TABLE 12A.1 SUMMARY OF MAJOR PROSPECTIVE TRIALS USING HORMONAL THERAPY AND RADIOTHERAPY | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
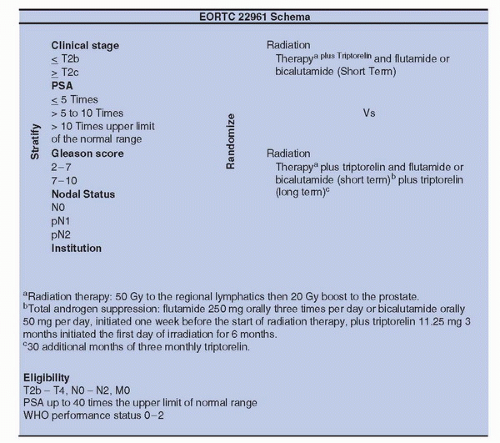
significant difference in overall survival (p = 0.02) and PFS (p = 0.005) in favor of the combined arm; this difference was mainly caused by lymph node positive tumors. In conclusion, patients with pathologically or clinically involved pelvic lymph nodes should be considered for RT plus immediate long-term HT.
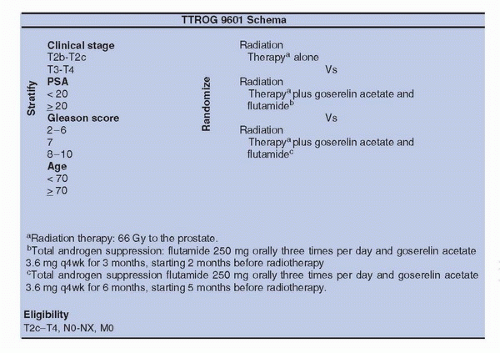
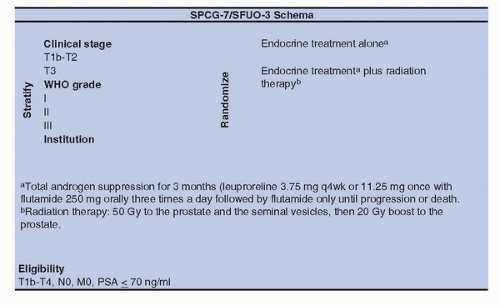
alone was not assessed so far (Fig. 12A.2). The SPCG-7/SFUO-3 trial has included 875 patients with T1b-2, G2-3, or T3 of any WHO histological grade (1,2,3) (78% of T3) with baseline PSA < 70 ng/mL; patients were randomly allocated to endocrine treatment alone (3 months of total androgen blockade followed by continuous treatment using flutamide [439 patients]) or to the same endocrine treatment combined with RT (436 patients). After a median follow-up of 7.6 years, the cumulative incidence at 10 years for prostate cancer-specific mortality was 23.9% in the endocrine alone group and 11.9% in the endocrine plus RT group for a relative risk of 0.44 (0.30-0.66); the cumulative incidence for overall mortality was 39.4% and 29.6% with a relative risk of 0.68 (0.52-0.89) (26). In conclusion, in patients with locally advanced or high-risk localized prostate cancer, the combination of RT to HT halved the 10-year prostate cancer-specific mortality and decreased overall mortality with fully acceptable risk of side effects, compared to HT alone.
done with PSA (lower than 20 ng/mL), histological grade, and nodal status. Patients were randomized between neodjuvant CAB, 2 months before conventional irradiation and 2 months during irradiation (66.6 Gy) versus irradiation alone. The 10-year overall survival was 62% for the combined approach versus 57% (p = 0.03). In conclusion, the addition of neoadjuvant and concomitant CAB—4 to 6 months—with irradiation (66.6-70 Gy) improved overall survival in men with intermediate-or poor-risk localized prostate cancer without moderate or severe comorbidity.
CAS. The hazard ratio (HR) for freedom from PSA failure was 0.67 (95% CI 0.53-0.85; p = 0.0007) for the dose escalated group and the 5-year control rate was 71% for the high dose compared to 60% for conventional dose group. Of note, there was also a trend for improved freedom from salvage AS, HR of 0.78 (0.57-1.07; p = 0.12); and metastases-free survival was 0.74 (0.47-1.18; p = 0.21) (41). We are eager to wait the results of EORTC trial 22991, which has accrued 820 patients, comparing 3D-CRT +/− IMRT alone with three levels of dose (70, 74, 78 Gy) versus the same regimen plus 6 months AS. In intermediate-risk localized prostate cancer, there is likely a room for dose escalation alone, provided the dose is ≥78Gy. The results of RTOG trial 94-08 in good and intermediaterisk localized prostate cancer have shown an improvement of 10-year overall survival (33) with 66.6 Gy plus 4 months of CAB, but we do not have data concerning a trial with a dose escalation RT alone arm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree