Management of Diabetic Kidney Disease
Mark E. Williams
Robert C. Stanton
The management of patients with diabetic nephropathy has become both increasingly complex and increasingly rewarding in the past 10 years. The elucidation of at least some of the underlying mechanisms has led to a number of therapeutic interventions that, when applied aggressively, will lead to a highly significant reduction in the number of patients who progress to end-stage renal disease (ESRD). Although these approaches have not led to either the complete prevention or cure of diabetic nephropathy, they have produced dramatic improvements in patients’ quality of life and mortality rates and have decreased healthcare costs. Much research remains to be done, but the promise of even more effective therapeutic approaches is on the horizon. This chapter reviews the current approaches to treatment and briefly anticipates future therapies.
The seriousness of diabetic nephropathy is demonstrated by the following. First, patients with diabetic nephropathy have a reduced life span. Second, diabetic patients with nephropathy have more comorbid events (such as worse cardiac and peripheral vascular disease) than do diabetic patients without nephropathy, events that significantly affect the patient’s lifestyle and ability to work. Third, diabetic nephropathy is now the major cause of ESRD in the United States and throughout most of the world (1). Fourth, the financial costs of caring for these patients are rapidly increasing, as treatment of ESRD (dialysis and transplantation) is paid for by national healthcare systems such as Medicare in the United States (2). Thus, for reasons of quality of life, morbidity and mortality, and financial costs, the prevention or slowing of the progression of diabetic nephropathy will significantly benefit both the patient and public heath.
In this chapter, we will review the current approaches to therapy. Although we cover all therapeutic interventions, our focus is primarily on hypertension and the use of medications that block the actions of angiotensin. The approach to hypertension and the use of angiotensin-blocking drugs has had a dramatic impact on the progression of both type 1 and type 2 diabetes. The use of these drugs in providing optimal treatment of diabetic nephropathy has increased in importance and complexity. Thus, a detailed analysis of these drugs follows.
DIAGNOSIS, SCREENING, AND NATURAL HISTORY
Diabetic kidney disease takes years to develop. It is rare to see diabetic nephropathy earlier than 3 years after the diagnosis of diabetes; it usually is seen after 5 to 15 years in patients with type 1 diabetes (2). For patients with type 1 diabetes, this time course is well understood because these patients present with very clear symptoms at diagnosis. The natural history of kidney disease in type 2 diabetes is less well understood, as patients can have relatively mild symptoms for quite some time before a diagnosis is made. It is likely, however, that in type 2 diabetes, as in type 1 diabetes, kidney disease develops only after a number of years of diabetes. From the physician’s standpoint, the
practical difference is that screening for kidney disease is not necessary before at least 3 years and usually not until 5 years after the diagnosis in patients with type 1 diabetes but should begin at the time of diagnosis in patients with type 2 diabetes because the duration of their disease is unknown.
practical difference is that screening for kidney disease is not necessary before at least 3 years and usually not until 5 years after the diagnosis in patients with type 1 diabetes but should begin at the time of diagnosis in patients with type 2 diabetes because the duration of their disease is unknown.
The earliest known manifestation of diabetic kidney disease is the presence of small amounts of albumin in the urine, called microalbuminuria (3). Protein excretion in the urine normally does not exceed 100 to 200 mg per 24 hours. Much of this protein comes from the tubules, but a percentage is filtered through the glomeruli. Studies done 20 years ago showed that urinary excretion of albumin, which is normally excreted in amounts of less than 30 mg per 24 hours, increases even before total urinary protein levels increase (4). This very important insight allowed physicians to detect diabetic nephropathy much earlier than they had in the past, thus permitting earlier therapeutic interventions to prevent progression. Microalbuminuria is the presence in the urine of 30 to 300 mg of albumin per 24 hours. When albumin levels exceed 300 mg per 24 hours, this condition is called proteinuria or overt nephropathy. Microalbuminuria has been called “early diabetic nephropathy” or “incipient nephropathy,” which to some suggests early, possibly insignificant, disease that does not require therapeutic intervention. It must be stressed, however, that microalbuminuria is the earliest marker we are aware of. Clearly, mechanistic changes over a number of years in the glomeruli of the kidney are now being expressed as the presence of albumin in the urine. An ideal situation would be to look at renal glomeruli during the course of diabetes to detect changes even before microalbuminuria is present (as ophthalmologists can detect early retinal pathology). Because this is not possible, we use as a marker a point at which the glomerular filter has been sufficiently damaged to permit the passage of albumin. The point is that microalbuminuria is kidney disease that needs to be treated and not simply monitored. It should be noted that not all patients with microalbuminuria progress to overt proteinuria and renal failure. Although a variety of attempts have been made to find markers (both genetic and physiologic) that can determine who will progress to overt proteinuria and kidney failure and who will not, there is not yet a standard that lets us ascertain this. Thus, at this time we strongly recommend treating all patients who have microalbuminuria as if they are going to progress to more severe kidney disease because preventing it from worsening has clear benefits and the treatments are all reasonably safe and prudent.
