RISK FACTORS FOR BREAST CANCER
Multiple factors are associated with an increased risk of developing breast cancer, but the majority of these factors convey a small to moderate increase in risk for any individual woman. It has been estimated that approximately 50% of women who develop breast cancer have no identifiable risk factor beyond increasing age and female gender. The importance of age as a breast cancer risk factor is sometimes overlooked. In 2009, it was estimated that 18,640 invasive breast cancers and 2,820 breast cancer deaths occurred in US women under age 45 compared with 173,730 cancers and 37,350 deaths in women aged 45 years and older.7
Familial Factors
A family history of breast cancer has long been recognized as a risk factor for the disease, but only 5% to 10% of women who develop breast cancer have a true hereditary predisposition. Many women with a family history overestimate their risk of developing breast cancer or harboring a predisposing genetic mutation. Overall, the risk of developing breast cancer is increased 1.5-fold to 3-fold if a woman has a mother or sister with breast cancer. Family history, however, is a heterogeneous risk factor with different implications depending on the number of relatives with breast cancer, the exact relationship, the age at diagnosis, and the number of unaffected relatives. Even in the absence of a known inherited predisposition, women with a family history of breast cancer face some level of increased risk, likely from some combination of shared environmental exposures, unexplained genetic factors, or both.
Inherited Predisposition to Breast Cancer
Mutations in the breast cancer susceptibility genes BRCA1 and BRCA2 are associated with a significant increase in the risk of breast and ovarian carcinoma, and account for 5% to 10% of all breast cancers. These mutations are inherited in an autosomal dominant fashion and have varying penetrance. As a result, the estimated lifetime risk of breast cancer development in mutation carriers ranges from 26% to 85%, and the risk of ovarian cancer from 16% to 63% and 10% to 27%, respectively, in carriers of BRCA1 and BRCA2.8 In a meta-analysis of 10 international studies, the cumulative risks to age 70 for breast cancer were 57% for BRCA1 and 40% for BRCA2 carriers.9 More than 700 different mutations of BRCA1 and 300 different mutations of BRCA2 have been described, and the position of the mutation within the gene has been shown to influence the risk of both breast and ovarian cancers.
Other cancers associated with BRCA1 or BRCA2 mutations include male breast cancer, fallopian tube cancer, and prostate cancer. Carriers of BRCA2 may also have an elevated risk of melanoma and gastric cancer. Management strategies available for risk reduction in BRCA1/2 mutation carriers include intensive surveillance, chemoprevention with selective estrogen receptor modulators (SERM), and prophylactic (breast and salpingo-ovarian) surgery, and these are discussed in a later section. There is a great interest in the role of environmental and lifestyle factors in the modification of cancer risk among BRCA1 or BRCA2 carriers; at present, however, the available data are inconsistent. It is worth noting that women with a significant family history of breast cancer (i.e., two or more breast cancers under the age of 50 years, or three or more breast cancers at any age), but who test negative for BRCA mutations have approximately a four-fold risk of breast cancer.10 In contrast, women in families where a BRCA mutation is present who test negative for the mutation are not at increased risk for breast cancer development in the absence of other risk factors, and do not require special surveillance.11
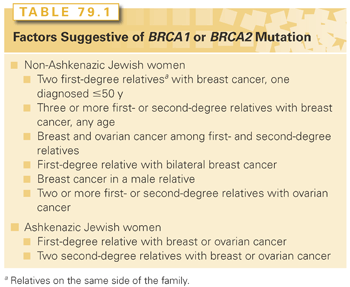
Women with BRCA1 mutations have a higher incidence of triple-negative/basal-like breast cancers (see the following), and cancers are more likely to be grade 3 tumors and to lack expression of the estrogen receptor (ER), progesterone receptor (PR), and HER2 overexpression than sporadic cancers.12 The phenotype of BRCA2 cancers does not differ from that seen in sporadic cancers. The presence of a BRCA1 or BRCA2 mutation may be suggested by the family history on either the maternal or paternal side of the family. The features considered by the 2005 US Preventive Services Task Force13 are listed in Table 79.1. Less-rigorous criteria for referral for genetic counseling are used for individuals of Ashkenazi Jewish ancestry, because the carrier frequency of specific BRCA1 (187delAG, 5385 ins C) and BRCA2 (6174delT) mutations in this group is 1:40, compared with 1:500 in the general population. These guidelines are particularly useful for individuals not affected with breast cancer. In the patient with newly diagnosed breast cancer, young age at diagnosis (≤40 years), bilateral breast cancer, Ashkenazi ancestry, or a malignancy consistent with the BRCA1 phenotype all constitute reasons for referral to a genetic counselor, particularly in the woman with a small number of female relatives. Models are available to estimate the likelihood of a BRCA1 or BRCA2 mutation based on family history. The implications of genetic testing for both individuals and their family members are considerable, and these issues should be discussed prior to undertaking genetic testing.
Other genetic mutations have been associated with breast cancer risk, although with a lower prevalence or penetrance than BRCA1 and BRCA2. TP53 and PTEN each account for <1% of cases, while mutations in CDH1 are associated with an autosomal dominant predisposition to diffuse gastric cancer and lobular breast cancer.14 Young women with TP53 mutations (Li-Fraumeni syndrome) seem to have a greater propensity for HER2+ breast cancers.15 Mutations in low-penetrance genes are thought to account for a significant number of non-BRCA1 or –BRCA2 breast cancers. A specific mutation of the checkpoint kinase 2 (CHEK2) gene was found in 11.4% of families with three or more cases of breast cancer diagnosed before age 60,16 but in a large study of 10,860 unselected breast cancer patients from five countries, the CHEK2 mutation was identified in only 1.9% of cases17 and 0.7% of controls (odds ratio, 2.34), and testing for CHEK2 is not routine. The increasing availability of next-generation sequencing has already resulted in commercially available panels of high and moderate penetrance genes, and is likely to change the approach to genetic screening in future years.
Hormonal Factors
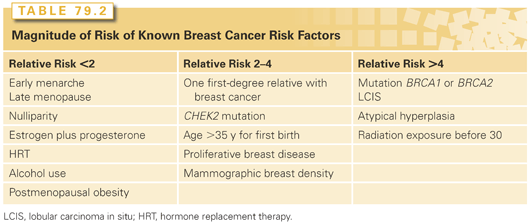
The development of breast cancer in many women appears to be related to female reproductive hormones, particularly endogenous estrogens. Early age at menarche, nulliparity or late age at first full-term pregnancy, and late age at menopause increase the risk of developing breast cancer. In postmenopausal women, obesity and postmenopausal hormone replacement therapy (HRT), both of which are positively correlated with plasma estrogen levels and plasma estradiol levels, are associated with increased breast cancer risk. Most hormonal risk factors have a relative risk (RR) of ≤2 for breast cancer development.
The age-specific incidence of breast cancer increases steeply with age until menopause, and then plateaus. There is substantial evidence that estrogen deprivation via iatrogenic premature menopause can reduce breast cancer risk. Premenopausal women who undergo oophorectomy without hormone replacement have a markedly reduced risk of breast cancer later in life, with an increasing magnitude of risk reduction as the age at oophorectomy decreases.18 Data from women with BRCA1 and BRCA2 mutations suggest that early oophorectomy has a substantial protective effect on breast cancer risk in this population also.19
Age at menarche and the establishment of regular ovulatory cycles are strongly linked to breast cancer risk; the total duration of exposure to endogenous estrogens seems important. There appears to be a 20% decrease in breast cancer risk for each year that menarche is delayed. Of note, hormone levels through the reproductive years in women who experience early menarche may be higher than in women who undergo a later menarche.20 Additionally, late onset of menarche results in a delay in the establishment of regular ovulatory cycles, which may contribute to protective effects.
The relationship between pregnancy and breast cancer risk appears more complicated. Women whose first full-term pregnancy occurs after age 30 have a two- to five-fold increase in breast cancer risk in comparison with women who have a first full-term pregnancy before approximately age 18.20,21 Nulliparous women are at greater risk for the development of breast cancer than parous women, with a RR of about 1.4. Breast cancer risk increases transiently for the 10 years after a pregnancy, but then declines.21 Abortion, whether spontaneous or induced, does not increase breast cancer risk.22 Breastfeeding, particularly for longer duration, lowers the risk of breast cancer diagnosis. The combined effects of reproductive history and breastfeeding may account for substantial fractions of the difference in breast cancer risk between developed and developing nations.
The use of combined estrogen and progestin HRT also increases breast cancer risk. In the Women’s Health Initiative, use of combined estrogen and progestin HRT was associated with a hazard ratio (HR) of 1.24 (p <0.001) for breast cancer development as compared to placebo.23 The effects of HRT were noted after a relatively short duration of use. An excess of abnormal mammograms was observed after 1 year of HRT use and persisted throughout the study, and an increase in breast cancer incidence was noted after 2 years. The cancers occurring in HRT users were larger and more likely to have nodal or distant metastases than those occurring in the placebo group (25.4% versus 16%; p = 0.04), although they were of similar histology and grade.23 The observational UK Million Women Study found that current use of HRT was associated with a RR of breast cancer development of 1.66 (p <0.001) and a RR of breast cancer death of 1.22 (p = 0.05).24
Dietary and Lifestyle Factors
Observational studies suggested that high-fat diets were associated with higher rates of breast cancer than low-fat diets. However, a meta-analysis of eight prospective epidemiologic studies failed to identify an association between fat intake and breast cancer risk in adult women in developed countries.25 Consistent with these findings, a randomized dietary modification in 48,835 women in the Women’s Health Initiative study did not result in a statistically significant reduction in breast cancer incidence after 8 years of follow-up.26 Breast cancer risk increases linearly with the amount of alcohol consumed.27 Decreased intake of nutrients such as vitamin C, folate, and β-carotene may enhance the risk related to alcohol consumption.
Obesity is associated with both an increased risk of breast cancer development in postmenopausal women and increased breast cancer mortality. Women with a body mass index of ≥31.1 have a 2.5-fold greater risk of developing breast cancer than those with a body mass index of ≤22.6.23 Weight and weight gain appear to play an important but complex role in breast cancer risk. During childhood, rapid growth rates decrease the age of menarche, an established risk factor, and result in greater attained stature, which has been consistently associated with increased risk. During early adult life, obesity is associated with a lower incidence of breast cancer before menopause, but no reduction in breast mortality. Weight gain after age 18 is associated with a graded and substantial increase in postmenopausal breast cancer, particularly in the absence of HRT.28
Benign Breast Disease
Benign breast lesions are classified as proliferative or nonproliferative. Nonproliferative disease is not associated with an increased risk of breast cancer, whereas proliferative disease without atypia results in a small increase in risk (RR = 1.5 to 2.0). Proliferative disease with atypical hyperplasia is associated with a greater risk of cancer development (RR = 4.0 to 5.0).29 Dupont and Page30 found a marked interaction between atypia and a family history of a first-degree relative with breast cancer. This subgroup of patients had a risk 11-fold that of women with nonproliferative breast disease, and an absolute risk of breast cancer development of 20% at 15 years, compared with 8% in women with atypical hyperplasia and a negative family history of breast carcinoma. Proliferative breast disease appears to be more common in women with a significant family history of breast cancer than in controls, further supporting its significance as a risk factor. Of note, however, the majority of breast biopsies done for clinical indications demonstrate nonproliferative disease. In the study of 10,000 breast biopsies by Dupont and Page,30 69% had nonproliferative changes and only 3.6% demonstrated atypical hyperplasia.
Breast Density
Mammographic breast density has emerged as an important predictor of breast cancer risk, and makes detection of cancer more difficult. A significant component of breast density is genetically determined, although density has also been shown to vary with the initiation and discontinuation of postmenopausal HRT. Women with >75% breast density have 4.7-fold increase in the odds of breast cancer development compared with those with >10% breast density.31 The risk was apparent even after adjustment for other risk factors.
Environmental Factors
Exposure to ionizing radiation increases breast cancer risk, and the increase is particularly marked for exposure at a young age. This pattern has been observed in survivors of the atomic bombings, those undergoing multiple diagnostic X-ray examinations, and in women receiving therapeutic irradiation. A markedly increased risk of breast cancer development has been reported in women who received mantle irradiation for the treatment of Hodgkin lymphoma before age 15 years.32 Additionally, in women with Hodgkin lymphoma who develop unilateral breast cancer, the risk of a contralateral cancer approximates the level of risk seen in BRCA mutation carriers.32 Well-conducted studies do not suggest that exposure to electromagnetic fields and organochlorine pesticides increase breast cancer risk. A summary of the magnitude of risk associated with known breast cancer risk factors is provided in Table 79.2.
MANAGEMENT OF THE HIGH-RISK PATIENT
There is no formal definition of what constitutes high risk. Without question, women who carry mutations in either BRCA1 or BRCA2, or who have a family history consistent with genetically transmitted breast cancer, are considered to be at higher risk than those in the general population. Other high-risk groups include women who have received mantle irradiation, usually for treatment of Hodgkin lymphoma, and those with lobular carcinoma in situ (LCIS) or atypical hyperplasia. Although a variety of hormonal factors (e.g., early menarche, late age at first full-term pregnancy) affect breast cancer risk on a population basis, these conditions have a relatively small effect on risk for any individual woman.
Many women overestimate their risk of developing breast cancer, so providing an accurate assessment of breast cancer risk will often allay anxiety and facilitate management decisions. It can be helpful to provide women who are concerned about their breast cancer risk with a numeric risk estimate. The Gail et al. model,33 which calculates a woman’s risk of developing breast cancer based on age at menarche, age at first live birth, number of previous breast biopsies, the presence or absence of atypical hyperplasia, and the number of first-degree female relatives with breast cancer, has been used in the National Surgical Adjuvant Breast and Bowel Project (NSABP) breast cancer prevention trials. Efforts to validate the Gail et al. model in different settings have produced variable results. It was highly accurate in the NSABP P1 prevention trial,34 with a ratio of observed to predicted cancers in study participants of 1.03. In general, the Gail et al.33 model is thought to underestimate risk in women with strong family histories, at least in part because it only incorporates a family history in first-degree relatives and does not include ovarian carcinoma. The Claus et al.35 model takes into account both first- and second-degree relatives, although it does not include other risk factors, and may be more useful for women at risk on the basis of family history.
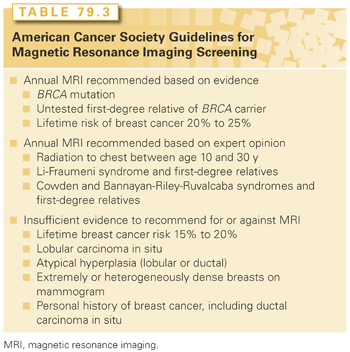
Management strategies available for high-risk women include intensive surveillance, chemoprevention with endocrine agents, and prophylactic surgery. Surveillance, consisting of monthly breast self-examination, annual screening mammography, and clinical breast examinations once or twice yearly, did not clearly result in early detection in high-risk women in the placebo arm of the NSABP P1 prevention trial where 29% of the women who developed breast cancer had axillary node metastases at diagnosis.34 Women at risk as a result of known or suspected BRCA1 or BRCA2 mutations warrant screening with magnetic resonance imaging (MRI), which results in earlier detection of breast cancer than conventional surveillance strategies, and has a higher specificity and sensitivity among high-risk women.36 Because the cancers detected by MRI are smaller and less likely to be associated with nodal positivity, it is likely that a survival benefit is present. However, there is no benefit for MRI screening in women with atypical hyperplasia or LCIS.37 An expert panel convened by the American Cancer Society in 2007 to develop guidelines for MRI screening recommended the use of MRI in addition to mammography for a small group of women at very high risk of breast cancer development, either due to genetic factors or a history of prior thoracic irradiation (Table 79.3). It has been estimated that only 1% of the population would be eligible for MRI screening using these criteria.38 For women with a <15% risk of breast cancer development, the American Cancer Society recommended against the use of MRI screening.39 In the remainder, they thought that the evidence was insufficient to recommend for or against MRI screening. Chemoprevention is an option in addition to surveillance strategies. Two SERMs, tamoxifen and raloxifene, have been shown to reduce the incidence of ER+ breast cancer. Four prospective, randomized trials have examined the effect of tamoxifen on breast cancer incidence (Tables 79.4 and 79.5).34,40–42 There is considerable heterogeneity in outcome among the trials, much of which can be attributed to differences in the populations studied. In an overview of the four studies, tamoxifen produced a 38% reduction in breast cancer incidence (95% confidence interval [CI], 8 to 46; p <0.001), and a 48% reduction in the incidence of ER+ breast cancers.43 No effect on the incidence of ER− cancers was seen in any of the trials, and the cancers occurring in women taking tamoxifen were not found to have had more positive nodes or to be larger in size than those in the placebo arm, providing reassurance that tamoxifen chemoprevention does not result in the occurrence of biologically more-aggressive cancers.
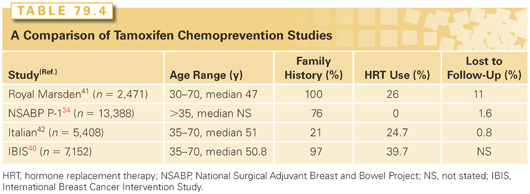
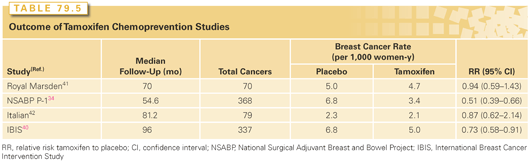
In the largest of these studies, the NSABP P1 trial, a 49% risk reduction was seen with tamoxifen, with 43.4 cancers per 1,000 women occurring in the placebo arm compared with 22.0 per 1,000 in the tamoxifen arm.34 The benefits of tamoxifen were observed for both invasive and noninvasive carcinoma, and were seen in women of all ages. A particular benefit was seen in those at risk because of atypical hyperplasia, with an 84% reduction in cancer incidence in this group. The risk reductions were similar in those at risk on the basis of a family history of breast cancer and those at risk from other factors. Controversy exists over the benefit of tamoxifen in BRCA mutation carriers,44,45 but it appears that it is the likelihood of expressing the ER that determines the efficacy of tamoxifen as a chemopreventive agent rather than the presence of a BRCA mutation.
The side effects of tamoxifen were well known from its use as a cancer treatment and were again observed in the prevention trials. In the combined analysis of the four studies, the RR of thromboembolic events in tamoxifen users was 1.9 (95% CI = 1.4 to 2.6; p <0.0001) and the RR of endometrial cancer was 2.4 (95% CI = 1.5 to 4.0; p = 0.0005).43 Significant elevation in endometrial cancer and thromboembolic events was limited to postmenopausal women. Thus, women most likely to have a favorable risk-benefit ratio for tamoxifen prevention include premenopausal women, younger postmenopausal women without a uterus, and those at risk on the basis of atypical hyperplasia or LCIS. Despite the proven efficacy, use of tamoxifen as chemoprevention has been limited because of concerns about side effects and the small absolute differences in outcomes.
Raloxifene is a SERM used for the treatment and prevention of osteoporosis that was noted to reduce the incidence of ER+ breast cancer in this population. The NSABP P2 trial, the Study of Tamoxifen and Raloxifene (STAR) trial, directly compared the chemopreventive actions and side effects of tamoxifen and raloxifene in 19,747 postmenopausal women at increased risk of breast cancer development.36 No difference in the incidence of invasive cancer was seen between women taking tamoxifen and those taking raloxifene (RR = 1.02; 95% CI = 0.82 to 1.28). More cases of noninvasive cancer were noted in the raloxifene group, with a cumulative incidence of 11.7 per 1,000 compared with 8.1 per 1,000 in the tamoxifen group at 6 years (RR = 1.4; p = 0.052). A more favorable side-effect profile was seen for raloxifene, with a reduction in the number of hysterectomies and endometrial cancers in the raloxifene group (RR = 0.62; 95% CI = 0.35 to 1.08), although the difference did not reach statistical significance. Significantly fewer thromboembolic events and cataracts occurred with raloxifene. Raloxifene is thus a viable alternative to tamoxifen for the chemoprevention of breast cancer in postmenopausal women at increased risk for the disease. In addition, the use of raloxifene in women with osteoporosis has the potential to lower breast cancer incidence in a group of women not considered at high risk.
Trials of aromatase inhibitors (AI) for breast cancer prevention suggest qualitatively similar results as seen with SERMs. The MAP.3 trial examined the use of the AI exemestane in postmenopausal women with a Gail risk score of 1.66, atypical hyperplasia, LCIS, or unilateral ductal carcinoma in situ (DCIS) treated with mastectomy. After a median follow-up of 3 years, a 65% reduction in invasive breast cancer was seen with exemestane (p = 0.002).46 The reduction in cancer incidence was also limited to ER+ cancers. At present, there are no chemopreventive agents that have been proven to be effective in reducing the incidence of ER− breast cancer.
The 2013 American Society of Clinical Oncology (ASCO) guideline on pharmacologic agents for breast cancer risk reduction47 recommends discussion of tamoxifen for breast cancer risk reduction with premenopausal women age 35 and older at increased risk for breast cancer development, and discussion of tamoxifen, raloxifene, and exemestane with high-risk postmenopausal women. Histories of deep vein thrombosis, stroke, pulmonary embolism, or transient ischemic attacks are considered contraindications to the use of both tamoxifen and raloxifene.
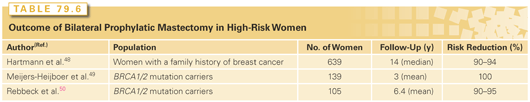
Prophylactic surgery, in the form of bilateral mastectomy or bilateral salpingo-oophorectomy, is another option for breast cancer risk reduction. The efficacy of prophylactic mastectomy has never been studied in a prospective, randomized trial. In a retrospective study of 639 women who had bilateral prophylactic mastectomy due to a family history of breast cancer,48 a 90% to 94% reduction in breast cancer incidence (95% CI = 71% to 99%) and an 81% to 100% reduction in breast cancer mortality with prophylactic mastectomy compared to unaffected sisters or Gail model predictions was observed. Prospective studies in BRCA mutation carriers (Table 79.6) demonstrate similar levels of risk reduction.48–50
Prophylactic bilateral salpingo-oophorectomy is an alternative risk-reduction strategy in women at risk on the basis of BRCA mutations, which has the added benefit of reducing the risk of ovarian carcinoma, a disease for which effective screening is not available. In a prospective study of the benefits of prophylactic salpingo-oophorectomy in 170 BRCA mutation carriers, Kauff et al.19 observed that the HR for breast cancer was reduced to 0.32 (95% CI = 0.08 to 1.20) and to 0.25 for gynecologic cancer (95% CI = 0.08 to 0.74) at a mean follow-up of 24 months. In a meta-analysis51 of risk-reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers, statistically significant reductions in breast cancer (HR = 0.49; 95% CI = 0.37 to 0.65 with similar risk reductions in BRCA1 and BRCA2 mutation carriers) and in the risk of BRCA1/2-associated ovarian or fallopian tube cancer (HR = 0.21; 95% CI = 0.12 to 0.39) were observed. More recently, recognition that BRCA-associated cancers arise in the fallopian tube rather than the ovary has led some to propose bilateral salpingectomy, with ovarian preservation, as a risk-reducing strategy, but the efficacy of this approach is uncertain.
The presence or absence of carcinoma in a suspicious clinically or mammographically detected abnormality can only be reliably determined by tissue biopsy. An abnormal MRI does not reliably indicate the presence of cancer, and a nonworrisome MRI does not reliably exclude carcinoma.52 Available biopsy techniques include fine needle aspiration (FNA), core needle biopsy, and excisional biopsy. Needle biopsy techniques (FNA or core biopsy) are preferred because they are more cost-effective than surgical excision, and because most breast lesions are benign, they avoid a surgical scar and potential cosmetic deformity.
FNA is easily performed, but requires a trained cytopathologist for accurate specimen interpretation and does not reliably distinguish invasive cancer from DCIS, a particular drawback for nonpalpable abnormalities, which are often DCIS. Core-cutting needle biopsy has many of the advantages of FNA, but provides a histologic specimen suitable for interpretation by any pathologist, and facilitates ER, PR, and HER2 testing. False-negative results from sampling error may occur with both core-cutting needle biopsy and FNA. When concordance between the core biopsy or FNA diagnosis, and the clinical and imaging findings is not present, additional tissue should be obtained, usually by excisional biopsy. Concerns about the false-negative rate of image-guided core biopsy have been resolved with the availability of large, vacuum-assisted biopsy devices that increase the extent of lesion sampling, coupled with the development of clearly defined indications for follow-up surgical biopsy. False-negative rates of core biopsy are now reliably <1%. Indications for surgical biopsy following core biopsy are listed in Table 79.7. Although the finding of atypical ductal hyperplasia on a core biopsy is uniformly accepted as an indication for open surgical biopsy, the need for surgical excision of all lesions showing atypical lobular hyperplasia (ALH) or LCIS remains controversial (discussed in the section “Lobular Carcinoma in Situ”). Papillary carcinoma in situ cannot always be readily distinguished from benign papillary lesions on a core biopsy, and radial scar may be difficult to distinguish from tubular carcinoma without complete removal of the lesion.
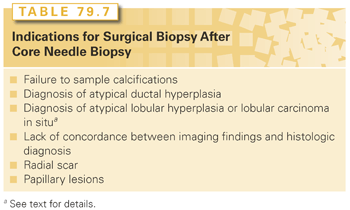
The use of core biopsy for the diagnosis of mammographic abnormalities is cost-effective and increases the likelihood that the patient will be able to undergo a single surgical procedure for definitive cancer treatment.53 Excisional biopsy as a diagnostic technique should be reserved for patients with imaging abnormalities that cannot be targeted for core biopsy. A core biopsy diagnosis permits a complete discussion of treatment options prior to the placement of an incision on the breast and allows the breast procedure and the axillary surgery to take place at a single operation.
When excisional biopsy is performed for diagnosis, a small margin of grossly normal breast should be excised around the tumor, orienting sutures should be placed, and the specimen should be inked to allow margin evaluation.
In 1941, Foote and Stewart54 published their landmark study of LCIS, describing a relatively uncommon entity characterized by an “alteration of lobular cytology.” They hypothesized that LCIS was a precursor lesion of invasive cancer, and, based on this, treatment with mastectomy was recommended. More recently, the term ALH has been introduced to describe morphologically similar, but less well-developed, lesions. Some centers use the term lobular neoplasia (LN) to cover both ALH and LCIS. Morphologically, LN is defined as “a proliferation of generally small and often loosely cohesive cells originating in the terminal duct-lobular unit, with or without pagetoid involvement of terminal ducts.”55
In the past, LCIS was most frequently diagnosed in women aged 40 to 50, a decade earlier than DCIS, but recent literature indicates that the incidence in postmenopausal women is increasing.56 Determining the true incidence of LCIS is difficult, as there are no specific clinical or mammographic abnormalities associated with the lesion and the diagnosis of LCIS is often made as an incidental, microscopic finding in a breast biopsy performed for other indications. The prevalence of LN in an otherwise benign breast biopsy has been reported as between 0.5% and 4.3%.57 LCIS is both multifocal and bilateral in a large percentage of cases.
In an analysis of nine studies including 172 patients with LCIS who were treated by biopsy alone, 15% developed invasive carcinoma in the ipsilateral breast and 9.3% carcinoma in the contralateral breast after 10 years of follow-up.58 This corresponds to a risk of development of invasive carcinoma of about 1% to 2% per year, with a lifetime risk of 30% to 40%. In this study (conducted prior to effective breast imaging), 5.7% of the patients developed metastatic breast cancer. In a more recent study of 776 women with LCIS followed in a high-risk screening program at Memorial Sloan-Kettering Cancer Center, King et al.37 reported that 13% had developed cancer at a median follow-up of 58 months, a rate similar to that seen in older studies. Cancers were detected at a median size of 0.8 cm (0.1 cm to 3.5 cm), and 78% were node negative. MRI screening had no significant impact on cancer stage at diagnosis in this cohort. Subsequent cancers are more often invasive ductal carcinoma than invasive lobular carcinoma (ILC), but the incidence of subsequent ILC is substantially increased compared with women without LCIS. Although the risk for development of breast cancer is bilateral, subsequent ipsilateral carcinoma is more likely than contralateral breast cancer, supporting the view that ALH and LCIS act both as precursor lesions and as risk indicators. The RR for development of subsequent breast cancer is lower in women diagnosed with ALH compared with LCIS. Therefore, although LN is a helpful term for collectively describing this group of lesions, specific classification into ALH and LCIS is preferable in terms of risk assessment and management.
LCIS is typically positive for ER and PR staining and negative for HER2/neu staining. LN (and ILC) characteristically lacks expression of E-cadherin, an epithelial cell membrane molecule involved in cell-cell adhesion. E-cadherin negativity serves as a fairly reliable means of distinguishing ductal from lobular disease, both in situ and invasive. Pleomorphic LCIS is a relatively uncommon variant of LCIS characterized by medium-to-large pleomorphic cells containing eccentric nuclei, prominent nucleoli, and eosinophilic cytoplasm. As with classic LCIS, it is usually ER+ and negative for E-cadherin; it also tests positive by immunohistochemistry (IHC) for gross cystic disease fluid protein-15. Pleomorphic LCIS can be associated with central necrosis, may be associated with mammographic microcalcifications, and may be difficult to distinguish from DCIS. Although pleomorphic LCIS has a more aggressive histologic appearance than classic LCIS, the relative rarity of this lesion and the lack of uniform diagnostic criteria make it difficult to know if pleomorphic LCIS has a different natural history than classic LCIS.
Genetic changes in LN have been evaluated in a number of studies using comparative genomic hybridization. In both ALH and LCIS, there was loss at 16q21-q23.1, an altered region previously identified in invasive carcinoma.59 This genomic signature, common to LN and ILC, further suggests that LN is a precursor lesion in some women.
Management of LN must address the bilateral risk, and options therefore include surveillance, chemoprevention, and prophylactic bilateral mastectomy. Surveillance is the strategy selected by most patients. Mammographic screening is the standard breast imaging technique for patients selecting surveillance. Breast MRI has been used, but, as noted previously, existing evidence does not support its routine use for patients with LCIS. Prophylactic mastectomy reduces breast cancer risk among high-risk women by approximately 90%. Chemoprevention with tamoxifen in patients with LCIS has been evaluated as part of the NSABP P1 study.34 Overall, tamoxifen reduced the incidence of breast cancer by 49% (p <0.00001), and a similar level of risk reduction was seen in the 826 participants with a history of LCIS. In the NSABP P2 (STAR) trial,36 893 participants gave a history of LCIS, and their rates of subsequent breast cancer were similar with tamoxifen and raloxifene. Benefit for exemestane in women with LCIS was also seen in the MAP.3 study.46
In the past, the finding of LN on a core needle biopsy usually led to a recommendation for surgical biopsy to rule out coexisting DCIS or invasive cancer. Recent studies have demonstrated that when the diagnosis of LN is concordant with the imaging findings, upgrade rates with surgical excision are ≤3%.60 Discordance between the pathology and imaging, and the presence of pleomorphic LCIS in a core biopsy remain indications for surgical excision. The recent recognition that, in some cases, LCIS may be a precursor lesion has led to confusion as to whether it should be treated like DCIS (i.e., excised to negative margins and irradiated). At this time, there are no data indicating that the incidence of subsequent cancer is reduced with this approach. When LCIS is seen on an excised tissue, it is not necessary to obtain negative margins of resection, and there is no established role for radiation therapy in patients with LN.
DCIS is defined as the proliferation of malignant-appearing mammary ductal epithelial cells without evidence of invasion beyond the basement membrane. Prior to the widespread use of screening mammography, <5% of mammary cancers were DCIS. At present, 15% to 30% of the cancers detected in mammography screening programs are DCIS, and the greatest increase in the incidence of DCIS has been seen in women aged 49 to 69 years. DCIS can present as a palpable or nonpalpable mass, Paget disease of the nipple, an incidental finding at biopsy, or, most commonly, as mammographically detected calcifications.
A central problem in the management of DCIS is the lack of understanding of its natural history and the inability to determine which DCIS will progress to invasive carcinoma during a woman’s lifetime. The concordance between risk factors for DCIS and invasive carcinoma suggests that they are part of the same disease process.61 Attempts to better characterize the natural history of DCIS on the basis of pathologic features have not been particularly successful. The traditional morphologic classification into comedo, papillary, micropapillary, solid, and cribriform types is confounded by the observation that as many as 30% to 60% of DCIS lesions display more than one histologic pattern. To overcome this difficulty, a number of classifications based on nuclear grade and the presence or absence of necrosis have been developed. No single classification scheme has been widely adopted and, most importantly, none of the classification systems have been prospectively demonstrated to predict the risk of development of invasive carcinoma. Molecular profiling studies in DCIS have been limited by the need for histologic examination of the entire lesion to reliably exclude the presence of invasive carcinoma. The Oncotype DX (Genomic Health, Redwood City, CA) assay using paraffin-embedded tissue was modified for DCIS, and an initial study suggested usefulness in predicting the risk of invasive in-breast recurrence. This finding has not been validated in other populations, nor has this test been shown to predict the benefit of radiotherapy (RT).62 The available data indicate that DCIS lesions share many of the genetic alterations of invasive carcinoma, but predictors of progression to invasion remain to be identified.
Treatment of the Breast
Cancer-specific survival for the woman diagnosed with DCIS exceeds 95%, regardless of the type of local therapy employed.63,64 Mastectomy, excision and RT, and excision alone have all been proposed as management strategies for DCIS. The appropriate therapy for the woman with DCIS depends on the extent of the DCIS lesion, the risk of local recurrence (LR) with each form of treatment, and the patient’s attitude toward the risks and benefits of a particular therapy.
Total or simple mastectomy is curative in approximately 98% of patients regardless of age, DCIS presentation, size, or grade.65 The primary medical indication for mastectomy in DCIS is a lesion too large to be excised to negative margins with a cosmetically acceptable outcome.66 The extent of DCIS is most accurately estimated preoperatively with the use of magnification mammography.67 Studies indicate that MRI both overestimates and underestimates the size of DCIS lesions, does not improve surgical planning when compared with diagnostic mammography, and does not decrease the rate of ipsilateral breast tumor recurrence (IBTR).68
For women with localized DCIS, management by excision alone and excision plus RT have both been employed. Four published prospective, randomized trials have directly compared these two approaches in >4,500 patients.63,64,69,70 In all four trials, the majority of participants had mammographically detected DCIS, and in all but the Swedish trial,70 negative margins (no ink on tumor) were required. A dose of 50 Gy of radiation was delivered to the whole breast in 25 fractions, and a boost dose to the tumor bed was not required. The results of these trials are summarized in Table 79.8. No differences in overall survival (OS) were seen between treatment arms. In all four studies, the use of RT resulted in a highly significant reduction in the risk of an IBTR, with proportional risk reductions ranging from 47% to 63%.40,63 Consistent with observations from many retrospective studies, approximately 50% of the recurrences in both groups were invasive carcinoma, and a benefit for RT was seen in the reduction of both invasive and noninvasive recurrences.
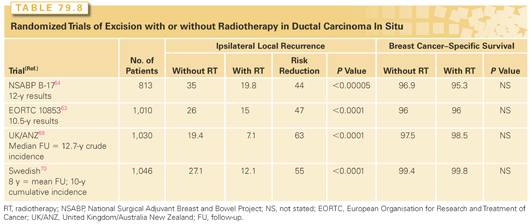
A meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) provides a concise overview of RT effect following lumpectomy for DCIS.71 With a median follow-up of 8.9 years, RT approximately halved the rate of ipsilateral breast events (rate ratio = 0.46; standard error [SE] = 0.05; 2p <0.00001). RT was effective in all subgroups. There were 291 “low-risk” cases of DCIS that were low-grade, >20 mm in size, and with negative surgical margins identified. Among them, the 10-year risk of an ipsilateral event in those allocated to lumpectomy alone was substantial at 30.1%, and even with this relatively small number of women, the effect of RT was highly significant (rate ratio = 0.48; SE = 0.17; 2p =0.002), with a 10-year absolute gain of 18.0% (SE = 5.5%).
The Radiation Therapy Oncology Group (RTOG) study 9804 sought to determine RT benefit after lumpectomy for patients with low-risk DCIS.72 In comparison to the other randomized trials, RTOG 9804 enrolled patients with smaller lesions, all low- or intermediate-grade DCIS, and had a much higher rate of adjuvant tamoxifen use (62%). Recurrence rates were 6.7% in the observation arm, compared to 0.9% in the RT arm, after a median follow-up of 7.2 years (HR = 0.11; 95% CI = 0.03 to 0.47; p = 0.0003).72 This suggests that even in low-risk DCIS, RT can lower the risk of in-breast recurrence. LR rates after excision and RT have decreased over time due to improvements in imaging and pathologic analysis. In a retrospective review of 246 consecutive patients treated at Dana-Farber Cancer Institute and Brigham and Women’s Hospital from 2001 to 2007, there were no LRs with a median follow-up time of 58 months.73
Despite the clear benefit of RT seen in these five trials, there was considerable interest in identifying patients who could be spared RT for DCIS. The Dana-Farber/Harvard Cancer Center conducted a single-arm, prospective trial of wide excision alone from 1995 to 2002 among 158 patients.74 Entry criteria included DCIS of predominant grade 1 or 2 with a mammographic extent of no greater than 2.5 cm and final margin width of at least 1 cm. Tamoxifen was not permitted. The study was closed to further accrual because the number of LRs (n = 13) met the predefined stopping rules. The median patient age was 51 years, and 94% had mammographically detected DCIS. The median follow-up was 11 years, and 143 patients were followed for >8 years. Nineteen patients developed an LR. Fourteen recurrences were in the same quadrant as the initial DCIS, and five were elsewhere in the ipsilateral breast. A total of 32% recurred with invasive disease. No patient developed distant metastasis. The 10-year cumulative incidence of LR was 15.6%.
Similar results were seen in the Eastern Cooperative Oncology Group single-arm trial of excision alone for DCIS. Eligibility criteria for this study included DCIS at least 3 mm in size, excised with a margin width of ≥3 mm as determined by sequential sectioning, and complete embedding. The study enrolled patients with low- or intermediate-grade DCIS ≤2.5 cm in size and high-grade DCIS (defined as nuclear grade 3 with necrosis) up to 1 cm in size. A postexcision mammogram was required for all patients. At a median follow-up of 8.8 years, the 10-year LR rate was 19.0% for patients with high-grade DCIS, and 14.6% for those with low- or intermediate-grade DCIS.62 Taken together, these prospective studies indicate that even patients with small low- to immediate-grade DCIS treated with excision to widely negative margins of resection have about a 15% 10-year risk of a LR in the absence of RT. No reduction in recurrence was observed for patients excised to margins of ≥1 cm compared to those with margins of 3 to 9 mm.
The 2014 National Comprehensive Cancer Network (NCCN) Guidelines® endorse lumpectomy and whole breast radiation therapy or total mastectomy as category 1 recommendations, and lumpectomy without RT as a category 2B recommendation.66 Clearly, patients should be included in the treatment decision making to learn what magnitude of risk reduction is meaningful to them. A detailed discussion of the pros and cons of the various treatment options is needed to allow each woman with DCIS to make an informed treatment choice.
Treatment of the Axilla
In situ carcinoma by definition does not metastasize, so theoretically, axillary staging should be unnecessary for DCIS. Studies of axillary dissection (ALND) in DCIS have demonstrated axillary nodal metastases in only 1% to 2% of patients, presumably due to unrecognized microinvasion. Data from the NSABP B-17 and B-24 studies confirm that the risk of isolated axillary recurrence with no axillary surgery is <0.1%, regardless of whether RT and tamoxifen are administered.75 These low rates of axillary recurrence argue against routine use of sentinel lymph node biopsy in DCIS. Selective use of sentinel node biopsy in patients with DCIS who are at significant risk of having coexistent invasive carcinoma is appropriate. Approximately 15% of patients diagnosed as having DCIS with large vacuum-assisted biopsy devices are found to have invasive cancer after complete excision of the lesion. The diagnosis of DCIS in a palpable breast mass and pathologic interpretation of a core biopsy specimen as suspicious, but not diagnostic, of microinvasion are circumstances in which invasive cancer is frequently found when the lesion is completely examined and so sentinel node biopsy should be considered.
Because patients undergoing mastectomy forfeit the opportunity for sentinel node biopsy if not performed concurrently, patients receiving mastectomy for DCIS should undergo sentinel node biopsy.
Endocrine Therapy
The ER is present in about 80% of DCIS lesions and is more frequent in noncomedo than comedo DCIS.76 Endocrine therapy might reduce LR after breast-conserving therapy (BCT) and prevent development of new primary breast cancers in the contralateral breast. Two trials have examined the use of tamoxifen in women with DCIS. In the NSABP B-24 trial,64 patients with DCIS were treated with excision and RT and randomized to tamoxifen 20 mg daily or a placebo for 5 years. Patients in the tamoxifen arm had a 32% reduction in the risk of an invasive LR (p = 0.025), a 16% reduction in the risk of a DCIS LR (p = 0.33), and a 32% reduction in contralateral breast cancer (p = 0.023) compared to the patients in the placebo arm. In the subset of women with ER+ DCIS, tamoxifen reduced the risk of any breast cancer event by 51% (RR = 0.41; 95% CI = 0.25 to 0.65; p = 0.0002); there was no benefit seen if the DCIS lesion was ER−.76 The United Kingdom/Australia New Zealand trial69 that randomized women to tamoxifen or to no tamoxifen and with a median follow-up of 12.7 years found that tamoxifen reduced ipsilateral events (HR = 0.78; 95% CI = 0.62 to 0.99), but more significantly, reduced contralateral events (HR = 0.44; 95% CI = 0.25 to 0.77) (In a subset analysis, the benefit of tamoxifen appeared restricted to patients who did not receive RT.) Taken together, these trials suggest that tamoxifen modestly reduces ipsilateral events with or without RT, and substantially reduce contralateral events. The addition of tamoxifen to RT is particularly attractive in young patients with ER+ DCIS, in whom the risk of LR is higher and the toxicity of tamoxifen is less than in older patients.
Evidence that the AIs reduce the incidence of contralateral breast cancer to a greater extent than tamoxifen has led to interest in their use in DCIS. Ongoing trials (NSABP B53 and IBIS II) are comparing tamoxifen with an AI; however, at present, there are no data for use of AIs in management.
In summary, patients with localized DCIS have treatment options ranging from simple excision to mastectomy, all of which have high survival rates but different risks of LR. Patient preference plays a major role in treatment selection, but available evidence indicates that patients have limited understanding of the nature of DCIS. In one study, women with DCIS estimated their risk of breast cancer death to be 39%.77 Perhaps related to this, Katz et al.78 found that although patients reported that their surgeon infrequently recommended mastectomy for DCIS, greater involvement of patients in the decision-making process was associated with higher rates of mastectomy.
The staging system for breast cancer was last updated in 2010.79 The American Joint Committee on Cancer (AJCC) system is both a clinical and pathologic staging system, and is based on the TNM system in which “T” refers to tumor, “N” to nodes, and “M” to metastasis. The current version is the seventh edition of the system and is provided in the following.79
The major changes in this edition were the inclusion of a new classification system for patients after neoadjuvant therapy and the creation of a new M0(i+) category for patients found to have circulating tumor cells, tumor in the bone marrow, or incidentally detected tumor deposits in other tissues not exceeding 0.2 mm in size. Patients in this category are staged according to T and N, and are not classified as stage IV.
The AJCC staging system provides a strategy for grouping patients with respect to prognosis. However, TNM staging, while still important, has been superseded by rapidly evolving molecular characterizations of breast cancers, which more precisely define subgroups with different outcomes, both in terms of prognosis and response to specific treatments. Increasingly, multigene diagnostic tests, such as Oncotype DX and MammaPrint (Agendia Inc. USA, Irvine, CA), are employed as part of treatment decision making for invasive breast cancer.
Tumor, Node, and Metastases Definitions
Definitions for classifying the primary tumor (T) are the same for clinical and for pathologic classification. If the measurement is made by physical examination, the examiner will use the major headings (T1, T2, or T3). If other measurements are used, such as mammographic or pathologic measurements, the subsets of T1 can be used. Tumors should be measured to the nearest 0.1 cm increment. The AJCC TNM staging system is illustrated in Table 79.9. Stage IIIC breast cancer includes patients with any T stage who have pN3 disease. Patients with pN3a and pN3b disease are considered operable and are managed as described in the section on stage I, II, IIIA, and operable IIIC breast cancer. Patients with pN3c disease, in which the ipsilateral supraclavicular nodes are affected by cancer, are considered inoperable and are managed as described in the section on inoperable stage IIIB or IIIC or inflammatory breast cancer (IBC).79 Pathologic stage after neoadjuvant therapy is designated with the prefix “yp.” Complete response is defined as the absence of invasive carcinoma in the breast and axillary nodes.


Historically, classification of invasive breast cancers has been based on the morphologic appearance of the cancer as seen by light microscopy.80 The most widely used such classification is that of the World Health Organization (second edition),55 based on the growth pattern and cytologic features of the invasive tumor cells. Although the classification system recognizes invasive “ductal” and “lobular” carcinomas, this is not meant to indicate that the former originates in the ducts and the latter in the lobules of the breast. Most invasive breast cancers arise in the terminal duct lobular unit, regardless of histologic type.
The most common histologic type of breast cancer is invasive (infiltrating) ductal carcinoma, comprising 70% to 80% of cases. The diagnosis of invasive ductal carcinoma is a diagnosis by exclusion (i.e., this tumor type is defined as a type of cancer not classified into any of the other special categories of invasive mammary carcinoma, such as invasive lobular, tubular, mucinous, medullary, and other special types). To emphasize this point, most classification systems use the term infiltrating ductal carcinoma, not otherwise specified (NOS) or infiltrating carcinoma of no special type. In practice, the terms invasive ductal carcinoma, infiltrating ductal carcinoma, and infiltrating or invasive carcinoma of no special type are used interchangeably.
Special types of cancers comprise approximately 20% to 30% of invasive carcinomas. At least 90% of a tumor should demonstrate the defining histologic characteristics of a special type of cancer to be designated as that histologic type. Breast cancer histologic classifications recommended by the AJCC Staging Manual include NOS; ductal; inflammatory; medullary, NOS; medullary with lymphoid stroma; mucinous; papillary (predominantly micropapillary pattern); tubular; lobular; Paget disease and infiltrating; undifferentiated; squamous cell; adenoid cystic; secretory; and cribriform. Tumor subtypes that occur in the breast, but that are not considered to be typical breast cancers, include cystosarcoma phyllodes, angiosarcoma, and primary lymphoma.
Invasive breast cancers can be further subclassified based on microscopic features. The most common subclassification has been grading, based either solely on nuclear features (nuclear grading) or on a combination of architectural and nuclear characteristics (histologic grading). In nuclear grading, the appearance of the tumor cell nuclei is compared with those of normal breast epithelial cells, and the tumor nuclei are classified as well differentiated, intermediately differentiated, or poorly differentiated. In current practice, histologic grading is the most commonly used method of grading. In histologic grading, breast carcinomas are categorized based on the evaluation of (1) tubule formation, (2) nuclear pleomorphism, and (3) mitotic activity. The grading system by Elston and Ellis,81 a modification of the grading system proposed by Bloom and Richardson in 1957, is recommended as part of AJCC staging. Tubule formation (>75%, 10% to 75%, and <10%), nuclear pleomorphism (small and uniform, moderate variation in size and shape, and marked nuclear pleomorphism), and mitotic activity (per field area) are each scored on a scale of 1 to 3. The sum of the scores for these three parameters is the overall histologic grade. Tumors with a sum of the scores of 3 to 5 are designated grade 1 (well differentiated), those with sums of 6 and 7 are designated grade 2 (moderately differentiated), and those with sums of 8 and 9 are designated grade 3 (poorly differentiated). Histologic grading, particularly the distinction between grades 1 and 3, has prognostic significance as discussed in the section “Prognostic and Predictive Factors in Breast Cancer.” In addition, breast cancers with pure tubular, mucinous, papillary, or cribriform features are recognized to have a more favorable outcome than the more common types of breast cancer.80 Micropapillary tumors are a recently recognized entity with a high incidence of lymphatic and vascular invasion, and systemic recurrence.82
LOCAL MANAGEMENT OF INVASIVE CANCER
The evaluation of the patient newly diagnosed with breast cancer begins with a determination of operability. The presence of distant metastases at diagnosis has traditionally been considered a contraindication to surgery. Some retrospective studies have suggested a survival benefit for surgery of the primary tumor in the patient presenting with metastatic disease,83,84 but systemic therapy remains the initial therapeutic approach. Extensive evaluations to look for metastatic disease are not warranted in asymptomatic patients with stage I and II cancer because of the low likelihood of identifying metastatic disease.85 In patients with stage III disease, occult metastases are more frequent, often estimated at 20% of cases, and staging studies are recommended.85
Patients with T4 tumors and those with N2 or N3 nodal disease are also not candidates for surgery as the first therapeutic approach and should be treated with systemic therapy initially (discussed in the section “Locally Advanced Breast Cancer and Inflammatory Breast Cancer” on page 1147). In the patient with clinical stage I, II, and T3N1 disease, the initial management is usually surgical. In these patients, the evaluation consists of a determination of their suitability for BCT and a discussion of the options of mastectomy with and without reconstruction. Initial systemic therapy may be used to shrink the primary tumor to allow BCT in a woman who would otherwise require mastectomy, but is not mandatory, as it is for women with locally advanced and inflammatory carcinoma. The current status of management approaches for primary operable breast cancer is discussed in detail in the following sections.
Breast-Conserving Therapy
The goal of BCT using conservative surgery (CS) and RT is to provide survival equivalent to mastectomy with preservation of the cosmetic appearance and a low rate of recurrence in the treated breast. Because of the wide acceptance of the Halstedian dogma, a relatively large number of randomized clinical trials were conducted comparing mastectomy and BCT, and they demonstrated equivalent survival. The long-term stability of this equivalence was confirmed by the 20-year follow-up reports of the two largest studies, the NSABP B-06 and Milan I trials.86,87 An overview of all the trials has also demonstrated comparable survival,88 indicating that survival for most patients with breast cancer does not depend on the choice of mastectomy versus BCT.
Medical contraindications to BCT are infrequent. In a population-based study of 1984 patients with DCIS, stage I and II breast cancer, only 13.4% were advised by their surgeon that mastectomy was medically necessary.89 In the 1,459 women in whom BCT was attempted, conversion to mastectomy occurred in 12%, and re-excision was not attempted in the majority. Thus, the available data indicate that a minority of patients have contraindications to BCT, and these are readily identified with standard clinical tools. Patient participation in the surgical decision-making process is an important factor in mastectomy use. In a population-based study of patients diagnosed with breast cancer in 2002 in two large metropolitan areas (Los Angeles and Detroit), more patient involvement in decision making was associated with a greater likelihood of undergoing mastectomy.90,91 The incidence of LR after BCT has declined over time, from 10-year rates of 8% to 19% seen in retrospective studies and the initial randomized trials of BCT, to 2% to 7% in patients excised to negative margins in more recent studies. Table 79.10 shows the 10-year rates of LR in node-negative NSABP trials.92 This decrease in LR rates is the result of a combination of improved mammographic and pathologic evaluation, and the more frequent use of adjuvant systemic therapy (discussed in detail in the section “Risk Factors for Local Recurrence Following Conservative Surgery and Radiation Therapy” on Page 1129). In contrast, rates of LR after mastectomy have remained stable over the same time period.
Recurrences in the breast are typically classified by their location in relation to the original tumor. Recurrences at or near the primary site (presumably representing a recurrence of the original tumor) are classified as either a true recurrence (within the boosted region), a marginal miss (adjacent to the boosted region), or elsewhere in the breast (occurring at a distance from the original tumor and presumably representing a new primary). The time course to LR in the patient undergoing BCT is prolonged. In one study, the annual incidence rate for a true recurrence/marginal miss recurrence was between 1.3% and 1.8% for years 2 through 7 after treatment and then decreased to 0.4% by 10 years after treatment. The annual incidence rate for recurrence elsewhere in the breast increased slowly to a rate of approximately 0.7% per year at 8 years and remained stable. It also appears that just as the time to development of distant metastases is more rapid in patients with ER− or HER2+ cancers than in those with ER+ and PR+ cancers, the time to LR also varies with receptor status. (These results have been contrasted to those seen after mastectomy, in which most LRs occur in the first 3 to 5 years after surgery.) In the Milan I trial, after 20 years of follow-up, the risk of any type of recurrence in the treated breast was 0.63 per 100 woman-years compared with a risk of 0.66 per 100 woman-years for contralateral cancer.87 This suggests that although whole-breast irradiation (WBI) is effective at eradicating subclinical multicentric foci of breast carcinoma present at the time of diagnosis, it does not prevent the subsequent development of new cancers. Thus, patients who elect BCT require lifelong follow-up to screen for the development of new cancers in both the treated and the contralateral breast.
Risk Factors for Local Recurrence Following Conservative Surgery and Radiation Therapy
Risk factors for recurrence after CS and RT can be subdivided into patient, tumor, and treatment factors.
Patient Risk Factors
The most important patient risk factors for LR recurrence are age and inherited susceptibility. Age (<35 or 40) is associated with an increased risk of LR after BCT. Young patient age is associated with an increased frequency of various adverse pathologic features, such as lymphatic vessel invasion, grade 3 histology, absence of ER/presence of HER2, and the presence of an extensive intraductal component (EIC). However, even when correction is done for the differing incidence of the pathologic features of the primary tumor between the age groups, young age is still associated with an increased likelihood of recurrence in the breast.93 However, young age is also a risk factor for LR after mastectomy and should not be considered a contraindication to BCT.
An inherited susceptibility to breast and ovarian cancer, and other cancers has been mainly linked to germline mutations in BRCA1 and BRCA2. Patients with breast cancer with a mutation have a substantial risk of contralateral and late ipsilateral breast cancers. In a retrospective study, outcome following CS and RT was compared for 302 stage I-III patients with breast cancer with germline BRCA1 or BRCA2 mutations and 353 stage I-III patients treated with mastectomy.94 With a follow-up time of 8.2 and 8.9 years for BCT and mastectomy patients, respectively, LR was significantly more likely in those treated with BCT compared to mastectomy, with a cumulative estimated risk of 23.5% versus 5.5%, respectively, at 15 years (p <0.0001); the 15-year estimate in carriers treated with BCT and chemotherapy was 11.9% (p = 0.08 when compared to mastectomy). Most LR events appeared to be second primary cancers. The risk of contralateral breast cancer was high in all groups, exceeding 40%. It is important to consider genetic testing in a patient with newly diagnosed breast cancer with a personal and family history suggestive of a BRCA1 or BRCA2 germline mutation. In patients with a mutation, the option of bilateral mastectomy should be strongly considered to avoid the long-term risk of a second breast cancer in either breast. Patients with breast cancer most likely to benefit from bilateral mastectomy are those who are young and have early-stage disease. Given the high risk of a contralateral breast cancer, unilateral mastectomy is generally not performed in a patient who is a candidate for BCT.
Tumor-Based Risk Factors
An important tumor risk factor is the margin of resection. A negative margin is defined by absence of cancer cells at inked surfaces, but there is no standard definition of a close margin. Margins need to be interpreted in conjunction with the operative findings. A close deep margin is not significant if the breast resection was carried down to the pectoral fascia; the same is true for a close anterior margin if the resection extended to the deep dermal surface. Patients with negative margins of excision have low rates of LR after treatment with CS and RT. The impact of close margins of resection on LR has been more controversial, resulting in the frequent use of re-excision to obtain margins more widely clear than no ink on tumor. A 2013 multidisciplinary consensus panel considered a meta-analysis of margin width and IBTR from a systematic review of 33 studies including 28,162 patients. The results of randomized trials, reproducibility of margin assessment, and current patterns of multimodality care were also considered. The panel concluded that positive margins (ink on invasive carcinoma or DCIS) were associated with a two-fold increase in the risk of IBTR compared to negative margins. This increased risk was not mitigated by favorable biology, endocrine therapy, or a radiation boost. The panel also concluded that more widely clear margins beyond “no ink on tumor” do not significantly decrease the rate of IBTR. There is no evidence that more widely clear margins reduce IBTR for young patients, unfavorable biology, lobular cancers, or cancers with an EIC. (When an EIC is present, young age and multiple close margins are associated with an increased risk of IBTR and can be used to select patients who might benefit from re-excision). Therefore, the use of no ink on tumor as the standard for an adequate margin in invasive cancer in the era of multidisciplinary therapy is associated with low rates of IBTR and has the potential to decrease re-excision rates, improve cosmetic outcomes, and decrease health-care costs.95
The underlying molecular subtype of the tumor is the most significant determinant of the likelihood of LR after BCT (and mastectomy), particularly among those treated in the modern era with surgery to achieve negative margins.96–98 Higher risks of LR are observed in patients with triple-negative breast cancer than in those with other subtypes, regardless of whether they are treated with BCT or mastectomy.99
There is interest in identifying molecular predictors of the risk of LR. The 21-gene recurrence score (Oncotype DX) predicts local-regional recurrence (LRR) in node-negative, ER+ breast cancer, regardless of type of surgery.100 The 10-year LR rate was 4.3% for patients with a low recurrence score (<18), 7.2% with an intermediate recurrence score (18 to 30), and 15.8% with a high recurrence score (>30).
Treatment-Based Risk Factors
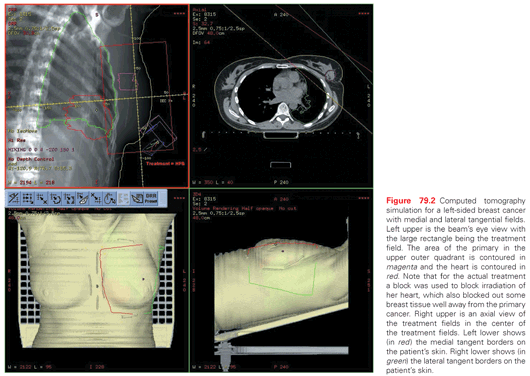
Other important treatment risk factors are the use of a boost and the use of adjuvant systemic therapy. A boost or supplementary irradiation to the area of the primary site is generally used. It is standard in RT after lumpectomy for patients to receive 45 to 50 Gy of WBI followed by a 10 to 16 Gy boost to the region of the tumor bed (Fig. 79.2). The use of a boost is supported by the large European Organisation for Research and Treatment of Cancer (EORTC) trial in which 5,318 patients with negative margins were randomized to a boost of 16 Gy or no boost following 50 Gy to the whole breast.101 With a median follow-up of 10.8 years, the cumulative incidence of ipsilateral breast recurrence was 10.2% without a boost and 6.2% with a boost (p <0.0001). This 41% proportional reduction in LR was similar in all age groups; however, the absolute benefit of the boost was greatest in young patients aged 40 years or less (24% decreased to 14%) and was smallest in patients over age 60 (7% decreased to 4%). Severe fibrosis was increased from 2% to 4% with the boost. Survival at 10 years was the same in both arms. A clinicopathologic study was performed on 1,616 patients in the EORTC trial. In multivariate analysis, high-grade invasive ductal carcinoma was associated with an increased risk of LR (HR = 1.67; p = 0.026), and the boost was effective in reducing LR in this subgroup.102
The use of adjuvant systemic therapy is a very important factor associated with recurrence in the treated breast in conjunction with CS and RT. This effect is clearly demonstrated in three randomized clinical trials. In the NSABP B-14 trial, node-negative, ER+ patients were randomized to receive tamoxifen or to a placebo. The 10-year rate of recurrence in the ipsilateral breast was 14.7% without tamoxifen and only 4.3% with tamoxifen.103 A similar result was seen in the Stockholm Breast Cancer Study Group among node-negative patients randomized to receive tamoxifen or a placebo.104 In the NSABP B-21 trial, patients with node-negative breast cancer measuring ≤1 cm treated with lumpectomy were randomized to tamoxifen alone, RT, or RT and tamoxifen. With a mean follow-up of 87 months, the 8-year rate of ipsilateral LR was 9.3% in the patients treated with RT and 2.8% in the patients treated with RT and tamoxifen.105 Similar results are seen with adjuvant chemotherapy. In the NSABP B-13 trial, node-negative, ER− patients were randomized to chemotherapy or to a no-treatment control group. Among the 235 patients treated with CS and RT, the 8-year rate of recurrence in the ipsilateral breast was 13.4% without chemotherapy and only 2.6% with chemotherapy given concurrently with the RT.106 The net result of the benefit of systemic therapy on local control is that between 1990 and 2011, LRR decreased from 30% to 15% of all recurrences seen in a population of 86,598 women enrolled in 53 randomized phase 3 trials.107
The standard approach is to use sequential chemotherapy and RT. Given the primary importance of preventing systemic relapse, it has been the standard to use initial chemotherapy followed by RT. Although concerns have been expressed about an increased rate of LR with this approach, the results of clinical trials in patients with negative margins have not shown this to be a problem even following 6 months of chemotherapy as with four cycles of doxorubicin and cyclophosphamide followed by four cycles of taxol, both given every 3 weeks.108
Preservation of a Cosmetically Acceptable Breast
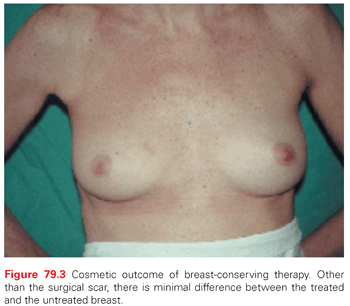
A major goal of BCT is the preservation of a cosmetically acceptable breast. When modern treatment techniques are used, an acceptable cosmetic outcome can be achieved in almost all patients (without compromise of local tumor control) (Fig. 79.3). Treatment-related changes in the breast stabilize at around 3 years. Evolution of the untreated breast, such as change in size because of weight gain and the normal ptosis seen with aging, continue to affect the symmetry. The major factor determining the cosmetic result is the extent of surgical resection.109 A variety of factors must be considered together (the size of the patient’s breast, the size of the tumor, the depth of the tumor within the breast, and the quadrant of the breast in which the tumor is located) to judge the feasibility of a cosmetically acceptable resection. For example, although the removal of a large tumor in the lower portion of the breast often results in distortion of the breast contour, this is only apparent with the arms raised and is acceptable to most women. A similar distortion in the upper inner quadrant of the breast, which is visible in most types of clothing, might not be as acceptable.
Guidelines for Patient Selection
Because of the potential options for treatment of early-stage breast cancer, careful patient selection and a multidisciplinary approach are necessary. Critical elements in patient selection for BCT are (1) history and physical examination, (2) mammographic evaluation, (3) histologic assessment of the resected breast specimen, and (4) assessment of the patient’s needs and expectations.
Recent (i.e., usually within 3 months) preoperative mammographic evaluation is necessary to determine a patient’s eligibility for BCT by defining the extent of a patient’s disease, the presence or absence of multicentricity, and other factors that might influence the treatment decision. If the mass is associated with microcalcifications, an assessment of the extent of the calcifications within and outside the mass should be made using magnification views. Mammography of the contralateral breast is also standard at the time of diagnosis to exclude synchronous lesions.
There is controversy regarding the role of additional imaging studies, particularly MRI of the breast, in selecting patients for BCT. A meta-analysis of 3,112 patients in nine studies with comparison cohorts treated with and without MRI found no difference in the need for re-excision or unexpected conversion to mastectomy, after age adjustment, in patients managed with and without MRI.110 The age-adjusted rate of initial mastectomy was increased three-fold in patients having MRI. Several other studies have shown an association between MRI use and greater, but unwarranted, use of mastectomy.111,112 MRI frequently identifies additional areas of involvement in the breast, but long-term clinical experience has demonstrated that the majority of this disease is controlled with RT. In addition, MRI has a substantial false-positive rate. Ideally, prospective trials demonstrating a clinical benefit in patients selected for BCT with MRI are needed before these examinations are routinely used for patient selection.
The patient and her physician must discuss the benefits and risks of mastectomy compared with those of BCT in her individual case. The following factors should be discussed:
1. The absence of a long-term survival difference between treatments
2. The possibility and consequences of LR with both approaches
3. Psychological adjustment (including the fear of cancer recurrence), cosmetic outcome, sexual adaptation, and functional competence
Psychological research comparing patient adaptation after mastectomy with that after BCT shows no significant differences in global measures of emotional distress. However, women whose breasts are preserved have more positive attitudes about their body image and experience fewer changes in their frequency of breast stimulation and feelings of sexual desirability. In addition, patients treated with BCT have better physical functioning compared with patients treated with mastectomy at the end of primary treatment.113
Absolute and Relative Contraindications to Breast-Conserving Therapy (National Comprehensive Cancer Network 2014)
Contraindications for BCT requiring radiation therapy include the following:
Absolute:
■ Radiation therapy during pregnancy
■ Diffuse suspicious or malignant-appearing microcalcifications
■ Widespread disease that cannot be incorporated by local excision through a single incision that achieves negative margins with a satisfactory cosmetic result
■ Positive pathologic margin
Relative:
■ Active connective tissue disease involving the skin (especially scleroderma and lupus)
■ Tumors >5 cm
■ Focally positive margin
■ Women with a known or suspected genetic predisposition to breast cancer:
■ May have an increased risk of ipsilateral breast recurrence or contralateral breast cancer with BCT
■ Prophylactic bilateral mastectomy for risk reduction may be considered
Preoperative Systemic Therapy for Operable Cancer
Women who desire BCT but are not candidates for the procedure because of a large tumor relative to the size of the breast should be considered for preoperative or neoadjuvant therapy. This approach does not allow BCT for patients with multicentric carcinoma or those with an EIC that precludes a cosmetic resection. Patients most likely to be converted to BCT with neoadjuvant chemotherapy are those with unicentric, higher-grade, HER2+ or triple-negative cancers, as such cancers often respond dramatically to chemotherapy.
Pathologic complete response (pCR)—defined as the absence of residual invasive cancer in the breast and axilla following preoperative therapy—is a common endpoint for clinical trials of preoperative treatment. Multiple studies have shown that patients who experience pCR with neoadjuvant treatment have, on average, better long-term outcomes, with lower risk of cancer recurrence, than women with residual cancer following neoadjuvant chemotherapy. The US Food and Drug Administration has recently indicated that pCR may be a surrogate for accelerated drug approval in neoadjuvant treatment of operable breast cancer.114
Prospective, randomized trials of patients with operable breast cancer have demonstrated that clinical response rates to neoadjuvant chemotherapy are high, ranging from 50% to 85% in many studies. pCRs in the breast range from 15% to 40%. Despite these high response rates, only 25% to 30% of patients who were not candidates for BCT at presentation were able to undergo the procedure after preoperative therapy.115,116 This is a reflection of both the difficulty of assessing the extent of residual viable tumor after preoperative chemotherapy and the often patchy nature of cancer cell death in response to chemotherapy. This type of response significantly decreases the total number of viable tumor cells, but viable tumor remains scattered throughout the same volume of breast tissue, precluding BCT. MRI is better than mammography or ultrasonography in evaluating the extent of viable tumor and its distribution, but may both underestimate and overestimate the extent of residual disease.
In patients who overexpress HER2, the preoperative administration of anti-HER2 therapy in combination with chemotherapy has been associated with high rates of pCR. Clinical trials have confirmed that adding trastuzumab to chemotherapy improves the pCR rate among women with HER2+ breast cancer receiving neoadjuvant therapy and improves long-term survival,117 consistent with the survival benefit observed for trastuzumab when given with chemotherapy in the adjuvant setting.
In 2013, the US Food and Drug Administration approved concurrent use of a second anti-HER2 antibody, pertuzumab, for use in combination with trastuzumab and chemotherapy in women receiving neoadjuvant therapy for HER2+ breast cancer, after it was shown that pertuzumab enhanced the rates of pCR.118
A meta-analysis of nine randomized trials of preoperative chemotherapy demonstrated no increase, or decrease, in survival with preoperative compared with postoperative treatment,119 but an elevated risk of LRR (RR = 1.22; 95% CI = 1.04 to 1.45) was noted. Some of the increase in LR was due to the inclusion of studies in the meta-analysis in which patients who had a clinical complete response did not have surgery. Even in patients undergoing surgery, an elevated risk of LR has been observed. In the NSABP B-18 study,115 LR rates were 15% in patients who required chemotherapy to undergo BCT compared with only 7% in those who were initially candidates for BCT. The increased rates of LR after neoadjuvant therapy likely reflect that in this setting, a negative margin may still be associated with a clinically significant residual tumor burden that is unlikely to be controlled by RT. Thus, an evaluation of both surgical margins and the extent of viable tumor elsewhere in the specimen is essential and may dictate resection of additional breast tissue even when margins are apparently tumor-free. Among women who have mastectomy after neoadjuvant chemotherapy, a pCR is a favorable prognostic finding, associated with a far lower risk of LRR than seen in women with residual cancer.120 Percutaneous placement of marker clips within the primary tumor prior to the initiation of chemotherapy will provide a landmark for localization and excision should a clinical and radiographic complete response occur. The lack of a survival benefit for neoadjuvant therapy and the increased complexity in determining the appropriate extent of resection suggest that for women who are candidates for breast conservation at presentation, neoadjuvant therapy outside the context of a clinical trial offers little benefit.
Neoadjuvant endocrine therapy has also been used to increase rates of BCT. In trials of postmenopausal women with ER+ cancers who were not considered candidates for BCT at presentation, roughly 30% to 40% of those who received 4 months of endocrine therapy were able to undergo BCT.121,122 These studies indicate that in postmenopausal women with hormone receptor-positive tumors, the preoperative use of an AI or tamoxifen significantly increases the likelihood of breast conservation. However, despite the proven survival benefit seen with adjuvant endocrine therapy, pCR is rare with the short duration of treatment used in the neoadjuvant setting. Patients who experience substantial tumor shrinkage with endocrine therapy, even short of pCR, may also have a more favorable prognosis.123 For women with ER+ breast cancer, a small randomized trial has suggested that either endocrine or chemotherapy can be equally effective.124 In clinical practice, neoadjuvant endocrine therapy is typically reserved for women not considered to be candidates for neoadjuvant chemotherapy.
Conservative Surgery Without Radiation Therapy
An unresolved question is whether RT is necessary in all patients with invasive breast cancer after CS. It is well known that RT after CS reduces LR by about 70%, but there has been uncertainty about whether this improvement in LR is important to survival and whether there is a subgroup of patients with a low risk of LR following CS alone. The impact of improving local control on overall long-term survival was greatly clarified with the findings of the EBCTCG meta-analysis125 first published in 2005, and updated in 2011.125 In this updated analysis, 17 trials including 10,801 women, 3,143 deaths, and 9.5 median woman-years at risk were included. Importantly, the EBCTCG moved from assessing the effect of RT on LR to its effect on first failure (or first recurrence, either LR or distant metastasis). (Although commonly employed in studies on the local treatment of breast cancer, actuarial calculation of time to LR is, strictly, not statistically valid.) RT proportionally reduced the annual rate of any failure (LR or distant metastases) over the first 10 years by about half (RR = 0.52) and proportionally reduced the annual rate of breast cancer death by about one-sixth. The absolute benefit of RT was greater in patients with the greater risk of recurrence. In node-negative patients, the absolute benefit was strongly correlated with age (inversely), tumor grade and size, and ER status, with very small absolute benefit seen in some subgroups. The updated EBCTCG analysis still demonstrates that RT after CS is not only important for local control, but also for maximizing long-term survival.
Attempts to identify a subgroup of patients (based on various clinical and histologic features) within the available clinical trials who have a low risk of LR after CS alone have been unsuccessful. LR rates are generally lower in trials that use more extensive surgery than in those using lumpectomy, and in older patients than in younger patients. The Joint Center for Radiation Therapy in Boston conducted a prospective single-arm trial of wide excision alone for patients with a tumor size of ≤2 cm, histologically negative axillary nodes, absence of either lymphatic vessel invasion or an EIC in the cancer, and no cancer cells within 1 cm of inked margins.126 The median age of patients in this trial was 66 years, 75% of cancers were detected by mammography alone, and the median pathologic size of the cancers was 9 mm. None of the patients received adjuvant endocrine therapy or chemotherapy. This trial was stopped shortly before it reached its accrual goal of 90 patients because of stopping rules ensuring against an excessively high LR rate. With a median follow-up time of 86 months among the 81 eligible patients, the crude rate of LR was 23%. The average LR rate was 2.8% per year. Of note, of the six patients with a tubular cancer, three had an LR. Examination of subsets of patients by age and tumor size did not find any statistically significant differences. Similar results were seen in a small randomized clinical trial from Finland. Based on the results of these prospective studies, it was concluded that even highly selected patients with breast cancer (based on patient and tumor characteristics) have a substantial risk of early LR after treatment with wide excision alone. Newer markers are needed to more reliably identify patients who can be safely treated with wide excision alone.
More recently, there have been five trials that have compared tamoxifen with and without RT after breast-conserving surgery (BCS; largely in ER+ patients), and their details are shown in Table 79.11.105,127–130 The LRR rates for these trials are shown in Table 79.12. The 5-year results seem reasonable, but the rate of LR appears increased after 5 years. In the Canadian trial,128 LR was 7.7% at 5 years, but 17.6% at 8 years. This raises the question of whether tamoxifen is merely delaying LR. As previously discussed, the combination of tamoxifen and RT provides a very low rate of LR. Tamoxifen alone has its greatest appeal in older patients (older than 70 years)129 where competing risks of other illnesses are substantial. In a prospective randomized trial of 636 patients age 70 and above with stage 1, ER+ breast cancers treated by lumpectomy and randomized to tamoxifen or tamoxifen plus WBI, no differences in OS or rates of breast preservation were observed at 10 years. WBI reduced the incidence of LRR from 10% to 2% in this population.129 In elderly patients, it is critical for the clinician to assess the patient’s particular cancer characteristics (especially tumor grade) as well as her comorbid illnesses and individual value system in determining the advisability of adding RT. (The website ePrognosis.com is useful is estimating life expectancy in older patients.)
Hypofractionated Whole-Breast and Accelerated Partial-Breast Irradiation
There have been a growing number of studies attempting to decrease the overall treatment time for RT after lumpectomy through the administration of fewer, but larger daily doses (hypofractionation) of RT delivered to the whole breast or only to the portion of the breast containing the primary tumor, typically given twice a day (accelerated).
Hypofractionated WBI was studied131 among women with invasive breast cancer who had undergone BCS and in whom resection margins were clear and axillary lymph nodes were negative. Women were randomly assigned to receive WBI either at a standard dose of 50 Gy in 25 fractions over a period of 35 days (the control group) or at a dose of 42.5 Gy in 16 fractions over a period of 22 days (the hypofractionated-radiation group). The risk of LR at 10 years was 6.7% with standard irradiation as compared to 6.2% in the hypofractionated regimen (95% CI = −2.5 to 3.5). At 10 years, 71.3% of women in the control group compared with 69.8% of the women in the hypofractionated-radiation group had a good or excellent cosmetic outcome. In a subset analysis, conventional fractionation had better results in patients with high-grade cancers. This trial was initiated before the value of a boost was established, and it is not clear how best to give a boost in patients treated with hypofractionated WBI. As noted previously, the value of a boost is very small in patients aged 60 years and greater, so at a minimum, it seems reasonable to treat patients aged 60 and greater with grade 1 or 2 node-negative breast cancer with accelerated WBI without a boost. A task force of the American Society for Radiation Oncology (ASTRO) developed guidelines for the use of hypofractionated WBI in 2011.132 The task force favored a dose schedule of 42.5 Gy in 16 fractions (“Canadian”) and its use in patients aged 50 years or older with pT1-2N0 cancer treated with BCS, and not treated with adjuvant chemotherapy where the dose homogeneity is within ±7% and the heart can be excluded from direct irradiation. There was no agreement on the use of a boost.
Ten-year results are now available from the similar START-B trial fully corroborating the results of the Canadian trial.133 In START-B, a regimen of 50 Gy in 25 fractions over 5 weeks was compared with 40 Gy in 15 fractions over 3 weeks. START-B enrolled 2,215 women. A boost dose was allowed in this trial. Median follow-up was 9.9 years, after which 95 LRRs had occurred. The proportion of patients with LRR at 10 years did not differ significantly between the 40 Gy group (4.3%; 95% CI = 3.2 to 5.9) and the 50 Gy group (5.5%; 95% CI = 4.2 to 7.2; HR = 0.77; 95% CI = 0.51 to 1.16; p = 0.21). In START-B, breast shrinkage, telangiectasia, and breast edema were significantly less-common normal tissue effects in the 40 Gy group than in the 50 Gy group. It is anticipated that the use of hypofractionated WBI will increase.
There are several potential benefits for accelerated partial-breast irradiation (APBI), including the following: (1) the quality of life of patients could be improved by relieving patients of the necessity of daily treatments for 5 to 6 weeks, (2) the underutilization of BCT could be reduced by making it more feasible for patients to receive RT, (3) the integration of local and systemic therapies could be simplified, and (4) long-term complications of RT could be decreased by limiting the volume of critical structures irradiated to high dose. The rationale for APBI is based on studies of the patterns of recurrence after standard whole-breast RT and after excision alone that demonstrate that the large majority of recurrences are in the immediate vicinity of the tumor bed.134 In addition, pathologic studies on the distribution of tumor cells in relation to the primary tumor demonstrate in most cases that the vast majority of tumor cells in the breast are found near the primary tumor.
These are a number of different APBI techniques, including interstitial brachytherapy, (three-dimensional conformal) external-beam irradiation, intracavitary brachytherapy, and intraoperative limited RT. At present, there are few long-term data, especially from randomized clinical trials, for APBI, and appropriate patient selection remains controversial. Successful application of this approach requires technical expertise. Two cooperative group studies—the recently completed NSABP/RTOG phase 3 trial comparing conventional RT versus APBI (allowing implant or external-beam techniques) and the National Cancer Institute of Canada RAPID study, which permitted external beam technique—will answer important questions about APBI. To provide some direction during this time of uncertainty, an ASTRO expert panel has defined a “suitable” group, for whom APBI outside a clinical trial was acceptable, as being patients135 meeting all of the following criteria: age 60 years or greater, BRCA1/2 mutation not present, unicentric invasive ductal carcinoma measuring ≤2 cm, margins negative by at least 2 mm, without lymphatic vessel invasion or an EIC, ERs present, and node-negative on pathologic examination. The panel also identified “cautionary” and “unsuitable” groups.
The most widely used form of APBI is with external beam irradiation. There is controversy whether cosmetic results are compromised relative to conventional whole breast external beam treatment. An interim cosmetic and toxicity analysis from the RAPID trial was published in 2013.136 Between 2006 and 2011, 2,135 women had been randomly assigned to APBI or WBI. Median follow-up was 36 months. Adverse cosmesis at 3 years was increased among those treated with APBI compared with WBI as assessed by trained nurses (29% versus 17%; p <0.001), by patients (26% versus 18%; p = 0.0022), and by physicians reviewing digital photographs (35% versus 17%; p <0.001). Grade 3 toxicities were rare in both treatment arms (1.4% versus 0%), but grade 1 and 2 toxicities were increased among those who received APBI compared with WBI (p <0.001). The authors concluded that external beam APBI increased rates of adverse cosmesis and late radiation toxicity compared with standard WBI.
The first results of a trial from the European Institute of Oncology in Milan testing APBI using intraoperative radiation with electron beam versus conventional whole-breast radiation were published in 2013. A total of 1,305 patients were randomized, and after a median follow-up of 5.8 years, the 5-year rate of LR (IBTR) was 4.4% in the APBI arm and only 0.4% in the conventional whole breast arm (HR = 9.3; 95% CI = 3.3 to 26.3). OS did not differ.137
Toxicities of Breast Radiotherapy
Breast RT is generally very well tolerated with very few long-term toxicities. Evidence has long been accumulating that RT involving the heart can result in premature ischemic heart disease, but interest peaked in 2013 when a case-control study found an increased risk for cardiac-related deaths in patients with breast cancer who received RT.138 This was a population-based case-control study involving 2,168 Scandinavian women treated between 1958 and 2001. It found that rates of major coronary events increased linearly with the mean dose to the heart by 7.4% per Gy (p <0.001), with no apparent threshold. The increase started within the first 5 years after RT and continued into the third decade after RT. The proportional increase in the rate of major coronary events per Gy was similar in women with and without cardiac risk factors at the time of RT, but the absolute increase was greater in patients with cardiac risk factors. The overall average of the mean doses to the whole heart in this study was 4.9 Gy.
It is important to note the major limitations of the study; mainly, it is a case-control study and as such, it does not provide the highest level of evidence. Also, there were limitations in design. The investigators developed virtual simulations of RT dose based on CT scans of patients with “typical anatomy,” which they used to construct idealized radiation fields and with which they estimated the doses to the heart.
It is also important to emphasize that despite the proportional relationship between RT dose to the heart and heart disease, the absolute increase was very small. For 50-year-old women without cardiac risk factors, the lifetime increased risk was 0.5% after 0.5 Gy, 0.2% after 1 Gy, and just 0.5% after 3 Gy delivered as a mean heart dose. Today, for most node-negative women having BCT, the mean heart dose is only about 1 Gy, although higher doses (still only about 2 Gy) are more common for women with left-sided postmastectomy RT.
It should also be noted that any cardiac mortality risk in current practice is small compared to the survival benefit from RT both in the setting of BCT and PMRT. The survival benefit seen for RT in these older trials includes the deleterious effects on the heart seen with doses as high as 10 Gy. This means that using current techniques that spare the heart, RT will provide even greater survival benefit. The issue of cardiac irradiation, however, has more importance in patients with DCIS, where the survival impact of RT is small at best.
Notwithstanding these study limitations and improvements in technique, radiation oncologists should operate on the principle that there is no totally safe radiation dose to the heart, and that the heart dose should be kept as low as possible. A number of maneuvers, such as using cardiac blocks, prone techniques, and deep inspiration breath holds, make radiation delivery much safer in current practice.
Mastectomy
Mastectomy, with or without immediate breast reconstruction, is the surgical approach for the patient with breast cancer who has contraindications to BCT or who prefers treatment with mastectomy. The mastectomy used today is a total or complete mastectomy, with removal of the breast tissue from the clavicle to the rectus abdominous and between the sternal edge and the latissimus dorsi muscles. A total mastectomy also removes the nipple-areolar complex (NAC), the excess skin of the breast, and the fascia of the pectoralis major muscle. When accompanied by an ALND, the procedure is termed a modified radical mastectomy. Mastectomy is an extremely safe operation. The 30-day mortality is approximately 0.25%, and the 30-day incidence of major complications is about 5%, the majority of which are related to wound healing. In contemporary American practice, roughly 30% of women underwent mastectomy for breast cancer owing to either contraindications to BCT or patient choice.90
Advances in plastic surgical technique have made immediate reconstruction an option for most patients who undergo mastectomy. There have been no prospective trials comparing mastectomy alone with mastectomy with immediate reconstruction, but the available retrospective data do not support concerns about the incidence or detection of LR in the reconstructed patient. The majority of postmastectomy recurrences occur in the skin or subcutaneous fat of the chest wall and present as palpable masses in the skin flap, so detection is not affected by the presence of the reconstruction.139 Skin-sparing mastectomy in which skin excision is limited to the NAC and the excisional biopsy scar (if present) is now routinely used to preserve the skin envelope of the breast and facilitate reconstruction. The reported rates of LR after skin-sparing mastectomy are comparable to those of patients treated with conventional mastectomy. This finding is consistent with prior observations that the extent of skin removal in patients treated with mastectomy alone is not a major determinant of the risk of chest wall recurrence. Traditionally, skin-sparing mastectomy has included resection of the NAC due to the need to leave breast tissue on the NAC to provide a blood supply and the risk of leaving behind malignancy within the ducts of the nipple. Nipple sparing mastectomy (NSM) preserves the NAC and is being used with increasing frequency due to the excellent cosmetic results that can be obtained with this technique. Intraoperative frozen section of the tissue beneath the nipple is often used to minimize the risk of residual cancer. To date, most studies of this procedure have consisted of highly selected patients who had relatively short follow-up periods, making the long-term safety of NSM, particularly in high-risk women such as those with BRCA mutations, difficult to ascertain. The reported incidence of occult involvement of the NAC in patients with known breast cancer ranges from 0% to 58%.140 Despite this, a review of 10 studies including 1,148 NSMs reported a 2.8% rate of LRR after a median follow-up of only 2 years.141 In a large series from the European Institute of Oncology, including 772 patients with invasive cancer, and 162 with DCIS treated with NSM and intraoperative RT to the NAC, at a median follow-up of 50 months, the 5-year rate of LR was 4.4% in the invasive cancer group and 7.8% in patients with DCIS.142 The majority of recurrences were at a distance from the NAC, suggesting that the more difficult exposure with this operation may result in retained breast tissue on the skin flaps. These studies indicate that NSM may be a viable option in highly selected women, specifically, patients with small, peripherally located, node-negative tumors with favorable histologic features. Most women in this category, however, are candidates for conventional BCT. The eligible population for NSM is further limited by the requirement that the nipple be in the appropriate position on the reconstructed breast. This is rarely the case for women with large, ptotic breasts, further limiting the application of this procedure.
In summary, immediate reconstruction with preservation of the skin envelope of the breast has not been shown to alter the outcome of mastectomy or to delay the administration of systemic therapy. Immediate reconstruction has the advantages of avoiding the need for a second major operative procedure and the psychological morbidity of the loss of the breast. The two major reconstructive techniques involve the use of implants and/or tissue expanders or the use of myocutaneous tissue flaps to create a new breast mound. The advantages and disadvantages of the techniques are summarized in Table 79.13. Implant reconstructions are best suited for women with small- to moderate-sized breasts with minimal ptosis, while flap reconstructions allow more flexibility in the size and shape of the reconstructed breast (Fig. 79.4). In the past, most breast implants were filled with silicone gel. However, after reports from uncontrolled studies suggested an increased incidence of connective tissue disease in women with silicone implants, the US Food and Drug Administration declared a moratorium on their use. Since that time, several epidemiologic studies have failed to demonstrate an increased incidence of connective tissue disorders in women with implants compared with matched control populations. Silicone implants are again available for use in patients with breast cancer, but many patients opt for saline implants or flap reconstructions as a result of the adverse publicity surrounding silicone implants.
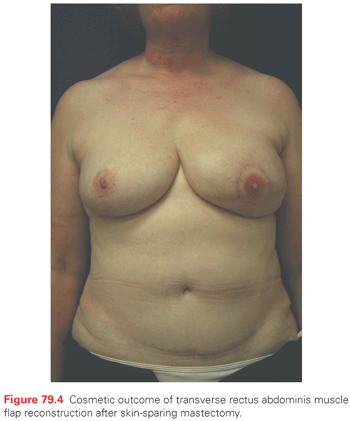
Reconstructive choices may be influenced by the possible need for postmastectomy RT. As indications for postmastectomy RT have expanded, the impact of RT on reconstruction has also become an issue. Immediate reconstruction can negatively impact the technical delivery of RT, possibly resulting in greater irradiation of the heart (in left-sided cancers) and lung, and undercoverage of the chest wall.143 There are a variety of strategies that have been proposed for selecting the type of reconstruction for a patient with a significant likelihood of requiring postmastectomy RT. There is considerable variability in outcome reported in the medical literature for the same approaches, and there are no prospective studies reported to date. The use of RT in patients who have been reconstructed with implants is associated with a higher risk of encapsulation and implant loss than in nonirradiated patients. In one study, however, Cordeiro et al.144 reported that after a mean follow-up of 34 months in 68 patients reconstructed with tissue expanders or implants who received RT, 80% had good to excellent aesthetic results and 72% would have chosen the same form of reconstruction again. The figures for nonirradiated patients were 88% (p = not significant) and 85%, respectively. Implant loss occurred in 11% of patients with irradiated implants and 6% of nonirradiated patients. The finding that the majority of patients who require RT after implant reconstruction have good cosmesis and are satisfied with their reconstruction choice has led some to advocate insertion of an expander at the time of mastectomy, which is inflated during chemotherapy and exchanged for a permanent implant prior to RT. In patients who are satisfied with the cosmetic outcome after RT, no further surgery is required. In patients with significant cosmetic deformity, a secondary flap reconstruction is performed. This approach has the advantage of allowing preservation of the breast skin and providing the patient with a breast mound during what may be a prolonged course of postoperative cancer therapy. However, additional favorable experience with irradiation of expanders or implants at other institutions is needed.
A primary flap reconstruction is another alternative for the patient who may require postmastectomy RT. Variable outcomes have been reported for patients who receive RT after transverse rectus myocutaneous flap or latissimus dorsi flap reconstruction. Complete flap loss is rare, but fat necrosis, fibrosis, and volume loss can occur. As in the native breast, the full cosmetic impact may not be evident until 3 years posttreatment. In one study, the 5-year incidence of major complications after transverse rectus myocutaneous reconstruction was 0% (n = 35) and 5% after tissue expander/implant (n = 50) reconstruction followed by RT.145 In contrast, 4 of 47 (9%) patients reconstructed with flaps and 6 of 15 (40%) implant patients underwent major corrective surgery a median of 8 months after RT in another series.146 An alternative approach is to perform sentinel node biopsy prior to mastectomy to identify patients with nodal involvement at highest risk for requiring postmastectomy RT and delay reconstruction in this subset of women until after the completion of oncologic therapy. This is an area that continues to evolve, and multidisciplinary consultation between the oncologic surgeon, reconstructive surgeon, and radiation oncologist will help to ensure optimal patient outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree