Malignant pleural mesothelioma
Daniel R. Gomez, MD  Anne S. Tsao, MD
Anne S. Tsao, MD  Haining Yang, PhD
Haining Yang, PhD  Harvey I. Pass, MD
Harvey I. Pass, MD
Overview
Malignant pleural mesothelioma is an aggressive malignancy with an incidence that may be peaking worldwide in the next 5–10 years. While the majority of patients do ultimately die of this disease, there have been substantial treatment and diagnostic shifts over the past decade that may improve long-term outcomes. These changes include worldwide interest in defining early detection biomarkers for the disease, a debate as to the optimal surgical approach (extrapleural pneumonectomy vs lung-sparing methods such as pleurectomy/decortication), further refinement of radiation techniques that allow for conformal treatment fields and the delivery of radiation with the involved lung intact, and the emergence of systemic therapies that are targeted in nature and allow for the increased individualization of care. Indeed, prospective clinical trials assessing the safety and efficacy of novel treatment approaches will be essential to the standardization of paradigms that improve the outcomes. It is hoped that the maturation of these efforts will lead to an optimal approach in which earlier-stage patients will benefit from multiple modalities that will provide synergistic control while limiting toxicity, thereby increasing the number of long-term survivors over the next one to two decades.
Incidence and epidemiology
The incidence of mesothelioma has been underestimated in mortality statistics.1 Presently, pleural mesotheliomas are responsible for approximately 15,000–20,000 deaths annually worldwide. Cases are clustered in areas of asbestos product plants and shipbuilding facilities, not only in the United States, but also in other industrialized countries, such as England.2, 3 Approximately, 3000 cases per year are diagnosed in the United States, and based on a latency period of approximately 20–40 years and estimated asbestos exposure rates, it has been projected that the incidence will peak in 2010–2015.4 This timeframe is similar to what has been predicted in other countries, such as the United Kingdom, Australia, and the Netherlands.5, 6 The male to female ratio is approximately 4 : 1, and 80% arise from the pleura.7 In autopsy studies, the frequency of malignant mesothelioma varies from 0.02% to 0.7%, with a rate of 0.2% in the largest series.8 In most hospital series, the pleura is more often involved than the peritoneum, with a predominance of the right side over the left (60 : 40).8 In some epidemiologic studies monitoring cohorts of asbestos workers, however, the peritoneal form is more common than the pleural. The mean age of patients is approximately 60 years, but the disease can occur at any age, including in childhood.9, 10
Etiology
It has been well established that one of the primary causes of mesothelioma is asbestos exposure. It has been estimated that beginning at 15 years after onset of exposure, approximately 6–10% of asbestos workers older than age 35 years will die of mesothelioma.11 Furthermore, from 1940 to 1979, approximately 27.5 million workers were occupationally exposed to asbestos in the United States, with a calculated annual death rate from mesothelioma of approximately 2000 in 1980 up to 3000 in the late 1990s.12 Insulation, construction, shipyard industries, and automobile brakes are among the many sources of occupational exposure.13 Although asbestos exposure and cigarette smoking act synergistically to produce lung cancer, smoking is not an established risk factor for mesothelioma, although an association between cigarettes using “micronite” filters has been postulated.14 Exposure can also occur by household contamination of women and children, usually through the work clothes of an asbestos’ worker. Other less-common causative agents include radiation therapy (RT), as has been observed in young adults who have received RT for Wilms tumor (WT) or for mediastinal lymphoma.15
The role of pleural plaques and their association with mesothelioma has been debated for several years. One recent study addressed this issue through a screening program in France for patients that had previous asbestos exposure. Specifically, 5287 males were followed for 7 years with CT (computed tomography) scans, and both the incidence of mesothelioma and subsequent survival were assessed. Overall, 17 patients were diagnosed with malignant pleural mesothelioma (MPM), and there was a significant association between pleural plaques, with a hazard ratio of 8.9. The authors concluded that the presence of pleural plaques appeared to be an “independent risk factor for mesothelioma.16”
Molecular biology of mesothelioma
Multiple cytogenetic and molecular abnormalities contribute to the development of MPM. Asbestos inhalation leads to deposition of fibers in the lung parenchyma with eventual migration and implantation of fibers in the pleural lining. Repeated episodes of inflammation and healing, oxygen free radical production from inflammatory cells and the iron moiety within asbestos, and direct damage to DNA by the fibers are generally accepted pathogenic features of asbestos exposure.17 Karyotype and comparative genomic hybridization (CGH) analyses of primary MPM tumors and cell lines detected frequent deletions, duplications, and translocations with genomic losses more common than gains.18 Deletions within chromosomes 1p, 3p, 4p, 4q, 6q, 9p, 13q, 14q, and 22q are common and notable for the loss of the tumor-suppressor genes p16/CDKN2A, p53, and NF2 located within these loci.19
HMGB1, tumor necrosis factor alpha (TNF-α) and NF-κ signaling play an important role in the survival of genetically damaged mesothelial cells.20 HMGB1 is a typical DAMP (damage-associated molecular pattern)21, 22 and a key mediator of inflammation.23 Moreover, it is a critical regulator in the initiation of asbestos-mediated inflammation leading to the release of TNF-α and subsequent NF-κB signaling.24 Phagocytic macrophages at sites of inflammation internalize asbestos and release mutagenic reactive oxygen species and numerous cytokines including TNF-α and interleukin-1 (IL)-1, which have been linked to asbestos-related carcinogenesis.20, 25 HMGB1 is also released by reactive macrophages, other inflammatory cells, and human mesothelial cells (HMC) upon exposure to asbestos.24 During programmed necrosis, HMGB1 binds several proinflammatory molecules and triggers the inflammatory responses that distinguish this type of cell death from apoptosis. Secreted HMGB1 stimulates RAGE, TLR2, and TLR4 (the three main HMGB1 receptors) expressed on neighboring inflammatory cells such as macrophages and induces the release of TNF-α and IL-1β. Asbestos-mediated TNF-α signaling then induces the activation of NF-κB-dependent mechanisms, promoting the survival of HMCs after asbestos exposure,24 and thus allowing HMCs with accumulated asbestos-induced genetic damage to survive, divide, and propagate genetic aberrations in premalignant cells that can give rise to a malignant clone. In addition, HMGB1 enhances the activity of NF-κB, which promotes tumor formation, progression, and metastasis.26 HMGB1 has been found to be elevated in the serum of asbestos-exposed individuals and MM (multiple myeloma) patients, and investigations are underway to confirm the molecule as a biomarker for asbestos exposure (Figure 1).
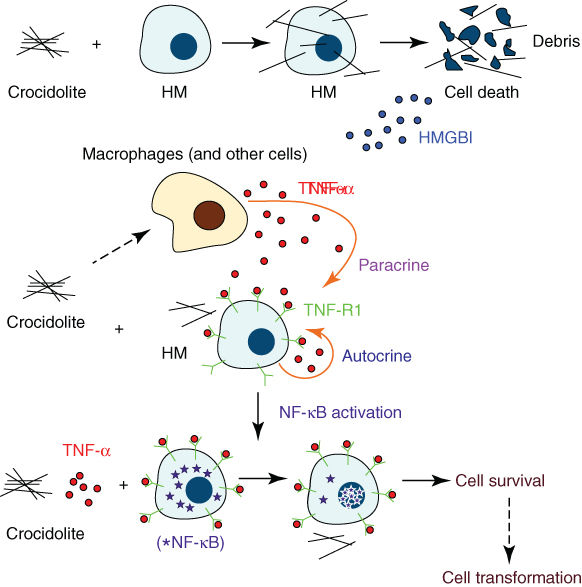
Figure 1 Mineral fiber carcinogenesis. Asbestos and erionite cause necrotic cell death in HM, which leads to the release of HMGB1 into the extracellular space. HMGB1 release causes macrophage accumulation, inflammatory response, and TNF-α secretion. TNF-α activates the NF-κB pathway, which increases HM survival after asbestos/erionite exposure. This allows HM with mineral fiber-induced DNA damage to survive and, if key genetic alterations accumulate, to eventually develop into MM.
Source: Reproduced with permission of Haining Yang PhD, and Michele Carbone MD, PhD.
Genetic predisposition to mesothelioma: the role of BAP1
Families have recently been identified in the United States, which appear to have unusual susceptibility to asbestos carcinogenesis, and these families have mutations of the BAP1 gene in their germline.27 The familial susceptibility is associated with the presence of uveal melanomas and other rare malignancies. BAP1 (BRCA-associated protein 1) has been identified as a novel MPM tumor-suppressor gene. BAP1 is located at the 3p21, a region frequently deleted in MM, and encodes for a deubiquitinase enzyme known to target histones and other proteins. Bott et al.28 originally described the association of somatic mutations of BAP1 in 23% of mesothelioma samples. In a larger series of patients, the same group confirmed that somatic BAP1 mutations occur in about 20% of pleural MM and reported that the only clinical variable significantly different among those with and without BAP1 mutations was smoking (former or current), with BAP1 mutations more prevalent among smokers (75% vs 42%).29 More recent studies have reported BAP1 gene alterations (either deletions or sequence-level mutations) in upward of 61% of MM samples,30 and Arzt et al.,31 with a separate cohort of 52 pleural MM, reported absence of BAP1 IHC staining in 60% of pleural MM, confirming previous results. In that study, there was no correlation between BAP1 expression and asbestos exposure, and these investigators suggested that expression of BAP1 in tumor samples is inversely correlated to survival.
BAP1 appears to exert its antitumor activities mainly in a BRCA-independent manner27 with regulation of the cell epigenome, via modulation of histone H2A ubiquitination and chromatin accessibility32; however, the relevance of BAP1 to the biology of normal and cancer cells remains largely unexplained. In fact, manipulation of BAP1 in cancer cells has often yielded unexpected or even contradictory results. For example, silencing of BAP1 in MM and uveal melanoma cell lines resulted in reduced cell growth.28, 33
Germline BAP1 mutations were first reported by Testa et al.34 in two US families having a high frequency of MPMs. In the same study, 22% sporadic MM tumors harbored somatic BAP1 mutations. These findings have promoted speculation that BAP1 germline mutations can cause a novel cancer syndrome characterized by a significant excess of both pleural and peritoneal MM, uveal and cutaneous melanoma, and possibly other tumors.
Diagnosing and staging the patient with possible mesothelioma
Symptoms and signs
The onset of mesothelioma is associated with chest pain, dyspnea, or cough (Figure 2). Progressive invasion of the chest wall often leads to intractable pain. Pleural effusion is present initially in up to 95% of cases. Often the fluid is viscous owing to high content of hyaluronic acid. Later, tumor growth usually results in complete obliteration of the pleural space and encasement of the lung. Late symptoms of bulky mesotheliomas include mediastinal invasion with dysphagia, phrenic nerve paralysis, pericardial effusion, and superior vena cava syndrome.35 Peritoneal involvement by mesothelioma is characterized by ascites and intestinal compromise leading to cachexia.
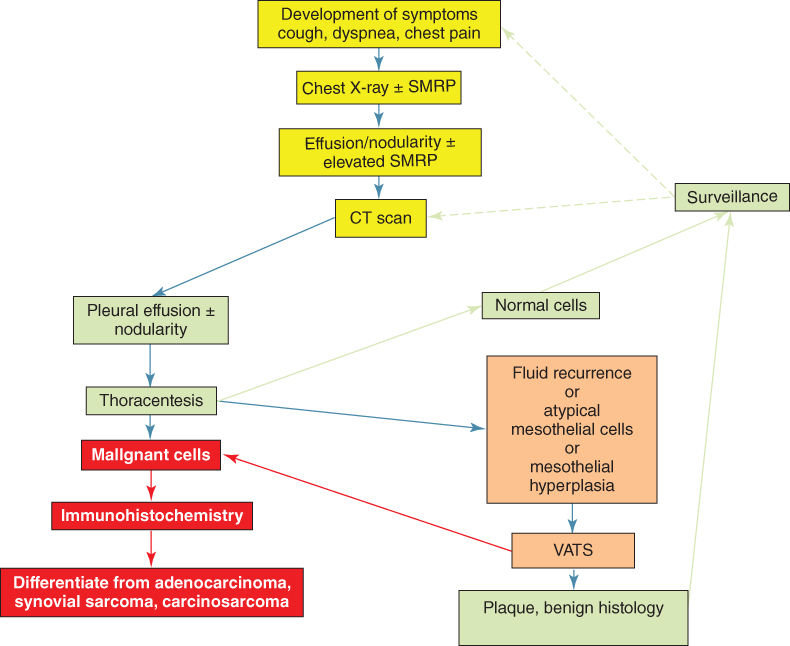
Figure 2 Algorithm for the work up of the asbestos-exposed individual who presents with new symptoms. Any new pleural effusion must have thoracentesis and immunohistochemical analyses. If atypical mesothelial cells are seen, thoracoscopy should be performed for histologic confirmation of malignancy or inflammatory disease.
Laboratory evaluation
There are several laboratory abnormalities associated with mesothelioma. Of these, the presence of thrombocytosis with platelet counts >400,000 is probably the most common. Others include hypergammaglobulinemia, eosinophilia, anemia of chronic disease, elevated homocysteine levels, folic acid deficiency, and Vitamin B12 and Vitamin B6 deficiency.35
Histologic subtypes
There are three primary types of mesothelioma. The epithelial type, the fibrous morphology type, also called the sarcomatoid type, and a combination called biphasic or mixed type. The majority of mesotheliomas (50–70%) are of the epithelial type, 10% are sarcomatoid, and the remaining mesotheliomas are mixed. Immunohistochemical (IHC) markers are necessary to help distinguish between metastatic carcinomas and mesothelioma. These markers include positive pankeratin, keratin 5/6, calretinin, and WT-1. Negative markers include CEA, CD15, Ber-EP4, Moc-31, TTF-1, and B72.3. Such markers are important as mesothelioma may be difficult to distinguish from other malignancies, that is, biphasic mesotheliomas from carcinosarcomas or fibrous mesothelioma from metastatic pleural sarcomas.36
Several studies have examined the role of histologic subtype on prognosis. It has consistently been shown that epithelioid type has a better prognosis than the biphasic or sarcomatoid subtypes.37 In addition to these histologies, several other, lower grade mesothelial tumors exist, such as papillary mesothelioma, which often occurs in the abdominal cavity, multicystic mesothelioma, which often presents in “clusters” and which can also present in the peritoneum, and adenomatoid mesothelioma, which is a benign mesothelial lesion in the genitourinary system. These subtypes typically have a more indolent course than the epithelioid, biphasic, or sarcomatoid histologies, though the clinical course can vary.38
Tumor markers
Two highly studied tumor markers for mesothelioma are osteopontin (OPN) and mesothelin (SMRP). OPN is a glycoprotein biomarker that is expressed by certain cancers, including lung, breast, colorectal, gastric, ovarian, and melanoma. OPN is also involved in cell-signaling pathways that are associated with asbestos-induced carcinogenesis. A recent study compared OPN levels in patients with mesothelioma with OPN levels in patients with asbestos-related diseases that were not malignant. This study revealed that OPN can be used to separate those patients that had asbestos exposure with mesothelioma from those who had asbestos exposure without malignancy.39 A recent meta-analysis examined the utility of ostepontin in the diagnosis of mesothelioma over seven studies, and found a pooled sensitivity of 0.57 (95% CI 0.52–0.61), specificity of 0.81 (95% CI 0.79–0.84), positive likelihood ratio (PLR) of 3.78 (95% CI 2.23–6.41), and a negative likelihood ratio (NLR) of 0.51 (0.38–0.67). These results support the above correlation between OPN and mesothelioma.40 OPN is limited by its lack of specificity and its performance is superior in plasma compared to serum.
SMRP originally described by Pastan,41, 42 is also a glycoprotein and is found in mesothelioma, ovarian cancer, and pancreatic cancer. Robinson et al.43 demonstrated in 48 patients that SMRP is a serum biomarker with 83% sensitivity and 95% specificity and was confirmed to be a promising marker for MPM in blood and pleural fluid.44 Serum SMRP levels were higher in MPM patients when compared to lung cancer patients, as were the SMRP levels in pleural fluid when compared to benign pleural fluid or other non-MPM pleural effusions.44 Indeed, prior studies have suggested that the accuracy of detection is superior in pleural fluid than in serum,45 though serum analysis is a more convenient measure of this factor. One recent study examined several combinations of serum markers as diagnostic tools for mesothelioma, and in particular to determine if other markers could enhance the diagnostic yield of using SMRP alone. The study found that combining SMRP and carcinoembryonic antigen (CEA) could improve the differentiation between mesothelioma and lung cancer, as well as the difference between mesothelioma and patients with asbestos exposure that do not have this disease.46 A large meta-analysis has confirmed the report that the sensitivity and specificity of SMRP are similar in many studies.47
In addition to being used as a diagnostic tool, several studies have focused on these markers in tracking the course of disease. In one prospective study, 41 patients had 165 samples collected and disease status temporally assessed using response evaluation criteria in solid tumors (RECIST) criteria. The authors found that while SMRP was significantly correlated with a response, as measured by absolute change, RECIST criteria, or modified RECIST criteria, no such associations were found with OPN.46 Another prospective study of 97 patients found that changes in soluble SMRP were associated with radiologic response, metabolically active tumor volume, and median survival.48 Further validation of SMRP as a prognostic marker and a measure of tumor response is ongoing.
Several other potential tumor markers have been explored as having utility in this clinical scenario. One high-profile tumor marker that has been explored recently is fibulin-3, an extracellular protein located in the basement membrane of the vasculature. When assessing plasma fibulin-3 levels, the investigators found that this marker could distinguish between healthy patients with exposure to asbestos from those patients with malignancy in a cohort of over 200 patients, and this was confirmed by blinded validation studies.49 Furthermore, when plasma fibulin-3 is analyzed in conjunction with that measured in the pleural effusion, it was found that the marker is effective in differentiating benign from both benign and other types of malignant effusions. The diagnostic ability of fibulin-3 has been confirmed in other studies.50 In one study that compared soluble SMRP and fibulin-3 as a tumor markers, the investigators found that while SMRP was superior in diagnosis, fibulin-3 was better in assessing prognosis, thus implying utility of both markers in a complementary manner.51
Imaging modalities
CT scan is very useful for diagnosis and disease assessment (Figure 3).52 Findings on CT scan include a pleural effusion, pleural nodularity, and concentric pleural thickening. However, investigators from Oxford recently estimated the positive and negative predictive values of approximately 400 patients undergoing a CT scan before thoracoscopy for diagnosis. They found that the positive predictive value of a CT scan that demonstrated “malignant” findings was 80%, while the negative predictive value was 65%. Thus, CT scan alone appears to be insufficient in determining which patients undergo invasive pleural biopsies.53
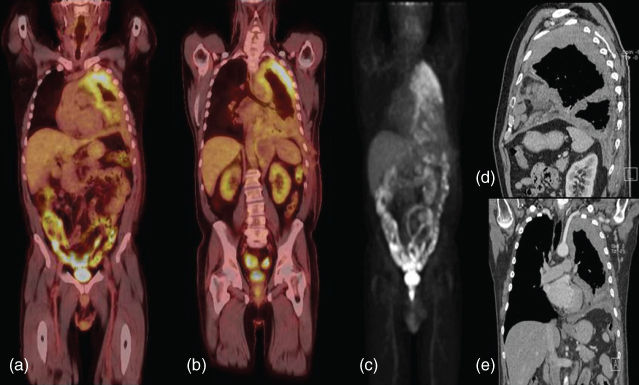
Figure 3 PET-CT and pleural mesothelioma. (a,b) Posterior and anterior views of the left chest reveals a bulky hypermetabolic tumor (c) maximum intensity projection (MIP) view confirming no disease outside the chest. (d) Sagittal view of CT image reveals bulky disease in the fissures. (e) Coronal view reveals abutment of the subclavian artery and possible diaphragmatic involvement.
Additional imaging modalities that can be used to stage mesothelioma include magnetic resonance imaging (MRI) and positron emission tomography (PET) scanning. MRI is not frequently used, but may be of additional benefit to detect diaphragm invasion, involvement of the endothoracic fascia, or an isolated area of chest wall involvement in patients that may be candidates for surgical resection. In addition, the role of PET/CT scanning has not been conclusively elucidated, and PET imaging is not recommended for diagnosis or staging in the 2014 National Comprehensive Cancer Network guidelines. However, many prior and ongoing studies are assessing this technique because of its utility in other thoracic malignancies such as lung cancer. In one study, PET was found to have a sensitivity of only 11% in detecting metastatic lymph-node disease54; however, the same study found that a high standardized uptake values (SUV) correlated with N2 disease at the time of resection. More recently, research from the same institution showed that an SUV value 10 greater correlated with a significantly shorter survival time and a 3.3 times greater risk of dying compared to SUV levels below 10.55 This suggests that PET results may help to stratify patients for different treatments according to their metabolic activity. Integrated CT-PET is more informative than PET alone. In a study of 29 MPM patients, integrated CT-PET correctly assigned the overall stage in 72% of cases, showed increased sensitivity for T4 disease, 67% versus 19% for PET alone, identified 7 patients with extrathoracic disease missed by conventional radiographic studies, and identified 12 patients that would have been precluded from surgical resection based on conventional studies.56 In addition, investigators from Memorial Sloan-Kettering Cancer Center examined the accuracy of PET scan in detecting histologic subtype. In 100 patients with MPM who underwent preoperative PET scans, they found that the mixed subtype of epithelioid mesothelioma had higher SUVs than patients with the nonpleomorphic subtype, thus supporting the conclusion that higher SUVs are correlated with aggressive disease.57 Another recent study examined the prognostic significance of PET scan after neoadjuvant chemotherapy for resectable MPM in 50 patients, all of whom underwent EPP (extrapleural pneumonectomy) and postoperative hemithoracic radiotherapy. Metabolic response after neoadjuvant chemotherapy was significantly correlated with the overall survival, and the authors concluded that response to therapy may be integrated into the decision-making process for surgical resection after neoadjuvant chemotherapy.58
Surgical diagnosis and staging in mesothelioma
The interventions that can be used for diagnostic purposes in mesothelioma include thoracentesis with closed pleural biopsy, VATS (video-assisted thoracoscopy surgery) with pleural biopsy, and open pleural biopsy. It is important to have adequate tissue to classify not only whether the biopsy reveals mesothelioma, but also possibly the subtype of mesothelioma. Although cytologic diagnosis can be difficult, electron microscopy and IHC staining on a cell block of the pleural fluid can be performed to increase diagnostic yield up to 84% in suspected cases. Video-assisted thoracoscopy with direct biopsy and assessment of the involvement of the lung and pleura remains the gold standard for diagnosis. Studies have confirmed the utility of this technique among patients undergoing surgery for mesothelioma, but have also pointed out the difficulty in obtaining correct histologic diagnosis.59 Information about parietal, visceral, and diaphragmatic pleural involvement can be obtained, and this might have prognostic value for survival.60 Open pleural biopsy is rarely performed, but may be required when there is no free pleural space.
Staging and prognostic indicators
The American Joint Committee on Cancer (AJCC) staging system for mesothelioma has been validated in a number of surgically based trials61 and has been used since the 1990s. In order to improve the current staging system, an international database of over 2000 staged patients with MPM was analyzed by the International Association for the Study of Lung Cancer, and covariates predictive of survival included best staging information, age, sex, histology (epithelioid or not), and type of surgical procedure (palliative vs EPP or pleurectomy decortication).62 Supplementary prognostic indicators complementing the international database include platelet count, white blood cell count, and the addition of adjuvant therapy.63 Gill,64 in a study of 88 epithelial MPMs having EPP, has expanded on the influence of tumor volume and survival in MPM stage-independent model of risk for death in patients having surgical cytoreduction as originally described by Pass et al.65 In Gill’s report, tumor volume, hemoglobin concentration, platelet count, pathologic TNM (tumour, node, and metastasis) category, and administration of adjuvant chemotherapy or RT were associated with survival.64 Richards, using the same MPM cohorts as Gill from the Brigham and Women’s Hospital series, has recently described a validated model for survival and time to recurrence in cytoreduced MPM using hemoglobin, CT volume, thickness of the fissures, and histological subtype (Table 1).66
Table 1 The International Mesothelioma Interest Group (IMIG) staging system for pleural mesothelioma
| T1 | |
| T1a | Tumor limited to the ipsilateral parietal ± mediastinal ± diaphragmatic pleura, no involvement of the visceral pleura |
| T1b | Tumor involving the ipsilateral parietal ± mediastinal ± diaphragmatic pleura, tumor also involving the visceral pleura |
| T2 | Tumor involving each of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features:
|
| T3 | Describes locally advanced but potentially resectable tumor |
Tumor involving all of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features:
| |
| T4 | Describes locally advanced technically unresectable tumor |
Tumor involving all the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic, and visceral pleura) with at least one of the following features:
| |
| N-Lymph nodes | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Metastases in the ipsilateral bronchopulmonar or hilar lymph nodes |
| N2 | Metastases in the subcarinal or the ipsilateral mediastinal lymph nodes including the ipsilateral internal mammary nodes |
| N3 | Metastases in the contralateral mediastinal, contralateral internal mammary, ipsilateral, or contralateral supraclavicular lymph nodes |
| M-Metastases | |
| MX | Presence of distant metastases cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
| Stage I | |
| Ia | T1a N0 M0 |
| Ib | T1b N0 M0 |
| Stage II | T2 N0 M0 |
| Stage III | Any T3 M0 |
| Any N1 M0 | |
| Any N2 M0 | |
| Stage IV | Any T4 |
| Any N3 | |
| Any M1 |
Abbreviations: T, primary tumor; T1, limited to ipsilateral pleura only (parietal pleura, visceral pleura); T2, superficial local invasion (diaphragm, endothoracic fascia, ipsilateral lung, and fissures); T3, deep local invasion (chest wall beyond endothoracic fascia); T4, extensive direct invasion (opposite pleura, peritoneum, and retroperitoneum); N, lymph nodes; N0, no positive lymph node; N1, positive ipsilateral hilar nodes; N2, positive mediastinal nodes; N3, positive contralateral hilar nodes; M, metastases; M0, no metastases; M1, metastases; blood-borne or lymphatic.
Source: Adapted from Rusch 1996.61
Treatment
The treatment options for mesothelioma depend on several parameters, including performance status, medical comorbidities, pulmonary function, stage, and age of the patient as well other factors. Surgical options are considered as long as the bulk of the disease can be removed without leaving gross disease behind. If this cannot be accomplished, then supportive measures for palliation can be used (Table 2).
Table 2 Treatment options for mesothelioma
| Supportive care | |
| Effusion control | Talc pleurodesis |
| Vats assisted | |
| Slurry | |
| Pleurex catheter | |
| Repeated thoracenteses | |
| Pain control | Narcotics |
| Permanent epidural catheter | |
| Localized radiation | |
| Surgery | Pleurectomy with decortication |
| Extrapleural pneumonectomy | |
| Chemotherapya | Pemetrexed (Alimta) and cisplatina |
| Gemcitabine and cisplatina | |
| Vinorelbine (Navelbine) | |
| Phase I/II trials with targeted agents | |
| Multimodality therapy | Induction chemotherapy, surgery, and postoperative radiotherapyb |
| Surgery and postoperative radiotherapy | |
| Surgery and postoperative chemotherapy | |
| Surgery and novel cytotoxic/targeted agentsb | |
| Induction Radiation followed by surgeryb | |
| Novel intrapleural therapies | Hyperthermic chemoperfusion,b chemotherapy, povidone-iodineb |
| Photodynamic therapyb | |
| Gene therapyb | |
| Novel radiation techniques at selected centers | Intensity-modulated radiation therapy |
a Most commonly used in recent phase II/III trials.
b Under investigation.
Supportive care
The median survival of MPM patients who select supportive care ranges only from 467 to 13 months,68 and is influenced by multiple factors including the biology of the tumor as well as whether survival time is recorded from the time of symptom initiation or from the time of definitive diagnosis. There are a number of options for the control of pleural effusion, including repeated thoracenteses, talc pleurodesis, or placement of a Pleur X (Denver Biomedical, Denver, CO) catheter.69 Failure of these techniques is usually associated with lung trapped by the tumor, a large solid tumor mass, a long history of effusion with multiple thoracenteses leading to loculations, age older than 70 years, or poor performance status. In such cases, the PleurX catheter with its one-way valve can be implanted under local anesthesia into pleural effusion, and patients can drain themselves at home using the available disposable drainage kits.70 Recently, a randomized trial examined the influence of pleural fluid palliation in mesothelioma. The MesoVats trial was a randomization between 2003 and 2012 of 87 VAT pleurectomy or 88 talc pleurodesis for patients who were not considered for maximal cytoreductive techniques. There was no survival difference, but pleural effusion was better controlled with pleurectomy patients, and a significant benefit in quality of life was recorded in favor of the pleurectomy group.71
Patients with chest-wall pain due to mesothelioma should be seen in consultation by a dedicated pain management team to consider narcotic control as well as novel pain-control techniques including outpatient epidural catheter use. Palliative radiotherapy is associated with a median survival of 4–5 months.72, 73
Surgery for pleural mesothelioma
Maximal cytoreductive procedures
The goal of cytoreductive surgery in MPM should be the removal of all visible or palpable tumor (R0 or R1) or a “macroscopic complete resection” (MCR), regardless of whether that involves EPP or a lung-preserving operation. The Mesothelioma Domain of the International Association for the Study of Lung Cancer has defined uniform nomenclature for pleural mesothelioma resections74 and includes EPP, extended pleurectomy and decortication (ePD) (resection of the diaphragm and/or pericardium), and pleurectomy decortication as potential MCRs. Suspicion and documentation of disease outside the involved hemithorax eliminates the use of an MCR. Patients who present with ECOG performance status 2 or greater, or who have limiting cardiac reserve either with nonreversible myocardial ischemia or compromised ventricular function (ejection fraction <50%) are usually not candidates for cytoreductive procedures. Moreover, patients with compromised pulmonary-function testing who are not felt to be candidates for pneumonectomy must be carefully evaluated for either extended P/D in that the cytoreduction should not compromise the existing lung function but may recruit trapped lung and possibly improve dyspnea.
Extrapleural pneumonectomy (EPP)
As detailed in a recent systematic review of the use of EPP for MPM,75 the median overall survival varies from 9.4 to 27.5 months, and 1-, 2-, and 5-year survival rates ranges from 36% to 83%, 5% to 59%, and 0% to 24%, respectively (Figure 4). Overall perioperative mortality rates ranged from 0% to 11.8%, and centers routinely performing the operation have mortality rates close to 3% through improvements in preoperative staging, anesthesia, resection, reconstruction, and perioperative management of procedure-related complications.76 The chief perioperative morbidities include atrial fibrillation, myocardial infarction, and pulmonary complications.77 EPP alone does not appear to prolong survival. In most EPP series, median survival is <2 years, but 10–20% of patients are 5-year survivors. Patients with sarcomatoid histology and/or lymph-node involvement have an even poorer prognosis and should not be considered as candidates for EPP.78

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