As previously noted, patients with type 1 diabetes should be screened 3 to 5 years after diagnosis, and patients with type 2 diabetes should be screened at the time of diagnosis. Patients should be tested yearly by examining the urine for albumin. At this time, we recommend using the albumin/creatinine ratio in a spot urine test, as this accurately reflects 24-hour total excretion in most circumstances. Although a 24-hour urine test for albumin can be done, it has a few drawbacks, including inconvenience for the patient and the possibility of an incomplete collection. The ratio has been shown to be quite accurate for both diagnosis and follow-up (3). The albumin/creatinine ratio is normally less than 30 μg of albumin per milligram of creatinine. A ratio of more than 30 μg of albumin per milligram of creatinine is abnormal. The current recommendation is to repeat the albumin measurement on two more occasions during a 3- to 6-month period; if microalbuminuria persists, the patient has diabetic nephropathy (5). Some causes of transient increases in albuminuria include exercise, dietary intake, pregnancy, drugs, and other factors that can alter both protein delivery and glomerular hemodynamics. Although transient increases of up to 300 mg of albumin in 24 hours have been seen, levels of greater than 150 to 200 mg per 24 hours usually remain elevated after one or two more measurements. Of note, dipsticks used in standard urinalysis for the determination of protein in urine are not sensitive enough to measure albumin excretion of greater than 300 μg per milligram of creatinine and thus are not sufficiently sensitive to measure levels of microalbuminuria of 30 to 300 μg per milligram of creatinine.
The diagnosis of diabetic nephropathy is usually quite evident in the appropriate clinical setting, but it is important to be aware of associations that support the diagnosis or suggest the possibility of another diagnosis for a patient with diabetes and renal disease. A diagnosis of diabetic kidney disease is supported by the following. First, a large majority of patients have albuminuria. It has been suggested that up to 100% of patients with diabetic nephropathy have albuminuria; however, this is clearly not the case. The absence of albuminuria should certainly prompt a search for an alternate diagnosis, but in the absence of clear signs to seek an alternate diagnosis, there is usually nothing else to consider. Second, the urinary sediment is characteristically unremarkable—i.e., there are usually no casts, no white blood cells, and no red blood cells—although red blood cells (2 to 15 per high-power field) may be seen in up to 30% of patients. Third, the majority of patients have retinopathy before the onset of diabetic kidney disease. Fourth, as previously noted, the duration of the disease is also important; it is relatively unusual to diagnose diabetic nephropathy before 5 years of diabetes. Deviations from any of the above associations should prompt a search for alternate diagnoses in a patient with diabetes and kidney disease. It is rarely, if ever, necessary to obtain a renal biopsy to make a diagnosis of diabetic nephropathy. Renal biopsy in a patient with diabetes is used principally when there is a question about whether the kidney disease is due to a disorder other than diabetes.
EPIDEMIOLOGY
Diabetic nephropathy occurs in 30% to 50% of patients with type 1 or type 2 diabetes (5). In the past, except in select ethnic populations, the incidence and prevalence of nephropathy have been lower in patients with type 2 diabetes than in those with in type 1 diabetes. In recent years, however, the incidence and prevalence of diabetic nephropathy have been steadily increasing, so that the percentage of patients with type 2 diabetes who have nephropathy is approaching that seen in patients with type 1 diabetes (6). ESRD due to hypertension and glomerulonephritis is relatively unchanged, but there has been an inexorable increase in the cases of ESRD due to diabetic nephropathy such that almost 50% of all cases of ESRD are caused by diabetes. The increasing incidence of diabetic nephropathy in patients with type 2 diabetes is in part the result of the greater success in decreasing mortality due to type 2 diabetes. Improved management of the cardiovascular complications of type 2 diabetes through better control of lipids and improved and more stringent blood pressure control has significantly increased life expectancy and has allowed time for other complications to develop. The increased prevalence of diabetic nephropathy is due in part to the current worldwide epidemic of diabetes.
Specific ethnic populations are at unusually high risk for developing diabetic nephropathy. Native Americans (e.g., the Pima Indians of the southwestern United States) (7) and African Americans have a high prevalence of diabetes and diabetic nephropathy (8,9). The reasons for the increased risk have not been clearly identified. It is likely that there is a very strong genetic basis for the observed susceptibility. But factors such as hypertension and diet also may play a role. In any event, a concerted effort to understand the specific susceptibility of these groups is of great importance to the prevention of the increased
morbidity and mortality associated with the onset of diabetic nephropathy.
morbidity and mortality associated with the onset of diabetic nephropathy.
In general, the incidence of diabetic nephropathy follows a pattern of increase about 5 years after diagnosis, with the highest incidence 5 to 15 years after diagnosis of type 1 diabetes. There is a gradual decline in the incidence of diabetic nephropathy with increased duration of type 1 diabetes so that by 20 to 25 years after the diagnosis the incidence of diabetic nephropathy decreases. The natural history of diabetic nephropathy in type 2 diabetes is less well characterized because of the indefinite start of the disease.
WHO IS GOING TO PROGRESS FROM MICROALBUMINURIA TO MORE SEVERE RENAL DISEASE?
A major question surrounding therapy is who will progress from microalbuminuria to frank proteinuria (>300 mg/24 hours) and to ESRD. Because not all patients progress, perhaps not all of them need to be treated aggressively. Although this is an important question, the answer is not clear. A few studies have tried to determine markers for progression. A recent study by Caramori et al. (10) suggested that the rate of increase of albuminuria was predictive; i.e., a rapid rate was predictive of subsequent progression of disease. However, although the correlation is good, the albumin excretion rate was too variable to be used routinely for clinical decision making. Also, Thomas and colleagues (11) reviewed data from the Diabetes Complication and Control Trial (DCCT) to evaluate the relationship between an increase in blood pressure and progression to albuminuria. The retrospective analysis showed that an increase in albumin excretion routinely preceded an increase in blood pressure to a hypertensive range (140/90 mm Hg). They concluded that an increase in blood pressure was not a good marker for progression. Interestingly, this relationship was observed only in the conventionally treated group; in the intensively treated group, an increase in blood pressure preceded an increase in albuminuria. Thus, at this time there are no clear markers for progression, and even the intensity of treatment may affect markers. Considering the potential seriousness of worsening disease and the negative effect of increased albuminuria and frank proteinuria on cardiovascular and renal outcomes, we recommend very aggressive treatment of any patient with established microalbuminuria or frank proteinuria. The study by Thomas et al. (11) also illustrates the important point that the changing treatments for diabetes are possibly altering the phenotypic expression of diabetic nephropathy. For example, diabetic nephropathy (biopsy proven) with no albuminuria and no preceding retinopathy is now being diagnosed in more patients.
CREATININE CLEARANCE SHOULD BE ESTIMATED IN PATIENTS WITH KIDNEY DISEASE TO ACCURATELY ASSESS KIDNEY FUNCTION
An important consideration when evaluating patients with diabetic nephropathy is the relation between serum creatinine and the actual glomerular filtration rate (GFR). The creatinine clearance overestimates the actual GFR because a percentage of the creatinine is secreted into the tubule rather than filtered. Nevertheless, the creatinine clearance offers a highly useful approximation of GFR. A 24-hour urine test to determine creatinine clearance can be done, but a number of formulas use a combination of weight (as a marker of muscle mass), age, sex, and other factors to estimate creatinine clearance (12). An excellent and relatively simple formula is the Cockcroft-Gault calculation of the estimate of creatinine clearance.
This formula often provides surprises to non-nephrologists, as the creatinine clearance determined is considerably lower than expected (especially in older women). For example, the clearance for a 70-year-old woman with a serum creatinine of 1.5 mg/dL and a weight of 50 kg is
Determining the creatinine clearance is important for evaluating the severity of diabetic kidney disease and knowing with certainty that the patient is receiving proper dosages of drugs. For example, treatment with metformin is not recommended for patients with creatinine clearances of less than 50 mL per minute, and the dosages of many medications need to be adjusted for patients with impaired renal function.
As noted above, the creatinine clearance (measured either by 24-hour urine assessment or by a formula) is quite useful but overestimates the GFR. The overestimate may be greater for patients with diabetic kidney disease than for patients with nondiabetic kidney disease. The reason for this difference is unclear (although it may be due to a reduction in muscle mass and thus in the production of creatinine in patients with diabetes as compared with that in nondiabetic patients). In any event, it is important to be aware of the relationship between serum creatinine and GFR, as some patients with apparently “normal” serum creatinine values actually have significantly impaired renal function.
TREATMENTS
Control of Blood Glucose Levels
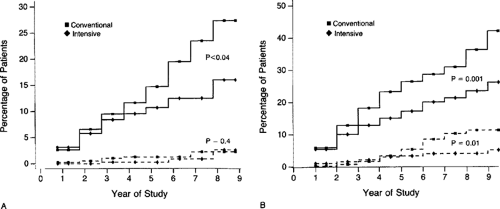
For many years, controversy existed over how stringent blood glucose control should be to prevent the development or progression of diabetic nephropathy. A number of studies have illustrated that intensive control of blood glucose levels is beneficial to kidney function. But the landmark DCCT study definitively demonstrated that tight control of the blood glucose significantly reduced the development of diabetic nephropathy and slowed progression of type 1 diabetes (13). In that study, 1,441 patients with type 1 diabetes were evaluated for a mean of 6.5 years and assigned either to a conventional therapy group [median hemoglobin A1c (HbA1c) of 9.1%] or to an intensive therapy group (median HbA1c of 7.2%). Intensive therapy led to a decrease in the presentation of microalbuminuria (defined as >40 mg per 24 hours) by 39% and led to a decrease in progression to albuminuria (defined as >300 mg per 24 hours) by 54% (Fig. 55.1). This dramatic reduction in the development and progression of diabetic nephropathy was achieved by diet, use of an external insulin pump, and/or the injection of three or more daily insulin injections associated with frequent monitoring of blood glucose level. A recent publication studied the same group of patients 4 years after the DCCT study to determine whether benefits of the DCCT persisted after completion of the trial (14). At the end of the DCCT, the patients in the conventional therapy group were offered intensive therapy, and the care of all patients was transferred to their own physicians. Nephropathy was evaluated on the basis of urine specimens obtained from 1,302 patients, approximately half from each group, during the third or fourth year. The median HbA1c values of the conventional therapy group (8.2%) and intensive therapy group (7.9%) were closer than in the original DCCT study. The authors reported that microalbuminuria was detected for the first time in 11% of 573 patients in the former
conventional therapy group as compared with 5% of 601 patients in the former intensive therapy group, representing a 53% odds reduction. The risk of new albuminuria was reduced by 86% in the intensive therapy group. Although blood glucose control worsened in the intensive therapy group, the importance of intensive control was still evident. Two significant observations can be made about these studies: Control of blood glucose to levels as close to normal as possible (without causing repeated hypoglycemic events) is beneficial; worsening of blood glucose control over years of diabetes therapy is quite common. This worsening of blood glucose control likely reflects a combination of increasing ineffectiveness of insulin due to multiple factors (e.g., changing metabolic requirements, resistance to effects of injected insulin, difficulty in maintaining the strict intensive regimen, age of the patient, genetic factors, and other as yet unanticipated factors). Thus, target blood glucose and HbA1c levels should be aimed for but may not be achieved. The chief adverse events associated with intensive therapy were a twofold to threefold increase in episodes of severe hypoglycemia and weight gain. In any event, the data strongly support the aggressive pursuit of close-to-normal blood glucose levels through an intensive mixture of diet, exercise, and insulin therapy in order so slow the progression of renal disease.
conventional therapy group as compared with 5% of 601 patients in the former intensive therapy group, representing a 53% odds reduction. The risk of new albuminuria was reduced by 86% in the intensive therapy group. Although blood glucose control worsened in the intensive therapy group, the importance of intensive control was still evident. Two significant observations can be made about these studies: Control of blood glucose to levels as close to normal as possible (without causing repeated hypoglycemic events) is beneficial; worsening of blood glucose control over years of diabetes therapy is quite common. This worsening of blood glucose control likely reflects a combination of increasing ineffectiveness of insulin due to multiple factors (e.g., changing metabolic requirements, resistance to effects of injected insulin, difficulty in maintaining the strict intensive regimen, age of the patient, genetic factors, and other as yet unanticipated factors). Thus, target blood glucose and HbA1c levels should be aimed for but may not be achieved. The chief adverse events associated with intensive therapy were a twofold to threefold increase in episodes of severe hypoglycemia and weight gain. In any event, the data strongly support the aggressive pursuit of close-to-normal blood glucose levels through an intensive mixture of diet, exercise, and insulin therapy in order so slow the progression of renal disease.
For type 2 diabetes, control of the blood glucose is also critical. A number of studies have shown he benefit of tight control of blood glucose in patients with type 2 diabetes with diabetic nephropathy (15). The large, comprehensive UK Prospective Diabetes Study Group (UKPDS) trial is of great importance (16). Patients in the conventional therapy group had an average HbA1c of 7.9%, whereas the average was 7.0% in the intensively treated group. In the intensively treated group, the reduction in risk for developing microalbuminuria was 11%, and the risk reduction for progression of microalbuminuria to proteinuria was 3.5%. Although these reductions are modest, the number of patients studied was large and the improvements were highly significant. The change in HbA1c was also relatively modest, and it is likely that greater reductions would have a greater protective effect on kidney function. The recommendation for blood glucose control in diabetic nephropathy in both type 1 and type 2 diabetes is to aim for HbA1c values of less than 7%.
Considering the central importance of hyperglycemia to the development of diabetic complications, it would appear that intensive control of blood glucose alone should be adequate to protect against the development or progression of diabetic kidney disease, but that is clearly not the case. Blood glucose control is only part of the management profile; blood pressure control is of paramount importance. In patients with type 2 diabetes in the UKPDS, controlling blood pressure was more important than lowering glucose levels in protecting the kidney. The next sections discuss in depth the management of hypertension, an essential part of treating a patient with diabetic nephropathy.
Management of Hypertension
More than 11 million people in the United States have coexistent diabetes mellitus and hypertension; however, hypertension in patients with diabetes remains underrecognized and undertreated. Hypertension and the risk of nephropathy form a continuum (17), so that all diabetic persons with hypertension, and even those with high normal blood pressure, are in a high-risk category (18). In addition, hypertension and diabetes combined are devastating to the cardiovascular system.
Hypertension is particularly prevalent in diabetic patients with nephropathy. Hypertension is now considered an early abnormality, not a late complication, of nephropathy. In patients with type 1 diabetes, a rise in systemic pressure may precede or predict the presence of diabetic nephropathy. In patients with type 2 diabetes, hypertension is common even without nephropathy, so that many patients with microalbuminuria and most of those with proteinuria are hypertensive (19) or are receiving treatment for hypertension (Table 55.1).
TABLE 55.1. Prevalence of Arterial Hypertension in Patients with Diabetes Mellitus | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
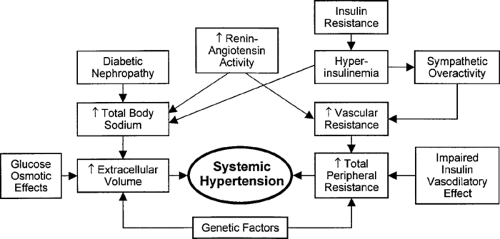
Several factors contribute to hypertension in type 1 and type 2 diabetes (20) (Fig. 55.2). The role of genetic factors in the clinical management of the diabetic patient with nephropathy has not yet been adequately defined.
Of further clinical importance is the link between hypertension and cardiovascular disease. Although diabetes and hypertension are both independent risk factors for cardiovascular disease, more than doubling the risk of cardiovascular mortality,
the risk is even greater when nephropathy is present. Cardiovascular causes account for more than half of the mortality associated with nephropathy (21). The diabetic patient with hypertension is already burdened with a greater prevalence of cardiovascular risk factors, such as dyslipidemia, hyperuricemia, thrombotic tendency, and left ventricular hypertrophy. The additional cardiovascular risk attributed to nephropathy is not adequately understood. However, aggressive therapy provides the opportunity to reduce excessive deaths due to cardiovascular disease (19).
the risk is even greater when nephropathy is present. Cardiovascular causes account for more than half of the mortality associated with nephropathy (21). The diabetic patient with hypertension is already burdened with a greater prevalence of cardiovascular risk factors, such as dyslipidemia, hyperuricemia, thrombotic tendency, and left ventricular hypertrophy. The additional cardiovascular risk attributed to nephropathy is not adequately understood. However, aggressive therapy provides the opportunity to reduce excessive deaths due to cardiovascular disease (19).
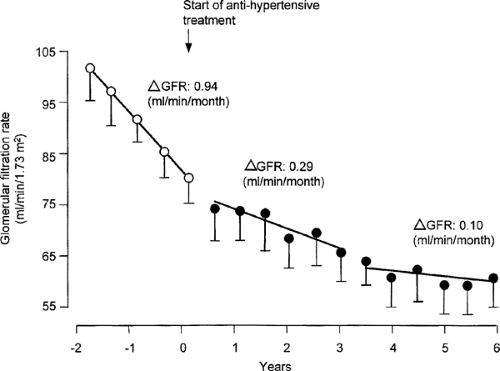
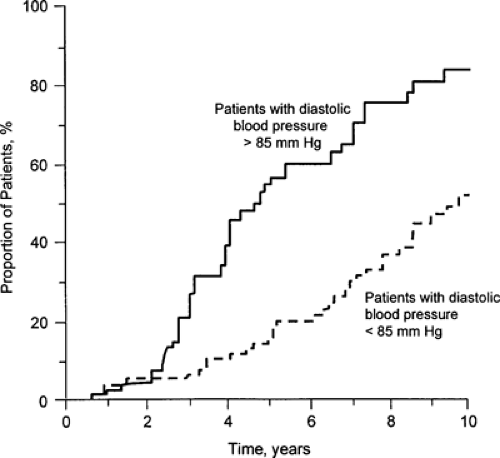
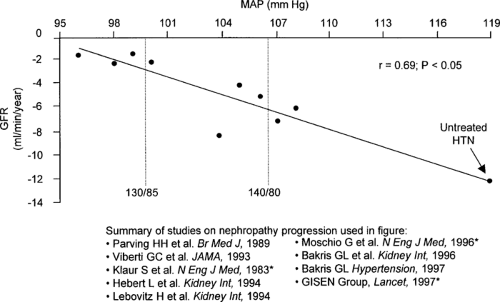
Effective antihypertensive therapy is generally regarded as the best inhibitor of diabetic nephropathy (22,23) almost regardless of the class of agent used (24,25). Convincing data now strongly support a role of hypertension in the progression of diabetic renal insufficiency, both for type 1 and for type 2 diabetes (26,27), as well as the benefit of early control of hypertension (Figs. 55.3, 55.4, 55.5). Although many studies of type 1 diabetic nephropathy have clearly demonstrated the benefit of controlling hypertension, the benefit in type 2 diabetes is not as clear. In a recent meta-analysis of 100 controlled and uncontrolled trials, about one third of the studies included only patients with type 2 diabetes. Overall, each 10 mm Hg decrease in mean arterial pressure was associated with an increase in GFR of about 4 mL per minute, which otherwise declined by 8 mL per minute (28). Angiotensin-converting enzyme (ACE) inhibitors were used in almost half of the groups. However, the duration of the study was greater than 1 year in only 13% of the study groups.
Hypertension, which exacerbates all the vascular complications of diabetes, worsens albuminuria and accelerates the decline in GFR. Because the afferent arteriole fails to autoregulate normally in patients with diabetic nephropathy, any increase in mean systemic pressure can be transmitted to the glomeruli (29). Further increases in glomerular capillary pressure derive from the effect of the increase in efferent arteriolar resistance by angiotensin (30).
Important management decisions that clinicians now face concerning the hypertensive diabetic patient with nephropathy include the following three questions (31,32):
When should treatment be initiated following the JNC-VI (Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) guidelines, in which hypertension is still defined as 140/90 mm Hg?
The blood pressure levels of diabetic patients need to be lower than those generally recommended to maximize the reduction in risk of renal disease. The JNC-VI recommended that all diabetic patients with blood pressure in excess of 130/85 mm Hg (mean arterial pressure, 100 mm Hg) be treated with both lifestyle modifications and drugs (Table 55.2). These guidelines were based on clinical trial results available in 1997 and have been supported by additional trials since the
guidelines were issued (33). Patients with hypertension and diabetes should be treated as if they already have target organ damage. Convincing studies conducted over more than two decades show that hypertension should be corrected at all levels of kidney function because aggressive control of hypertension is of key importance in slowing the loss of renal function. The effectiveness of blood pressure control appears to be related to the blood pressure achieved. A measurable decrease in proteinuria, a likely sign of renal protection, is also achievable by a reduction in systemic blood pressure.
TABLE 55.2. Risk Stratification and Treatment of Diabetic Patients with Nephropathya
Blood pressure stages (mm Hg)
Diabetes, with or without other risk factors (part of risk group C)
High-normal (130–139/85–89)
Lifestyle modification and drug therapy
Stage 1 (140–159/90–99)
Lifestyle modification and drug therapy
Stages 2 and 3 (>160/>100)
Lifestyle modification and drug therapy
aBy JNC-VI (Sixth Report of the Joint National Committee). All patients with diabetes and/or renal insufficiency should be instructed on lifestyle modification. All those with blood pressure >130/>85 mm Hg should also be started on drug therapy.
Adapted from the Sixth Report of Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 2000;160:1277–1283.
What should the target blood pressure be?
Although the unique sensitivity of diabetic nephropathy to systemic pressure has gained acceptance, the target blood pressure is still a source of some confusion (34). The primary goal is to lower blood pressure to the desired target levels. Guidelines of the JNC-VI (The 1997 report) reduced the target blood pressure for patients with diabetes to 130/85 mm Hg, values below those for nondiabetic hypertensive patients. Furthermore, recent treatment recommendations by a working group of the National Kidney Foundation based on subsequent tests have urged a slightly lower target blood pressure, 130/80 mm Hg, to better preserve kidney function.
Adults with proteinuria of greater than 1 g per 24 hours should maintain pressure goals as low as 125/75 mm Hg in the absence of contraindications (35). Clinical trial data confirm that the mean arterial pressure should be lowered to at least 92 mm Hg (corresponding to a blood pressure of about 130/70 mm Hg) for optimal renal protection (36). The lower the pressure, as tolerated, the greater the potential for preservation of renal function. Data from the Modification of Diet in Renal Disease (MDRD) study (37), the Hyper-tension Optimal Treatment (HOT) trial (38), the UKPDS (39), and the Appropriate Blood Pressure Control in Diabetes (ABCD) trial (40) have demonstrated that reducing the target blood pressures can preserve renal function or reduce cardiovascular events. (However, no trials that target the lower values of 125/75 mm Hg have been completed.) Concern for increased cardiovascular risk at lower blood pressures (J-curve) has so far not been supported by evidence (41,42). In fact, although recommendations for target blood pressure have been based on the effect of lowering systemic pressure on renal function, it is now evident that reductions in blood pressure decrease associated cardiovascular morbidity and mortality (43). In the diabetic subgroup of the HOT study, for example, the groups with the lowest target blood pressure (diastolic ≤80 mm Hg) had the lowest rate of cardiovascular events (38). Tight control of blood pressure also reduced the overall rate of complications in the UKPDS study, in which tight blood pressure control (144/82 mm Hg) was associated with a 37% reduction in microvascular endpoints and a 44% reduction in the rate of stroke (39).
Which classes of drugs are preferable?
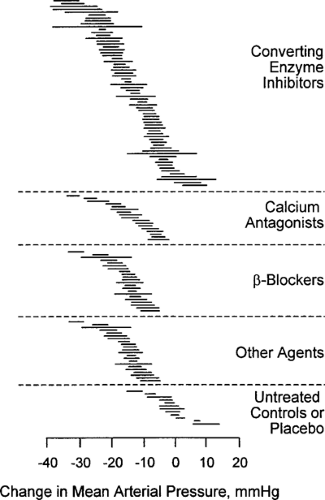
Successful blood pressure correction is more important than the specific treatment used. However, agents in specific classes of antihypertensive drugs have been investigated in clinical trials involving patients with type 1 and type 2 diabetes with various stages of diabetic nephropathy. Treatment with ACE inhibitors, calcium antagonists, and β-blockers may have similar effects on mean arterial pressure (29) (see following discussion), with no class having a greater antihypertensive effect than other classes (Fig. 55.6), and all are effective in reducing cardiovascular events (43). Most patients will require an “antihypertensive cocktail” that includes several drugs. Nonetheless, the quality of blood pressure control remains poor (44,45), and adequate control is seldom achieved, with only a minority of patients achieving the lower blood pressure goals for diabetic patients with nephropathy (46). Among the barriers that must be overcome to achieve target levels include cultural, emotional, and economic issues contributing to noncompliance by the patient (47); insufficient knowledge base and time limitations among physicians; and the inadequacy of blood pressure measurement outside the medical setting (48). Many hypertensive patients with persistent proteinuria should be referred to a nephrologist.
The subsequent sections will review ACE inhibitors and comparable therapies (49). Blood pressure reduction can be achieved by several classes of drugs. There are many special considerations in selecting antihypertensive therapy for the patient with diabetes (Table 55.3). Therapy with a diuretic, although no longer justified as monotherapy for the diabetic patient, is frequently required for the hypertensive diabetic with nephropathy. Loop diuretics are necessary for patients with impaired renal function. Potential side effects limit their benefit, including deleterious metabolic effects on lipids, glucose, and electrolytes. In addition to these unwanted
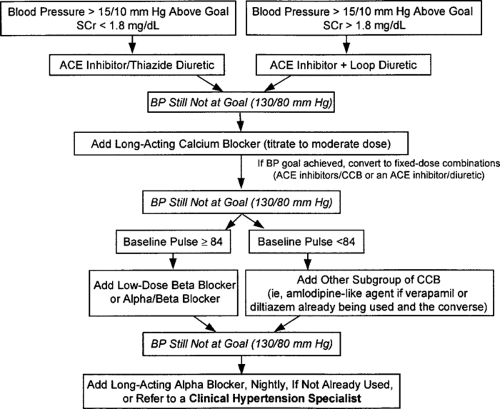
side effects, diuretics could also cause reactive stimulation of the renin-angiotensin system (RAS). Potassium-sparing diuretics are contraindicated in the presence of impaired renal function because of the risk of hyperkalemia. Adequate long-term data are not available on protection from diabetic complications such as nephropathy with other classes of antihypertensive drugs, such as α-blockers, central α-agonists, and β-blockers. Although their use in diabetic patients is discouraged, the effectiveness of β-blockers (50) is supported by the UKPDS study, in which atenolol was as effective as captopril in lowering blood pressure and protecting against microvascular complications (39). A recently suggested algorithm for reaching blood pressure goals in diabetic patients with renal insufficiency is shown if Figure 55.7.
side effects, diuretics could also cause reactive stimulation of the renin-angiotensin system (RAS). Potassium-sparing diuretics are contraindicated in the presence of impaired renal function because of the risk of hyperkalemia. Adequate long-term data are not available on protection from diabetic complications such as nephropathy with other classes of antihypertensive drugs, such as α-blockers, central α-agonists, and β-blockers. Although their use in diabetic patients is discouraged, the effectiveness of β-blockers (50) is supported by the UKPDS study, in which atenolol was as effective as captopril in lowering blood pressure and protecting against microvascular complications (39). A recently suggested algorithm for reaching blood pressure goals in diabetic patients with renal insufficiency is shown if Figure 55.7.
TABLE 55.3. Special Considerations in Selecting Antihypertensive Therapy for the Diabetic Patient with Nephropathy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Antihypertensive agents that both lower blood pressure and decrease proteinuria (see further on) include ACE inhibitors, angiotensin-receptor blockers, nondihydropyridine calcium-channel blockers, and β-blockers (51). High sodium intake reduces the antiproteinuric effect of both ACE inhibitors and calcium-channel blockers (33). Furthermore, ACE inhibitors, angiotensin-receptor blockers, and nondihydropyridine calcium-channel blockers attenuate both the abnormal permeability of the glomerular basement membrane and the morphologic damage associated with diabetic nephropathy. ACE inhibitors attack a fundamental abnormality in the hypertensive diabetic—capillary vasoconstriction—and pro-vide nearly uniform renal protective effects that surpass those of blood pressure control along (51,53). ACE inhibitors should be titrated to moderate or high doses as tolerated.
Many diabetic patients will tolerate angiotensin-receptor blockers better than they do ACE inhibitors. Several studies, primarily in patients without diabetes, have indicated that the renal protective effects of angiotensin-receptor blockers are similar to those of ACE inhibitors in reducing proteinuria. At present, comparative studies with ACE inhibitors are incomplete.
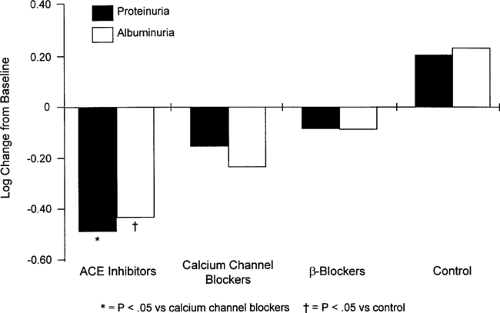
The ability of conventional antihypertensive agents to lower proteinuria is intermediate between that of ACE inhibitors and no therapy (Fig. 55.8), and conventional agents may have no effect on proteinuria independent of that due to blood pressure reduction. Renal protection provided by calcium-channel blockers is less uniform (see further on); first-generation dihydropyridine calcium-channel blockers, for example, may increase renal progression despite blood pressure control, and their use is more controversial because of safety concerns (40,54). The selection of additional agents will depend on the presence of extracellular volume expansion, in which case diuretics should be added, and on secondary issues such as metabolic profiles, drug safety, and comorbid conditions (43). ACE inhibitors (55), non-dihydropyridine calcium-channel blockers (56), and probably
angiotensin-receptor blockers do not adversely affect glucose or lipid metabolism, which might worsen microvascular and macrovascular complications. Combination therapy will generally be required and can be more effective than mono-therapy (57). The lower blood pressure goals recommended by JNC-VI not only may be effective in slowing renal progression and cardiovascular events but also may be cost- effective in the long run (46).
angiotensin-receptor blockers do not adversely affect glucose or lipid metabolism, which might worsen microvascular and macrovascular complications. Combination therapy will generally be required and can be more effective than mono-therapy (57). The lower blood pressure goals recommended by JNC-VI not only may be effective in slowing renal progression and cardiovascular events but also may be cost- effective in the long run (46).
Angiotensin-Converting Enzyme Inhibitors
The optimal drug management of diabetic nephropathy is still evolving (58,59,60). ACE inhibitors have, in recent years, gained widespread acceptance as first-line therapy to improve the prognosis for hypertensive diabetic patients with early or advanced nephropathy (5,61,62). Several lines of evidence, including animal models and human clinical trials, have led to the conclusion that ACE inhibitors, a class that includes more than 20 comparable compounds, provide selective protection in diabetic nephropathy independent of their ability to lower systemic blood pressure. Furthermore, the benefit of ACE inhibitors in patients with diabetes appears to exceed that in patients without diabetes. In contrast, other antihypertensive agents have little or no effect after beneficial influences on blood pressure are accounted for (28,63).
Nonetheless, several unresolved issues remain. The more definitive ACE inhibitor trials have generally been placebo controlled rather than comparisons with other available agents, although additional drugs frequently were necessary for blood pressure control. Not all patients will benefit equally from therapy with an ACE inhibitor (64,65), and questions about the accuracy of the assumption that all ACE inhibitors are equivalent remain unanswered (66,67). Critical review suggests that these agents may merely postpone ESRD rather than prevent it indefinitely. As a result, multiple interventions are likely to be necessary, particularly with current low blood pressure targets, and an individualized approach is necessary for maximum benefit to slow long-term progression. Furthermore, although three large trials in patients without progressive renal disease [ABCD (40), Captopril Prevention Project (CAPPP) (68), and Fosinopril versus Amlodipine Cardiovascular Events Randomized Trial (FACET) (54)] suggest a special advantage of ACE inhibitors for cardiovascular events, the most common determinants in diabetic patients with nephropathy (68), the overall long-term efficacy and safety profiles of ACE inhibitors in diabetic patients with nephropathy have not been determined.
Studies continue to elaborate on the complex role of angiotensin in diabetic glomerulosclerosis. As the key player in the pathophysiology of diabetic nephropathy, angiotensin II (ATII) has important hemodynamic and nonhemodynamic actions (70). The direct effects of ATII on increasing systemic vascular resistance and vascular tone have a unique role when applied to renal hemodynamics, in which vasoconstriction of the outgoing efferent glomerular arteriole causes increased glomerular capillary pressure. Brenner (71) proposed that the resulting glomerular hypertension is the basis for renal damage and glomerular proteinuria in diabetic nephropathy. Angiotensin also has direct structural and functional effects independent of those on renal hemodynamics. Exposure of proximal tubular, mesangial, and smooth muscle cells to ATII stimulates matrix molecular synthesis and cell hypertrophy, predominantly in response to increased production of transforming growth factor-β (TGF-β) by ATII itself. Angiotensin also stimulates filtration of proteins across the glomerular capillary wall. Metabolically, angiotensin may help reduce blood glucose levels by serving as an insulin-sensitizing vasoactive hormone (72).
These effects occur despite the apparent suppression of the circulating RAS in diabetes. However, the kidney may be exposed to excessive intrarenal actions by ATII through compartmental localization of the intrarenal RAS (73). Significant angiotensin may also be generated by pathways alternative to the ACE pathway; in patients with diabetes, more than one third of ATII formation may occur through these alternative routes.
These complex effects suggest that the actions of ACE inhibitors, the most important clinical innovation for slowing the progression of diabetic nephropathy, are complicated (Table 55.4). The benefits, clarified in experimental and animal studies, are due not only to control of blood pressure but also to renal hemodynamic and cellular effects. ACE inhibitors are known to decrease levels of ATII and inhibit actions mediated by the ATII receptor. ACE inhibitors block circulating and tissue levels of ATIIs. Systemically, ACE inhibitors decrease vascular resistance by antagonizing ATII-mediated vasoconstriction, potentiating the vasodepressor kinin system, and inhibiting effects of the sympathetic nervous system. Although the renal effects of ACE inhibitors may not be completely understood (74), ACE inhibitors, more than other antihypertensive drugs, complement their systemic hypotensive action by lowering the high intraglomerular pressure that is fundamental to diabetic nephropathy. ACE inhibitors also inhibit the effects of ATII on renal cells independent of hemodynamics. They retard glomerular basement membrane thickening (75) and tubular interstitial injury (76) in diabetic rats without hypertension, modulate intrinsic properties of the glomerular basement membrane (68) such as functional clearance of neutral dextran molecules, and ameliorate glomerular size-selective dysfunction. Nonhemodynamic benefits also include the blocking of the growth factor properties of ATII (77) and morphologic protection (78) through reduced matrix protein synthesis, decreased formation of matrix collagen, and decreased glomerular cytokine release (TGF-β, platelet-derived growth factor, and endothelin). Recent laboratory data indicate that activation of TGF-β may mediate some of the hypertrophic and proliferative effects of ATII on the diabetic kidney (79). Data also suggest that reduction in the production of TGF-β by ACE inhibitors may mediate some of these beneficial effects (80). In animal models of nephropathy, ACE inhibitors reduce glomerular production of TGF-β coincident with slowing the progression of renal disease. In patients with type 1 diabetes, ACE inhibitors-related reduction in serum levels of TGF-β correlate with long-term renal protection (79). Of particular importance for diabetic patients, ACE inhibitors may improve insulin sensitivity.
TABLE 55.4. Differences between the Clinical Effects of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II (type 1) Receptor Blockers | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
Experimental studies in animal models preceded important clinical trials in patients with type 1 and type 2 diabetes (82). Early studies by Zatz et al. (83) clearly established a beneficial effect of ACE inhibitors in experimental diabetes. Other data have shown that ACE inhibitors decrease the detrimental
effects of ATII in the kidney (84) and ameliorate functional and structural changes that produce clinical renal failure.
effects of ATII in the kidney (84) and ameliorate functional and structural changes that produce clinical renal failure.
Human interventional trials, the largest of which have used the ACE inhibitors captopril and enalapril, are best classified by the type of diabetes and stage of nephropathy. Long-term trials have recently increased the emphasis on the significance of reductions in proteinuria as a surrogate renal outcome, which may correlate with conventional outcome measures such as creatinine clearance or GFR. Proteinuria is now considered to be an independent risk factor for progression of renal disease. Although it has yet to be proven that a reduction in proteinuria indicates a better a long-term prognosis in diabetic nephropathy, proteinuria is increasingly accepted as a promoter of progressive loss of renal function by directly damaging the kidneys (85) through tubular cell injury and interstitial damage. Proteinuria increases over time in untreated patients. Higher levels of proteinuria correlate with decreased GFR in diabetic patients (86).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree