Clinical Assessment of Pain
Certain general principles should be followed in evaluating cancer patients with pain.56 Lack of attention to these general principles is the major cause for misdiagnosis of a specific pain syndrome. Adequate assessment is a critical component for defining the appropriate therapeutic strategy for each patient. The general principles are the following:
■ Believe the patient’s complaint of pain.
■ Take a careful history of the patient’s pain complaint.
■ Evaluate the patient’s psychological state.
■ Perform careful medical and neurologic examinations.
■ Order the appropriate diagnostic studies and personally review the results.
■ Treat the pain to facilitate the appropriate workup.
■ Reassess the patient’s response to therapy.
■ Individualize the diagnostic and therapeutic approaches.
■ Discuss advance directives with the patient and family.
Critical to the management of the patient with cancer pain is the establishment of a trusting relationship with the physician. The complaint of pain is a symptom, not a diagnosis. Pain perception is not simply a function of the amount of physical injury sustained by the patient but is a complex state determined by multiple factors. The diagnosis of a specific pain syndrome and a complete understanding of the patient’s psychological state are not always accomplished during the initial evaluation. In fact, it may take several weeks to define its nature because of the lack of radiologic or pathologic verification. It may take a similar period to fully comprehend each patient’s psychological makeup.
Take a Careful History of the Patient’s Pain Complaint
A careful history of the patient’s pain complaint should include the patient’s description of site of pain, quality of pain, exacerbating and relieving factors, temporal pattern, exact onset, associated symptoms and signs, interference with activities of daily living, effect on the patient’s psychological state, and response to previous and current analgesic therapies.56 Patients should be asked to describe the intensity, frequency, and severity of their baseline pain and episodes of breakthrough pain. Routine pain assessment tools should be provided to patients and families to record the patient’s pain experience and provide easy recording of analgesic drug use and the use of rescue medications. Multiple pain complaints are common in patients with advanced disease and must be ranked, classified, and recorded.
Evaluate the Patient’s Psychological State
The patient’s current level of anxiety and depression must be clarified and his or her past history of such symptoms must be defined. Knowledge of the patient’s previous psychiatric history and need for past hospitalization for psychiatric care helps to clarify the patient’s potential psychological risk. Information on how the patient has handled previous painful events may provide insight into whether the patient has demonstrated chronic illness behavior or has a past history of a chronic pain syndrome. It is important to know about a personal or family history of alcohol or drug dependence to understand why the patient may be fearful of taking or refuses to take opioid drugs.
Because each patient has his or her own understanding of the meaning of pain, it is useful to have the patient elaborate this meaning.57–60 Does he or she think it represents a recurrent tumor, or is he or she convinced it is simply arthritis? Evidence suggests that when patients have a clear understanding of the meaning of their pain as representing a recurrent tumor, they have increased psychological distress.
The importance of defining the psychological makeup of the patient with pain is supported by a variety of studies that have focused on the effect of suffering in patients with pain.57–62 Psychological factors play a significant role in accounting for the differences in pain experiences in cancer patients. A series of psychiatric syndromes have been described for cancer patients, with depression occurring in as many as 25% of patients.57,62 The depression presents either as an acute stress response or as a major depression. Awareness of the common psychiatric syndromes when evaluating the pain complaint expands the physician’s understanding of such a complaint.
Although it is critical to know as much as possible about each patient with pain, some information may not be readily available in the first interview; in some instances, it may never be available because of some patients’ inabilities to clearly define the various components of their pain. It is often necessary to verify the history by consulting a family member who may provide additional information. The family may be more objective in assessing a disability of a patient who underreports symptoms. Similarly, in a patient who is a poor historian, family may be able to provide essential information that may alter the diagnostic approach. All attempts should be made to compile a careful history and define the medical, neurologic, and psychological profile of the pain complaint. In geriatric patients with compromised cognitive function, geriatric pain and symptom scales should be used.24
It is critical to define the goals of any pain treatment. Some patients harbor unreasonable expectations, whereas others fail to critically consider the various options available to them. Complete relief is typically impossible, and the degree of relief must be balanced with any treatment’s adverse effects. Before starting any intervention, review carefully with patients the risks and benefits to provide them with a reasonable expectation of the potential outcome of this approach. As patients become more active in defining advance directives and as they focus on the quality of life, it is critical to ask them to define what they would do if the pain were intractable or intolerable.62 Did the patient have a family member who died a painful death? In the authors’ experience, patients who have had such an experience are particularly fearful of their own deaths. Does the patient have suicidal thoughts or a pact with a family member? Does the patient have a family history of suicide? Does the patient have drugs in reserve or a gun in the house that he or she might use in desperation? In a study by Chochinov et al.,62 pain alone did not correlate with patients’ suicidal ideation. Significant depression appeared to be the major correlating factor, although pain clearly played a role in the development of depression in some patients. This series of questions allows patients to discuss openly their fears of death and their intention to take matters into their own hands rather than trust the health-care professional. Such open discussions can allow the physician to better define for the patient the options for care and to reassure the patient of the physician’s commitment to care. Because patients rarely offer this information unless requested, it is critical to develop specific questions that can be readily integrated into the initial history taking by the physician. Discussing with the patient how he or she would die and engaging the patient in a discussion of his or her concerns and desires can address the commonly heard comment of patients, “I have never died before, how do I do it?”
Perform Careful Medical and Neurologic Examinations
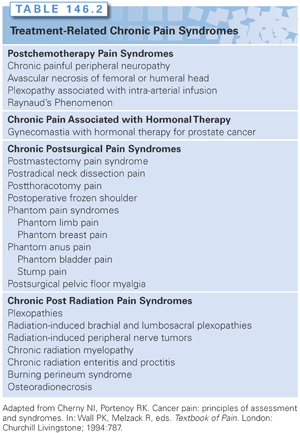
Medical and neurologic examinations help provide the necessary data to substantiate the history. They also provide a direct assessment of the cognitive status of the patient. Knowledge of the referral patterns of pain and the common cancer pain syndromes can direct the examinations. For example, the commonly described pain syndromes associated with postmastectomy states can readily be defined as separate from tumor infiltration of the brachial plexus.63
The physical and neurologic examinations allow a physician to visually inspect and palpate the site of pain and to look for the associated physical and neurologic signs that might help to better define the nature of the pain symptom. Defining the degree of motor or sensory changes can help identify the specific site in the nervous system that may be involved. Similarly, in patients with sensory loss, the presence of allodynia and hyperesthesia can further identify the nature of the sensory problem and define a neuropathic pain syndrome. Moreover, the degree of muscle spasm, gait instability, and impaired coordination can only be fully assessed by such an evaluation. In patients with neuropathic pain, the use of quantitative sensory testing can help define the underlying mechanism and determine selection of drug therapy.
Order the Appropriate Diagnostic Studies and Personally Review the Results
Diagnostic studies confirm the diagnosis and define the site and extent of tumor infiltration, when applicable. Computed tomography (CT) and magnetic resonance imaging (MRI) are the most useful diagnostic procedures for evaluating cancer patients with pain. Positron-emission tomography (PET) helps to further define the tumor and differentiate the tumor from radiation injury and postsurgical injury. The bone scan is a useful screening device and is more sensitive for demonstrating abnormalities in the bone before changes appear on a plain radiograph. However, a negative finding on bone scan does not rule out bony metastatic disease, nor does a positive finding on bone scan confirm the diagnosis of metastatic tumor. In patients with collapsed vertebral bodies, an MRI can distinguish osteoporotic from tumor-induced bony changes. The physician should review the results personally with the radiologist to correlate any pathologic change with the site of pain. Also, the measurement of tumor markers such as carcinoembryonic antigen (CEA), cancer antigen 125 (CA-125), and prostate-specific antigen, among others, can be useful in a patient in whom a recurrent tumor is suspected. In certain pain syndromes, the presence of recurrent disease is closely associated with the onset of pain (e.g., the appearance of late postthoracotomy pain syndrome in a patient after initial resolution of the postoperative pain).19
Treat the Pain to Facilitate the Appropriate Workup
Inadequate evaluations sometimes occur in the setting of severe pain. Early aggressive pain management while the source is investigated markedly improves the patient’s ability to participate in any necessary diagnostic procedures. During the initial evaluation, early consideration of the use of alternative methods of pain control, including anesthetic and neurosurgical approaches, should be considered (e.g., the temporary use of a local anesthetic via an epidural catheter to manage sacral pain).
Reassess the Patient’s Response to Therapy
A continual reassessment of the patient’s response to the prescribed therapy provides the best method to validate the initial diagnosis as correct. If relief is less than predicted or if the pain worsens, a reassessment of the treatment approach or a search for a new cause of the pain should be considered. Ideally, use the same assessment instrument at each time point; for example, if you are monitoring pain using a 0 to 10 numerical rating scale, continue to use that for each follow-up visit.
Individualize the Diagnostic and Therapeutic Approaches
An evaluation of the patient must be closely linked to the patient’s level of function, ability to participate in the diagnostic workup, willingness to undergo the necessary diagnostic approaches, objective evidence that treatment approaches may be beneficial, and life expectancy. Careful judgment is required to select diagnostic approaches that will have a direct effect on the choice of the therapeutic strategy or will answer a specific question. The random use of diagnostic procedures in these patients, particularly those with advanced cancer and significant pain, is inappropriate and costly. Open discussion with the patient about the need for assessment as well as the therapeutic options is critical to allow the patient to be part of the decision-making process. For some patients, diagnostic procedures such as an MRI are inappropriate because they simply confirm the existence of a disease for which no treatment is available, or for which the treatment would be a major surgical procedure (e.g., vertebral body resection) that would be inappropriate for a dying patient. Patient refusal of an evaluation or treatment must be respected when the physician has fully explained the options and is convinced that the patient has an accurate understanding of the implications of undertaking no further workup or treatment.
Discuss Advance Directives with the Patient and Family
When treatment approaches are being developed, there must be an open discussion about advance directives so that the physician has a clear understanding of the patient’s goal for therapy. The physician must have unconditional positive regard for the patient, placing the control of symptoms of pain and treatment of psychological distress in the highest regard. Knowledge of the patient’s decisions about resuscitation, living wills, and symptom management should he or she become incapacitated improves the physician’s ability to appropriately and humanely care for the dying patient with advanced disease.59
Evidence-based cancer pain management follows a systematic strategy of applying demonstrated interventions in the context of individual needs, circumstances, and preferences. The basic approach employs three categories of agents: opioid analgesics, nonopioid analgesics, and adjuvant therapies. The foundation of this approach is an individualized combination of opioid and nonopioid drugs; to this foundation is added tailored care that selects from a broad array of adjuvant interventions to achieve maximum comfort for the individual patient. Guidelines are available to assist the clinician in determining a foundation treatment plan (the reader is referred to the National Comprehensive Cancer Network, the American Pain Society guidelines, ESMO guidelines, and the British Pain Society as excellent sources of cancer pain management guidelines). 8,11–16 To enhance the effectiveness of this foundation, many pharmacologic and nonpharmacologic options exist for tailoring adjuvant care to individual exigencies.
The sections that follow will emphasize the principles of pharmacologic, nonpharmacologic, procedural, and manual management of cancer pain and briefly describe anesthesia and neurosurgical approaches to pain management to which the oncologist might refer a patient for specialized procedures.
Pharmacologic Management of Cancer Pain
Analgesic Drug Therapy: The Mainstay of Cancer Pain Management
Cancer pain management combines treatment of the primary disease with (1) analgesic drug therapy and (2) specific approaches that may include anesthetic, neurosurgical, rehabilitative, psychological (cognitive–behavioral), psychiatric, or complementary and alternative methods. The clinician begins with determining a patient-specific appropriate analgesic drug plan. Titration to effective pain relief and individualization of treatment represent hallmarks at this stage in the therapeutic approach. In recent years, standard analgesic drug therapy has become more effective due to increased sophistication in the use of analgesic drugs, coupled with research to understand the underlying mechanisms of pain. These new and more effective analgesic practices include the use of novel means of drug administration, particularly transdermal and transmucosal delivery; novel methods, such as the use of bisphosphonates and calcitonin for bone pain, radiopharmaceuticals, and novel approaches to neuropathic pain, such as antidepressants and anticonvulsants; and anesthetic, neurosurgical, psychological, and psychiatric approaches concurrently applied in the overall continuum of care.
As noted previously, numerous guidelines for the management of cancer pain have been issued by various organizations and researchers.8,11–16 These guidelines have each defined analgesic drug therapy as the mainstay of treatment and have articulated the aims of drug therapy as the achievement of adequate pain relief safely within an acceptable time frame, minimization of side effects of treatment, and ongoing analgesia by the most convenient and least noxious means available. Yet, despite available guidelines and an agreed upon approach to cancer pain management, determination of the best treatment path for the individual patient remains a complex, individualized process.
The World Health Organization Cancer Pain Guidelines
Although frequently debated, the WHO guidelines on cancer pain continue to provide a framework approach for pharmacologic management of cancer pain management. Field testing of these guidelines, as well as clinical experience, has shown that 70% to 90% of cancer patients’ pain can be controlled using a simple and inexpensive method described as the three-step analgesic ladder.64,65 The ladder describes a process for combining nonopioid, opioid, and adjuvant drugs, titrated to meet the individual needs of the patient according to the severity of pain and its pathophysiology. A randomized controlled trial of an algorithm based on the WHO ladder demonstrated that a standardized approach to cancer pain management using the algorithm provided more effective analgesia than routine oncology care.66 These new studies suggest that a two-step ladder is equally effective for cancer pain management, and the WHO is beginning a process of guideline revision and updates.67
Step 1 of the WHO ladder focuses on analgesic drug therapy for patients with mild-to-moderate cancer pain. Such patients should be treated with a nonopioid analgesic that may or may not be combined with an adjuvant drug, depending on the specific pain pathophysiology. For example, in a patient with mild pain from a peripheral neuropathy, the combination of a nonopioid with a tricyclic antidepressant or an anticonvulsant drug would be appropriate.
Step 2 of the WHO ladder focuses on patients with moderate pain who do not experience adequate pain relief from a nonopioid analgesic. These patients are candidates for a combination of a nonopioid, such as aspirin, acetaminophen, cyclooxygenase 2 (COX-2) inhibitors, or other nonsteroidal anti-inflammatory drug (NSAID), and low doses of opioid analgesics, such as codeine, oxycodone, or morphine, usually dosed at less than 60 mg oral morphine equivalents (OME) daily. These patients often require adjuvant drugs, depending on the pain pathophysiology.
Step 3 pertains to patients who report either severe pain (often gauged as greater than 7 on a 0 to 10 scale) or moderate pain that is inadequately managed after appropriate administration of drugs at the second step of the WHO ladder. For these patients, nonopioids are often used in combination with more potent doses of opioids to mitigate the opioid effect, and adjuvants are administered depending on the pain pathophysiology or need to control other concurrent symptoms in the individual patient. Opioid doses on step 3 are commonly greater than 60 mg OME daily.
In short, the analgesic drug ladder of the WHO defines a method for using drug combinations in three categories: nonopioids, opioids, and adjuvants; it is based on previously well-tested pharmacologic principles. However, controversy in the application of the WHO ladder continues to surround the choice of analgesic drug for the individual patient.63–66 For example, one meta-analysis of the role of NSAIDs in bone pain found them to be no more or less effective than opioids, although they are commonly used.68
Cancer Pain Management Using the World Health Organization Three-Step Analgesic Ladder
Nonopioid Analgesics for Cancer Pain Management. The nonopioid analgesics include acetaminophen and the NSAIDs, of which aspirin is the prototypic agent. These compounds are most commonly administered orally. Their analgesia is limited by a ceiling effect, such that increasing the dose beyond a certain level (900 to 1,300 mg per dose of aspirin) produces no increase in peak effect. Tolerance and physical dependence do not occur with repeated administration. Aspirin and the other NSAIDs have analgesic, antipyretic, anti-inflammatory, and antiplatelet actions. Some NSAIDs, such as choline magnesium trisalicylate, lack the antiplatelet effects of aspirin and may have less collateral toxicity in cancer patients already at risk of bleeding. Others (e.g., ibuprofen) appear to produce fewer gastrointestinal side effects than aspirin. The COX-2 inhibitors potentially offer less gastrointestinal (GI) toxicity and without affecting platelet function. However, the drug currently marketed as a COX-2 inhibitor—celecoxib—has not been systematically studied in cancer patients to define its particular role in pain management; concerns regarding cardiovascular side effects have limited use of this class of agents.69 More recently, cardiovascular effects have been recognized across even nonselective COX inhibitors, including ibuprofen; naproxen may pose a slightly lower risk.70 Acetaminophen, an analgesic and antipyretic agent equipotent to aspirin, is much less effective as an anti-inflammatory agent but does not interfere with platelet function or pose cardiovascular risk.
The nonopioid analgesics are generally thought to produce analgesia by inhibiting activation of peripheral nociceptors through their prevention of the formation of prostaglandin E2, a known sensitizer of peripheral receptors to nociceptive stimulation from tissue injury. The NSAIDs differ from one another both in duration of their analgesic action and in their pharmacokinetic profile. Ibuprofen and fenoprofen have short half-lives and the same duration of action as aspirin, whereas diflunisal and naproxen have longer half-lives and are longer acting. Because clinical experience has found that some patients respond better to one NSAID than to another, each patient should be given an adequate trial of one drug on a regular basis before switching to another. Survey data from the WHO demonstration projects suggest that 20% to 40% of patients obtain pain relief with the use of nonopioid analgesics alone.71
These drugs are thought to play a special role in the management of bone pain because numerous studies have shown that aspirin inhibits tumor growth in an animal model of metastatic bone tumor. A meta-analysis of studies using NSAIDs to treat bone pain did not demonstrate them to be more effective than weak opioids such as codeine, oxycodone, and propoxyphene.68 Recent data that detail cancer-related bone pain as a neuropathic pain model have challenged the dogma of NSAIDs for bone pain.39
Numerous studies have elucidated the major risk factors for GI toxicity, particularly ulcerative complications, associated with NSAIDs. These risk factors include advanced age, higher doses, concomitant administration of corticosteroids, and history of either ulcer disease or previous GI complications from NSAIDs. Various prophylactic therapies are administered to prevent GI complications. Misoprostol is the only U.S. Food and Drug Administration (FDA)–approved medication for this indication; a Cochrane systematic review supports its use for the prevention of NSAID-induced GI complications.72 More commonly, proton-pump inhibitors are prescribed, which have been associated with significantly decreased frequency of NSAID-related dyspepsia and fewer ulcers diagnosed by endoscopy.73 Whether this leads to fewer GI bleeds and reduced health-care utilization is still being debated. An empiric approach at this time is to administer misoprostol or a proton-pump inhibitor to all patients who are receiving NSAIDs with significant risk factors for GI complications.74
Nonopioid drugs represent the front-line approach for cancer pain management, but the choice and use of nonopioids must be individualized. Although several NSAIDs are FDA approved for use as analgesics for mild to moderate pain (Table 146.3), guidelines for the use of NSAIDs in patients with cancer are largely empirical. According to a 2004 Cochrane systematic review of 42 trials involving cancer pain patients, the evidence demonstrated at best a nonstatistically significant trend toward improvements when NSAIDs and opioids were combined; conclusions were limited by the short duration of the studies.75 The clinician must select an agent based on evidence, individual patient history, and patient-specific considerations, and then must give the patient an adequate trial of that nonopioid analgesic before switching to an alternative one. Such a trial should include the administration of the drug to maximum levels at regular intervals. Because there is a great variability among patient responses to different drugs, patients may require trials with several NSAIDs before finding an effective drug and dose regimen. If pain relief is not obtained, adding an opioid to a nonopioid provides additive analgesia.
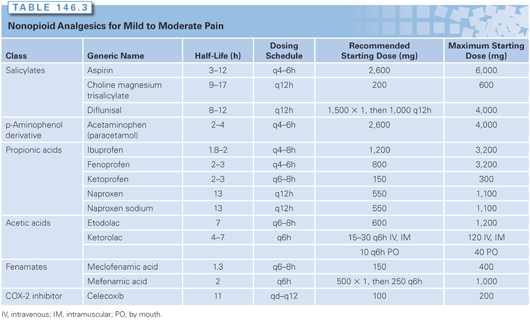
Combinations containing codeine, oxycodone, and propoxyphene are available, but these combinations often contain less than the full dose of 650 mg of aspirin or acetaminophen. Prescribing each drug separately provides a better method for individualizing pain control; this is particularly important when the patient requires escalation of the combination to provide analgesia, in which case the additional amount of the NSAID or acetaminophen may become excessive.
Opioid Drugs for Cancer Pain Management. The opioid analgesics, of which morphine is the prototype, vary in potency, efficacy, and adverse effects. These drugs produce their analgesic effects by binding to discrete opiate receptors in the peripheral and central nervous systems. In contrast to the nonopioid analgesics, opioid analgesics, at least the opioid pure agonists, do not appear to have a ceiling effect (i.e., as the dose is escalated on a log scale, the increment in analgesia is linear to the point of loss of consciousness). There are also series of drugs that are pure antagonists (i.e., they block the effect of morphine at the receptor). The antagonist drug most commonly used in clinical practice is naloxone, which is administered to reverse respiratory depression and other complications associated with opioid overdose.
Effective use of opioids requires the balancing of the most desirable effects of pain relief with the undesirable effects of nausea, vomiting, mental clouding, sedation, constipation, tolerance, and physical dependence. These undesirable effects impose a practical limit on the dose useful for a particular patient and have led to the concept of opioid responsiveness.
The use of opioids in the management of cancer pain remains controversial.5,76–90 Some of the controversies include their role in the management of neuropathic pain, which has been suggested to be opioid resistant, the specific choice of opioid drug, the use of sequential trials of opioids, routes of administration, the development of tolerance, risk of addiction, economic factors influencing these controversies, and the concern that opioids are agents of physician-assisted suicide and euthanasia. The following principles take into account these controversies while laying out a basic approach to the use of opioids in cancer pain management (Table 146.4).
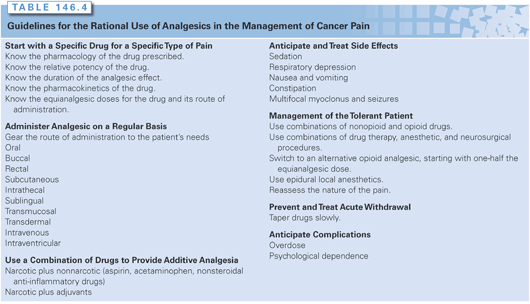
1. Start with a specific drug for a specific type of pain. As defined by the WHO three-step analgesic ladder, the specific drug chosen depends in part on the degree of pain intensity and the type of pain. Cancer patients commonly have multiple sites and types of pain. A continuum of opioid responsiveness, rather than an all-or-none phenomenon, has been clearly observed. Opioid responsiveness is defined as the degree of analgesia achieved during dose escalation to either intolerable side effects or adequate analgesia. Patient characteristics and pain-related factors, as well as drug-selective effects, influence this variable response.89
It has been suggested that neuropathic pain, which accounts for 15% to 20% of pain problems that are difficult to manage, is opioid resistant and that opioid drugs should not be used in this patient population.82 In fact, studies that explored reasons for inadequate pain treatment have identified that up to two-thirds of cancer pain patients have some contribution of neuropathic pain to their global pain syndrome66; when the concept of bone pain as a neuropathic pain syndrome is included, this number increases.39 Studies of cancer patients with both nociceptive and neuropathic pain, as well as controlled studies of nonmalignant neuropathic pain, demonstrate the variable responsiveness of neuropathic pain to opioid analgesics. Hence, the contribution of neuropathic pain must be considered when assessing opioid responsiveness.
A wide range of adjuvant analgesics has been suggested to provide analgesia alone or in combination with opioid drug therapy, and there are specific adjuvants for bone pain and neuropathic pain. The choice of a specific drug is dictated not only by pain intensity and type of pain, but also by the patient’s prior opioid exposure and history of allergy or side effects.
The WHO’s Cancer Pain Relief Program has developed cancer pain guidelines that designate morphine as the drug of choice based on practical, not scientific, considerations. The introduction of the WHO program rapidly demonstrated the limited availability of morphine worldwide for the oral treatment of chronic cancer pain.6,17 Morphine consumption worldwide is now used as an indicator of the success of the WHO Cancer Pain Relief Program.18 Controlled-release oral morphine is currently available in a wide range of doses from 15 to 200 mg; differing products provide options for every 8-, 12-, and 24-hour administration. These preparations provide analgesia comparable to that of immediate-release forms and offer increased convenience, improved compliance, and a reduction in the duration of pain. Historically, the dogma was to titrate patients to adequate pain relief using 4-hourly doses and then combine into sustained-release doses that provide an equal amount of opioid in 24 hours. In a recent randomized clinical trial, it was demonstrated that for cancer pain patients receiving greater than 60 mg OMEs daily, titration with a sustained release product was equally efficacious and yielded fewer side effects.91
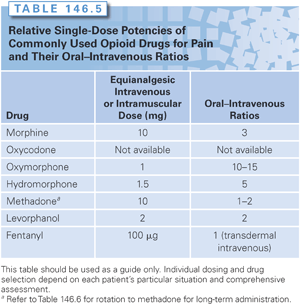
The clinician’s armamentarium for managing cancer pain now encompasses a series of opioid alternatives to morphine, including congeners of morphine—hydromorphone, oxycodone, and oxymorphone—as well as methadone, levorphanol, fentanyl, and buprenorphine. The choice of agent depends on the clinician’s knowledge about how to use the drug, patient factors such as age and renal function, route of delivery, opioid availability, and cost (Tables 146.5 and 146.6). Recently, new formulations of these drugs have been marketed with various combinations to deter tampering and to reduce their diversion.92
Hydromorphone has poor oral availability and a short half-life. Its high solubility and availability in high-potency parenteral form (10 mg per milliliter) make it a useful choice for chronic subcutaneous administration. Because of its short half-life, it is commonly used in the elderly patient. Myoclonus has been reported after high doses, possibly due to an accumulation of its metabolites (3-0 methyl-glucuronide and hydromorphone-6-glucuronide).93 When compared in a double-blind trial of patient-controlled analgesia, no differences in analgesia or side effects were noted between morphine and hydromorphone; these findings have been confirmed in several settings.94,95 Although cognitive performance was poor in the hydromorphone group, patients reported better mood than those who received morphine. A slow-release 24-hour formulation was recently released, but clinical experience thus far remains limited, and its cost is high.
Oxycodone, which is commonly administered in a 5-mg dose at the second step of the WHO analgesic ladder, can also be used in the third step at higher doses. It is available in a slow-release preparation.96 Its half-life is 3 to 4 hours. Oxymorphone is its active metabolite. Oxymorphone is currently available in oral sustained release, intravenous, and rectal preparations and serves as an alternative to morphine and its other congeners. Oxymorphone has a reduced histamine effect and may be of use in patients who complain of headache or itch after the administration of other opioids.
Levorphanol has high bioavailability but a long plasma half-life (12 to 16 hours). It should be used cautiously because, with repeated administration, accumulation may occur.
The role of methadone in managing cancer pain also remains controversial.5,76,87,88,90 Methadone represents a second-line drug for cancer pain patients who have had prior exposure to opioids. It is a relatively inexpensive oral analgesic, but its name has negative connotations for cancer patients, who view methadone as a drug used to treat addicts. The bioavailability of methadone is higher than that of morphine (85% versus 35%, respectively). Its analgesic potency also differs, with a parenteral to oral ratio of 1:2 in contrast to 1:6 for morphine. Moreover, the plasma half-life of methadone is 17 to 24 hours, with reports of up to 50 hours in some cancer patients, but with an analgesic duration of only 4 to 8 hours. Significant adverse effects have been reported in cancer patients receiving methadone by various routes. Most notably, reports of drug-induced long QT syndrome have increased concern, especially in cancer patients who may be on multiple agents that can prolong the QT interval.97–99 The discrepancy between the analgesic duration and plasma half-life of methadone has made it a difficult drug to use in the naïve patient because of the need for careful titration. In a randomized trial, the initial treatment of cancer pain with morphine versus methadone provided equal analgesic efficacy.100
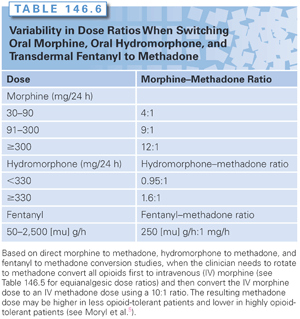
A number of case reports have highlighted the possibly greater analgesic potency of methadone than the often quoted 1:1 equivalency with morphine.76,87,88,90 Studies of interindividual differences in response to opioid analgesics have demonstrated that dramatically reduced dosages of methadone are required to produce analgesia in patients chronically taking morphine or hydromorphone. Several authors have shown marked reductions in the equianalgesic dose of methadone when patients with either uncontrolled pain or extreme side effects were switched to methadone.5,90 These clinical survey studies suggested up to a 75% reduction in the methadone equianalgesic dose when switching from hydromorphone to methadone (see Table 146.6).
In a prospective study, Ripamonti et al.90 developed a specific dose ratio based on the patients’ morphine doses. For patients taking 30 to 90 mg of morphine, the dose ratio is 4:1; for those taking 90 to 300 mg daily, the dose ratio is 6:1; and for those taking 300 mg or more, the dose ratio is 8:1. Bruera et al.76 developed a similar ratio for hydromorphone based on survey data. In patients who received more than 330 mg of hydromorphone, the dose ratio is 1.6:1.0; and in those who received less than 300 mg, the dose ratio is 0.95:1.0.
Studies of the use of methadone by experienced clinicians in caring for advanced cancer patients at home report fewer dose escalations and good pain control, which supports the use of methadone in home settings.87 Methadone can be administered by a variety of routes, but the subcutaneous route is associated with adverse effects, including cutaneous hypersensitivity.101 Due to its long half-life, some offer the suggestion that methadone be administered every 8 to 12 hours, whereas others have demonstrated analgesic efficacy and safety in acute 3- to 4-hour dosing intervals.102 Ongoing studies are attempting to better elucidate the clinical pharmacology of methadone to facilitate its broader use.
Meperidine is a drug that should not be used chronically in the management of patients with cancer pain. Meperidine has a poor parenteral to oral ratio (1:4). It is available in oral and intramuscular preparations, but repetitive intramuscular administration is associated with local tissue fibrosis and sterile abscess. Repetitive dosing of meperidine (more than 250 mg per day) can lead to the accumulation of normeperidine, an active metabolite that can produce central nervous system hyperexcitability.103 This hyperirritability is characterized by subtle mood effects followed by tremors, multifocal myoclonus, and occasional seizures. It occurs most commonly in patients with renal disease but can also occur after the repeated administration in patients with normal renal function. Naloxone does not reverse meperidine-induced seizures, and its use in meperidine toxicity is controversial. There have been some case reports that the use of naloxone has precipitated generalized seizures in individual patients. In rare instances, central nervous system toxicity characterized by hyperpyrexia, muscle rigidity, and seizure has been reported after the administration of a single dose of meperidine to patients receiving treatment with monoamine oxidase inhibitors. Recent reports of potentially fatal serotonin syndromes due to concurrent use of meperidine with selective serotonin reuptake inhibitors (SSRI) or with the antimicrobial linezolid raise further concern about the safety profile of this medication.104–106
The availability of transdermal patches and various transmucosal preparations facilitates the use of fentanyl for the management of both acute and chronic pain.107 The half-life of fentanyl is 1 to 2 hours. Published guidelines for fentanyl use summarize available data.85 In chronic cancer pain management, relative potency comparisons have not been fully established, but the common dosing guideline is that 10 mg of intravenous morphine is equivalent to 100 μg of intravenous fentanyl (see http://www.aahpm.org/pdf/equianalgesictable.pdf). The uniqueness of the available fentanyl preparations facilitates the management of patients who are unable to take drugs by mouth by providing them with continuous opioid analgesia. Patches are currently available in 12.5 to 100 μg per hour doses and are changed every 72 hours. When a patient is started on the fentanyl patch, there is up to a 12- to 15-hour delay in the onset of analgesia, and a 24- to 72-hour equilibration period; alternate approaches must be used to maintain pain control during this early period. Patients should be cautioned about factors that can impact the absorption rate of the drug from the patch, such as heating pads, sweating, or fever; deaths likely relating to these issues led to an FDA advisory. Proper disposal is also paramount, given the risk posed by unintentional exposure to caregivers or children. Of note, reliable drug absorption requires adequate subcutaneous fat to be present; topical fentanyl may not be appropriate in the setting of cachexia. Specific guidelines for switching to the fentanyl patch after an intravenous infusion of fentanyl have been developed and are based on use of a 1:1 conversion ratio.85 Fentanyl can also be used as an anesthetic premedication, as well as intravenously for pain control. Oral transmucosal and sublingual formulations have demonstrated effectiveness in treating breakthrough pain in cancer patients.85,108,109
Buprenorphine, a mixed agonist/antagonist opioid drug, is used to treat chronic noncancer pain and drug addiction and is also available in a transdermal preparation for cancer pain management. It is reported to be a useful agent in patients with moderate to severe pain, and it does not accumulate in patients with renal dysfunction. Its place in the management of cancer pain among the other opioids is not yet fully clarified.110
2. Know the equianalgesic dose of the drug and its route of administration. Knowing the equianalgesic dose (i.e., the dose of one analgesic drug that is equivalent in the pain-relieving potential of another analgesic drug) can ensure more appropriate drug use. The equianalgesic dose guides the recommended starting dose, with the optimal dose for each patient determined by dose adjustment. Relative potency is the ratio of the doses of two analgesics required to produce the same effect. Estimates of relative potency allow for a calculation of the equianalgesic dose, which provides the basis for selecting the appropriate dose when switching drugs or changing the route of administration of the same drug. The values in Table 146.5 are based on studies using 10 mg of morphine as the standard dose.111 There is now evidence to suggest that relative potency may differ in single-dose and repeated-dose studies. For example, for morphine, a 1:6 relative analgesic potency ratio should be used for patients with acute pain, whereas a 1:2 or 1:3 ratio is more appropriate in patients treated with repeated doses on a chronic basis. Lack of attention to differences in drug dose is the most common cause of undermedication of pain.
3. Administer analgesics regularly after initial titration. Medication should be given regularly to maintain the plasma level of the drug above the minimum effective concentration for pain relief. In the initial titration, patients should be advised to take their medication as needed to determine their total 24-hour requirements. During this time, the patient should reach the steady-state level of drug, which depends on the drug’s half-life. For morphine, a steady state can be reached in 24 hours; with methadone, it may take up to 5 to 7 days to reach a steady state. In patients on a fixed schedule, rescue medications equivalent to one-half of the standing dose should be available for breakthrough pain.
Continuous intravenous and subcutaneous opioid infusions to manage both acute and chronic cancer pain are commonly administered using a patient-controlled analgesic pump programmed to the patient’s need with a set lock-out time to prevent overdosing. This method of drug administration is especially useful in managing patients with breakthrough pain. It is a significant advance in facilitating adequate titration of analgesics in chronic cancer patients, allowing discharge to home and hospice settings.
4. Gear the route of administration to the patient’s needs. Various methods of opioid drug delivery have been developed in order to maximize pharmacologic effects and minimize side effects. Most patients require at least two routes of drug administration, and 20% need up to four approaches during the course of their cancer pain treatment.
The oral route is preferable and easy. Orally administered drugs have a slower onset of action, delayed peak time, and longer duration of effect. Drugs given parenterally have a rapid onset of action but a shorter duration of effect. Slow-release preparations of morphine, hydromorphone, and oxycodone allow more convenient dosing every 8 to 12 hours, or every 24 hours.
For cancer pain management by the sublingual route, well-absorbed drugs include fentanyl and methadone.112 Oral transmucosal fentanyl citrate preparations and intranasal sprays have been widely studied for the management of breakthrough pain and are increasingly available.
For the rectal route, oxymorphone, hydromorphone, and morphine are available in suppository form. Oxymorphone suppositories produce analgesia equivalent to 10 mg of parenteral morphine. Slow-release oxycodone and morphine preparations have also been demonstrated to be effective rectally, and ongoing studies with rectal methadone suggest that this drug is well absorbed by the rectal route.
The transdermal route is a convenient way to deliver a potent short-acting opioid on a continuous basis. Drug is released through the skin patch at a nearly constant amount per unit time with a concentration gradient from patch to skin. Serum fentanyl concentrations increase and steady-state levels are approached at 12 to 24 hours.109 After patch removal, the drug persists in the skin, with falling blood levels over 24 hours. Innovative transdermal delivery systems are in phase 3 testing, which include systems for immediate-dose delivery using iontophoresis and drug reservoirs. Iontophoresis is the transfer of ionic solutes through biologic membranes under the influence of an electric field. It offers an alternative system for parenteral administration and has been shown to allow for comparatively rapid achievement of fentanyl dose levels using a transdermal system.
Various parenteral routes include intermittent and continuous subcutaneous, intravenous, epidural, intraventricular, and intrathecal infusions. The use of intermittent and continuous subcutaneous infusions is most appropriate for patients who cannot tolerate oral analgesics because of GI obstruction or intractable nausea and vomiting, and for those who do not have intravenous access. The utility of this technique has been demonstrated using morphine, heroin, hydromorphone, levorphanol, and fentanyl. The administration of methadone by this route is associated with the development of a cutaneous hypersensitivity syndrome.101
Patient-controlled analgesic pumps designed to infuse continuously but with options for bolus administration are connected to a 27-gauge butterfly needle that the patient can insert into a new subcutaneous site every 3rd to 6th day. Limited pharmacokinetic studies have demonstrated that, for example, systemic absorption of the drug at a steady state reaches 87% bioavailability from subcutaneous infusion of hydromorphone.95 Intermittent and continuous intravenous infusions are used if intravenous access is available and more commonly in patients who are hospitalized. Specific guidelines for the use of continuous infusions have been developed.5,113
The use of intermittent and continuous epidural and intrathecal opioid infusions is based on the demonstration of opioid receptors in the dorsal horn of the spinal cord and the availability of opioid drugs to suppress noxious stimuli at the spinal cord level. Localized selective analgesia is produced without motor or sensory blockade. An analysis of the pharmacokinetics of epidural opioid administration demonstrates that there is significant systemic uptake after epidural injection, comparable with that after an intramuscular injection of the same drug and dose. However, distribution of the drug directly into the cerebrospinal fluid is 10 to 100 times greater. Existing studies demonstrate that this approach is used with approximately 10% of cancer patients to maximize analgesia and minimize side effects. This technique is commonly used in patients who have mixed nociceptive neuropathic pain syndromes and in whom combinations of local anesthetics and opioids are administered epidurally. A 3-year retrospective outcome study of the use of epidural catheters in the management of chronic cancer pain identified the occurrence of technical problems and infection, including epidural abscesses, in a significant number of patients. The study suggests that epidural catheters be used in patients with limited life expectancy.114
Smith et al.115 completed a randomized clinical trial in which an implantable drug delivery system was compared to comprehensive medical management of refractory cancer pain. The patients using the implantable system reported better pain relief with fewer drug side effects and improved survival. Cost-effectiveness studies have not been done, but there is clearly the added cost of the pump, and the costs of the system need to be compared to the costs of nonpump medicines, with the potential savings from prevention of hospitalizations for pain management analyzed. Rarely, intraventricular opioid infusion has been used to manage patients with pain in the cervical and craniofacial region from tumor infiltration.116 Doses between 1.0 and 7.5 mg per 24 hours have been used, and excellent results have been reported in 70% of patients. At present, there is not a clear indication that this intraventricular route offers special advantages over systemic approaches.
5. Use a combination of drugs. By using a combination of drugs, the physician can increase analgesic effects without escalating the opioid dose. Combinations that produce additive analgesic effects include an opioid plus a nonopioid, an opioid plus an antihistamine (100 mg of intramuscular hydroxyzine), and an opioid plus an amphetamine (10 mg of intramuscular dextroamphetamine).117–119
6. Anticipate and treat side effects. The side effects of the opioid analgesics often limit their effective use. The most common side effects are sedation, respiratory depression, nausea, vomiting, constipation, and multifocal myoclonus and seizures.
Sedation and drowsiness vary with the drug and dose and may occur after both single and repeated administration. They are mediated through activation of opiate receptors in the reticular formation and diffusely throughout the cortex. Management of these effects includes reducing the individual drug dose while giving the drug more frequently, or switching to an analgesic with a shorter plasma half-life. In controlled trials, amphetamine, methylphenidate, and caffeine have been demonstrated to counteract opioid-induced sedative effects. It is important to discontinue all other drugs that might exacerbate the sedative effects of opioid analgesics, including a wide variety of medications such as cimetidine, barbiturates, and other anxiolytics.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








