organ side) border (Figs. 13.3 and 13.4). While a range of diagnostic criteria have been reported for use in diagnosing lymph node metastasis, the method most generally accepted is the size of the lymph node depicted. Metastatic lymph nodes tend to be bigger than nonmetastatic lymph nodes [32]. When the maximum diameter of lymph nodes is compared on a histogram, however, there is a significant amount of crossover between metastatic and nonmetastatic lymph nodes [33], making it difficult to establish a strict cutoff value. For this reason, signal heterogeneity and irregular border are used, among other non-size-related morphological criteria, with reports suggesting that they are useful [34, 35]. Morphological determinations become more difficult, however, the smaller the size of the lymph node, and at present, it is believed difficult to diagnose lymph node metastasis merely using criteria relating to size and morphology.
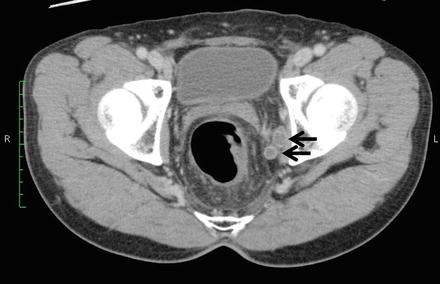
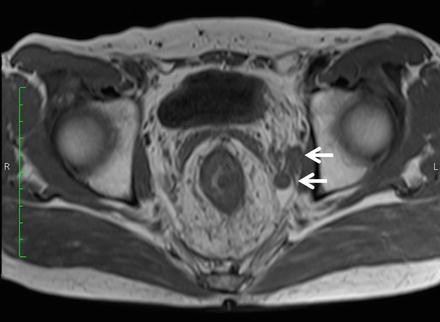

Fig. 13.3
CT findings of lateral pelvic lymph node metastasis. Uneven swelling noted in the left lateral pelvic lymph nodes (arrow)

Fig. 13.4
MRI findings of lateral pelvic lymph node metastasis. Uneven swelling noted in the left lateral pelvic lymph nodes (arrow)
More recently, diagnostic methods including qualitative elements, such as MRI diffusion-weighted imaging and FDG-PET, have proven useful in regard to diagnosing lymph node metastasis. In particular, FDG-PET has a sensitivity of between 40 and 50%, which is slightly lower than that of EUS, CT, and MRI, but an extremely high level of specificity at 90% [36], suggesting it may be a potentially effective supplementary method of morphological diagnosis (Fig. 13.5).
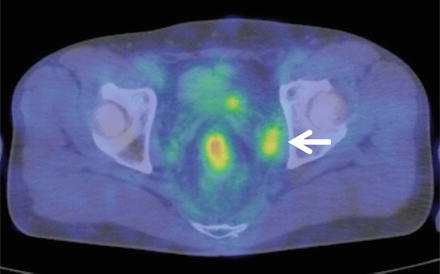

Fig. 13.5
FDG-PET findings of lateral pelvic lymph node metastasis. High accumulation image noted in the left swollen lateral pelvic lymph nodes (arrow)
Prognosis in Cases with Lateral Pelvic Lymph Node Metastasis and the Effect of Dissection
Sugihara et al. analyzed multicenter data in Japan and found that among cases in which LPLN dissection was implemented, the 5-year overall survival (OS) rate among patients with LPLN metastasis was 45.8%, which is significantly worse than the 71.2% of stage III cases with no LPLN metastasis (where lymph node metastasis was only inside the mesorectum) [17]. Furthermore, assuming that local recurrence occurs when LPLN metastasis is not removed and left, leading to the cause of death, it is calculated that LPLN dissection would have reduced T3–T4 rectal cancer local recurrence by 50%, improving the 5-year survival rate by 8%. Fujita et al. report [37] that upon analysis of 91 cases of LPLN metastasis in which dissection was performed, the 5-year OS rate was 39%, while the 5-year disease-free survival rate (DFS) was 27%. Japanese reports of this type state that the 5-year OS for cases of LPLN metastasis in which dissection was performed is around 40% (Table 13.1).
Akiyoshi et al. retrospectively analyzed more than 10,000 cases of lower rectal cancer, dividing the LPLN into the internal iliac lymph node (internal LPLN) and categorizing everything else as the external LPLN (obturator lymph node, external iliac lymph node, common iliac lymph node, lymph node below the aortic bifurcation, and midline sacrum lymph node) and then studying the prognoses in cases that metastasized. The 5-year OS and cancer-specific survival (CSS) in metastasized internal LPLN cases were 45% and 49%, respectively, which was comparable to the 5-year OS (45%) and 5-year CSS (51%) for cases in which lymph node metastasis was confined to the inside of the mesorectum with the number of metastasized node between 4 and 6, defined according to the AJCC Cancer Staging Manual as N2a. Furthermore, the 5-year OS and CSS for cases in which metastasis occurred in the external LPLN were 29% and 34%, respectively, which was worse than cases in which metastasis remained in the internal LPLN, but roughly the same as the 5-year OS (32%) and CSS (37%) of cases defined by AJCC as N2b, and significantly better than the 5-year OS (24%) and CSS (27%) of stage IV rectal cancer cases undergoing R0 resection. These results indicate that even if there is metastasis in the LPLN, provided it is removed, the prognosis is comparable to cases in which it is limited to the inside of the mesorectum and better than stage IV cases; in other words, they lead to the conclusion that LPLN metastasis is not a systemic disease, but should rather be considered a regional disease, and that it is important that it be removed if at all possible [38]. Furthermore, analysis of the prognostic factors indicates that LPLN dissection is an independent prognostic factor, equivalent to the stage and number of dissected lymph nodes, and that LPLN dissection can improve OS and CSS. Therefore, while cases with LPLN metastasis have a relatively poor prognosis in reports from Japan, there are indications that removal can improve prognosis. The USA Guidelines 2000 for Colon and Rectal Cancer Surgery also state that if LPLN metastasis is suspected clinically, then dissection should be attempted if it is considered possible [39].
Prophylactic Dissection of Lateral Pelvic Lymph Node
The indications above are that dissection may improve prognosis in cases of metastasis to the LPLN. The fact that the rate of metastasis to the LPLN is around 15% means that there are no oncological benefits to LPLN dissection for the majority of patients. It is, however, impossible to make a 100% accurate diagnosis of LPLN metastasis either preoperatively or from intraoperative findings. For this reason, we need to consider whether or not there is any meaning in prophylactic LPLN dissection for cases in which it is determined that there is no LPLN metastasis. An RCT (JCOG0212 trial) is currently being carried out in Japan in order to answer this question [40]. This study involves patients with advanced lower rectal cancer, in whom it has been clinically determined that no LPLN metastasis has occurred, and is a trial to validate the non-inferiority of TME alone without prophylactic LPLN dissection to TME with prophylactic LPLN dissection. Cases of rectal cancer in which the lower edge was between the peritoneal reflection and the anus, in clinical stages II–III, and with no swelling of the LPLN equal to or greater than 10 mm observed upon CT or MRI, which were operated on without any preoperative treatment such as CRT, with macroscopic R0 resection, and in which it was determined based on intraoperative findings that no LPLN metastasis had occurred, were randomly divided into those in whom a LPLN dissection was performed and those in whom it was not. The primary endpoint was the relapse-free survival, with an analysis expected to be conducted in 2015. The secondary endpoints are overall survival, local recurrence-free survival, occurrence of adverse events, surgery time, hemorrhage volume, and the occurrence of sexual dysfunction/urinary function problems. A report was made in 2012 of the short-term results [40], which indicated that the LPLN dissection group had significantly longer surgery time than those not undergoing LPLN dissection (360 min vs. 254 min, p < 0.0001), as well as greater blood loss (576 ml vs. 337 ml, p < 0.0001). There was no significant difference in the incidence of grade 3–4 adverse events, although the group undergoing LPLN dissection had slightly more (22% vs. 16%, p = 0.07). In terms of the primary endpoint of relapse-free survival, if the non-inferiority of TME without LPLN dissection is proven, it will be possible to determine that TME without LPLN dissection is an effective therapy for cases in which it is determined that LPLN metastasis has not taken place.
Preoperative Chemoradiotherapy and Lateral Pelvic Lymph Node Dissection
Preoperative CRT in cases of locally advanced rectal cancer has been demonstrated in multiple clinical trials to reduce postsurgical local recurrence [9–13]. In these clinical trials, TME was the basic surgery, with no routine LPLN dissection carried out. In Japan, on the other hand, TME accompanied by LPLN dissection has become standard, without preoperative CRT, and reports indicate that it achieves a similar or lower rate of local recurrence to that of TME after CRT in Europe and the USA [17, 18, 41] (Table 13.2). Given this, discussions are now underway regarding whether or not it is oncologically acceptable to omit LPLN dissection if preoperative CRT has been implemented. However, few reports exist that consider this clinical question.
Table 13.2
Impact of treatment modalities on local recurrence and survival
Study | Year | Design | Treatment | 5-year local recurrence (%) | p | 5-year overall survival (%) | p |
|---|---|---|---|---|---|---|---|
Western series | |||||||
Swedish trial [10] | 2005 | RCT | Surgery alone | 26a | <0.001 | 30b | 0.008 |
sRT | 9a | 38b | |||||
Dutch trial [57] | 2001 | RCT | Surgery alone | 10.9 | <0.001 | 63.5 | NS |
sRT | 5.6 | 64.2 | |||||
EORTC22921 [12] | 2006 | RCT | RT | 17.1 | 0.002 | 64.8 | NS |
CRT | 8.7 | 65.8 | |||||
FFCD9203 [13] | 2006 | RCT | RT | 16.5 | 0.0004 | 67.9 | NS |
CRT | 8.1 | 67.4 | |||||
Japanese series | |||||||
Moriya et al. [41] | 1995 | Retrospective | Surgery alonec | 9.3 | – | 70.0 | – |
Sugihara et al. [17] | 2006 | Retrospective | LLND (−) | 5.4 | <0.0001 | 76.9 | 0.0017 |
(+) | 10.9 | 82.6 | |||||
Kobayashi et al. [18] | 2009 | Retrospective | LLND (−) | 7.4 | NS | 79.5 | NS |
(+) | 10.5 | 75.8 | |||||
Watanabe et al. divided patients with advanced lower rectal cancer on which curative removal had been implemented into four groups, depending on whether or not they had undergone preoperative radiotherapy and whether or not they had undergone LPLN dissection, and retrospectively compared their prognoses. Overall, while an improvement in disease-free survival (DFS) was noted in patients undergoing preoperative radiotherapy, the fact that there was no difference in DFS between the group undergoing lateral dissection without radiotherapy and those undergoing radiotherapy, but no lateral dissection, indicates that preoperative radiotherapy may be a replacement for LPLN dissection [42].
Nagawa et al. compared prognosis and function between random groups comprising rectal cancer patients in whom no LPLN metastasis was diagnosed prior to surgery, who were given radiotherapy and then underwent rectal resection and then either underwent or did not undergo LPLN dissection [43]. There was no difference in either OS or DFS between the two groups. Furthermore, the group undergoing LPLN dissection had significantly higher rates of urinary function disorders and male sexual dysfunction. Given these results, it is possible that there is no need for patients diagnosed with rectal cancer and no LPLN metastasis to undergo LPLN dissection following radiotherapy. This clinical study is the only RCT verifying the preventative effects of LPLN dissection following radiotherapy; that said, it involved only 45 cases, and as such, the results thereof need to be interpreted with caution.
Kim and Takahashi et al. studied the additional oncological benefits of LPLN dissection and postsurgical CRT on TME in facilities in Japan and Korea. While there was no difference in OS or DFS, local recurrence occurred in 2.2 times the number of cases in the LPLN dissection compared with the postsurgical CRT group. From this result, it appears that postsurgical CRT is more effective in controlling local recurrence than LPLN dissection, that LPLN dissection alone is insufficient, and that the group on which LPLN dissection was performed also requires CRT. This trial, however, has several identified problems, including the fact that CRT was performed postsurgically and that the definition of lower rectal cancer differed between the institutions [44].
Kim et al. analyzed the local recurrence patterns in rectal cancer patients who were given CRT prior to TME and found that LPLN recurrence was the most common form of local recurrence. Local recurrence was noted in 7.9% of patients, and of these, it occurred in the LPLN in 82.7% of cases. Risk factor analysis was implemented for LPLN recurrence, with the result that, assuming that ypN+ and LPLN swelling are risk factors in recurrence, discussions are now ongoing regarding the potential benefits of lateral dissection after CRT for patients with these risk factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree