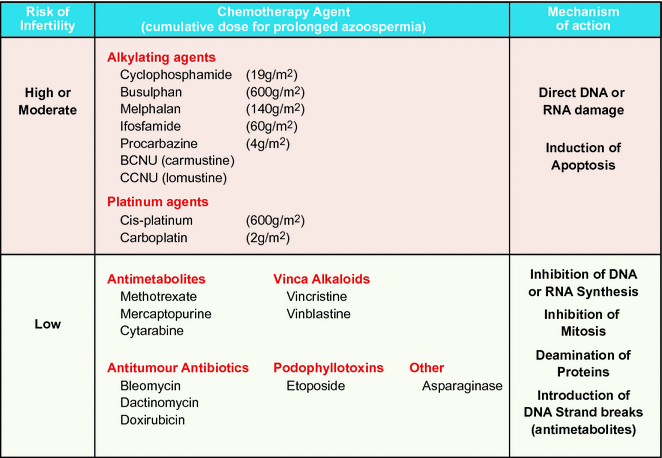
Fig. 12.1
Effect of cytotoxic therapies on the hypothalamic–pituitary–testicular axis in children and adults. Differences in hormonal profile, cellular composition and cellular maturity can result in differential effects of cancer treatment in children compared with adults. SSC—spermatogonial stem cell. * Insufficient evidence to determine effects of cancer treatment
Cancer Treatment and Hypothalamic-Pituitary Function
Following cranial irradiation, gonadotropin deficiency is the second most common pituitary hormone abnormality after growth hormone deficiency. Although in the short term, doses of 30–50 Gy may result in precocious puberty (more frequently in young girls than boys), and doses of at least 30 Gy may result in true gonadotropin deficiency in the longer term [35]. In a study of 45 children treated with cranial radiation, severe gonadotropin deficiency was observed in 11% of cases, resulting in lack of, or slow progression of puberty, with a reduced gonadotropin response to GnRH [36]. Studies in post-pubertal patients have demonstrated that in some the serum levels of testosterone are reduced, and LH and FSH levels may be low. However, demonstration of a preserved pituitary response to GnRH [37] and growth hormone-releasing hormone [38] following cranial irradiation suggests initial hypothalamic hypogonadism, with pituitary insufficiency occurring later.
Cancer Treatment and Spermatogenesis
Chemotherapy-Induced Damage to the Germinal Epithelium
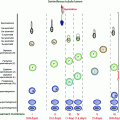
Testicular cells, especially rapidly dividing germ cells, are highly sensitive to cytotoxic treatment (Fig. 12.2). Low doses of these treatments deplete the pool of differentiating spermatogonia, while reserve spermatogonial stem cells (SSCs) survive, and spermatocytes and spermatids continue their maturation into sperm [39] (Fig. 12.2). Testicular involution after such gonadotoxic damage is a slow process that among sexually mature men occurs over several weeks until temporary or permanent azoospermia ensues.
Recovery of sperm production after a cytotoxic insult in puberty or adulthood depends on the ability of mitotically quiescent SSCs to survive and resume mitotic activity and to produce differentiating spermatogonia. If the damage is severe (e.g. as a result of a high cumulative dose of alkylating agents), all SSCs may commit to apoptosis and the patient becomes permanently infertile. Spermatogonia have been shown to be similarly susceptible to such depletion at all stages of life.
Alkylating agents, including cyclophosphamide and the combination of mechlorethamine and procarbazine, have been associated with increased risk of impaired spermatogenesis [48, 49]. Agents with the potential to cause impaired spermatogenesis include also ifosfamide at doses >60 g/m2 [43, 50–52]. Higher doses have greater risks compared to lower doses [48, 49, 53]. The threshold dose of cyclophosphamide, in relation to infertility, has been shown to be between 7.5 and 10 g/m2 [10, 54, 55]. Recent observations suggest that slow recovery of spermatogenesis may occur even after these high doses, and permanent sterility may appear after 19–20 g/m2 of cyclophosphamide [40, 41]. Similar threshold doses for incipient testicular failure have also been reported for other alkylating agents [41, 45, 55, 56], carboplatin and cisplatin [5, 44]. However, a recent large study of non-irradiated childhood cancer survivors failed to identify any threshold dose for alkylating agent exposure that predicted impaired spermatogenesis or azoospermia after a median follow-up of 21 years [53]. There may be other factors, in addition to absolute doses and regimen, such as genetic variation in drug metabolising pathways, which modulate the impact of alkylating agent exposure on spermatogenesis or its recovery [53].
Spermatogenetic Failure After Treatment of Haematological Malignancy
At diagnosis, between 40 and 70% of children with acute lymphoblastic leukaemia (ALL) and 50% of those with acute myeloid leukaemia (AML) are considered to be standard risk, and as a result will receive treatment that combines chemotherapy agents from a number of different classes [57]. These regimens typically involve the use of antimetabolite or vinca alkaloid chemotherapy which inhibits DNA and RNA synthesis and mitosis. Evaluation of testicular biopsies of ALL patients immediately following termination of therapy has revealed that 40% exhibit severe impairment of the tubular fertility index (with less than 40% of their seminiferous tubules containing spermatogonia; [58]). This index tends to improve for most patients as more time elapses after treatment [58, 59]. Long-term follow-up of childhood leukaemia survivors revealed that treatment with these cell cycle-specific cytotoxic drugs, without a high dose of alkylating agent cyclophosphamide, does not totally deplete SSCs, and that spermatogenesis is reinitiated from the surviving reserve stem cell population [40, 60].
Gonadotoxicity following exposure to the ABVD (doxorubicin, vinblastine, dacarbazine and bleomycin) protocol, without a high dose of alkylating agent for HD, is relatively mild with 90% of adult patients having normal gonadotropin levels and sperm counts 12 months after therapy [19, 61]. However, more than 3 courses of alkylating agent (cyclophosphamide, nitrogen mustard, or procarbazine)-based chemotherapy for HD is known to cause azoospermia or result in inhibin B and FSH levels consistent with oligozoospermia in 70–90% of patients [21, 49, 61–63]. Standard-risk non-Hodgkin lymphoma (NHL) patients who are treated with chemotherapy including antimetabolites, vinca alkaloids and low-dose alkylating agents have a low risk of developing testicular insufficiency similar to that of ALL patients.
Patients initially classified as standard-risk leukaemia or NHL may be re-assigned to a more gonadotoxic treatment regimen in the case of treatment failure/relapse. 30–40% of patients with acute leukaemia carry high-risk features. To be cured, these patients require treatment with allogeneic HSCT. Conditioning for HSCT is associated with a significant risk of germ cell failure with azoospermia in 85% of men and oligozoospermia in the remainder [64–66]. Germ cell failure with raised serum levels of FSH and decreased testicular growth in puberty is observed among most of the male patients irrespective of the type of conditioning therapy [65, 67]. The probability for recovery of spermatogenesis after HSCT is associated with the type of conditioning therapy, age of the patient, time interval since transplantation and absence of chronic graft versus host disease [65, 66]. One-third of adult HSCT patients receiving high-dose cyclophosphamide treatment had sperm in the ejaculate after a recovery period of 1 year, while those patients receiving cyclophosphamide combined with busulfan or thiotepa showed first recovery after 3 years. Following total body irradiation (TBI), recovery of spermatogenesis never occurred before the 4th year after transplantation [65]. Similar findings have recently been reported in the paediatric population. HSCT conditioning with busulfan or cyclophosphamide was associated with a larger adult testicular volume, lower serum levels of FSH and the more frequent presence of spermatozoa compared to those conditioned with TBI [68].
Spermatogenetic Failure After Testicular Cancer
The other group of patients in whom the effects of chemotherapy on testicular function have been widely investigated is those with testicular cancer [44, 69–71]. To attempt to delineate which abnormalities are a result of cytotoxic chemotherapy, several of these studies also examined pre-treatment testicular function, or compared chemotherapy-treated patients with those who underwent orchidectomy alone [69–71]. All have demonstrated greater testicular dysfunction in the cytotoxic-treated groups, with evidence of germinal epithelial damage indicated by raised FSH levels and/or reduced sperm counts. Lampe and colleagues [44] analysed data from 170 patients with testicular germ cell cancers who underwent treatment with either cisplatin- or carboplatin-based chemotherapy. 40 (24%) were azoospermic pre-treatment, and a further 41 (24%) were oligozoospermic. For a median of 30 months after the completion of chemotherapy, only 64% of those who were normospermic before therapy remained normospermic, whilst 54 (32%) of the total cohort were azoospermic and 43 (25%) were oligozoospermic. The probability of recovery to a normal sperm count was found to be higher for men with a normal pre-treatment sperm count, those who received carboplatin- rather than cisplatin-based therapy, and those treated with less than five cycles of chemotherapy. Brydøy and colleagues [72] reported that the paternity rate, by natural conception or using assisted reproductive techniques (ART), when sperm reappeared in their semen, was approximately 10% at 5 years in the patient group having received high-dose cisplatin (total cisplatin dose >850 mg), and about 35% in the group with low-dose cisplatin (total cisplatin dose ≤850 mg). However, even in the high-dose cisplatin group, the paternity rate increased to 85% after 15 years post-treatment. These data showed that the time necessary for the restoration of spermatogenesis in order to father children was as long as 5–10 years or more.
Irradiation-Induced Damage to Germinal Epithelium
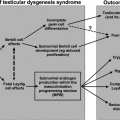
The germinal epithelium is very susceptible to irradiation-induced damage (Fig. 12.3). This depends on the field of treatment, total dose and fractionation [73, 74]. The progenitor and differentiating spermatogonia are radiosensitive to scattered doses as low as 0.1 Gy leading to short-term cessation of spermatogenesis [9]. Doses of 2–3 Gy also affect SSCs and cause long-term azoospermia [9, 75–77]. Doses in excess of 6 Gy deplete the SSC pool and lead to permanent infertility [9, 78].
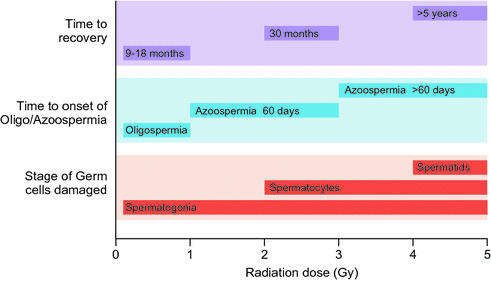

Fig. 12.3
Dose-dependent effects on spermatogenesis following single-dose irradiation in adults
TBI, as conditioning for haematological stem cell transplantation, is associated with significant germ cell failure [64]. Following treatment with TBI (10 or 13 Gy), azoospermia was found in 85% of men, and oligozoospermia occurred in the remainder [65]. Following TBI, recovery of spermatogenesis never occurred before the 4th year after transplantation; therefore, azoospermia after hematopoietic stem cell transplantation may be overestimated if semen samples are evaluated too soon after transplantation [65]. Fractionation of radiotherapy has been shown to increase germ cell toxicity possibly because of repeated hits to activated reserve stem cells [78–80]. However, a long-term follow-up study of 106 male survivors after paediatric allogeneic HSCT does not support this notion [68]. The mean adult testicular volume of 6 mL after single-fraction TBI was significantly smaller than the 11 mL after fractionated TBI. Lower serum levels of FSH after fractionated TBI further suggest that fractionation may slightly decrease the detrimental effect of irradiation on the testis [68].
Whilst most of the literature concerning radiotherapy effects on testicular function concerns the use of traditional radiotherapy techniques, recent technological advances have provided new methods for delivering radiotherapy which result in more localised treatment with less scatter dose [81]. These improvements will likely reduce damage to the testis for many treatments targeting radiation to the pelvic region without delivering radiotherapy to the gonad.
Cancer Treatment and Leydig Cell Function
While effects on the seminiferous epithelium are manifest by impacts on spermatogenesis and fertility, Leydig cell dysfunction may manifest as raised LH levels with normal or low–normal testosterone levels. Such Leydig cell effects are usually associated with much higher doses of radiotherapy/chemotherapy than those which damage seminiferous epithelium.
Chemotherapy and Leydig Cell Function
Evidence for Leydig cell dysfunction following chemotherapy has been described in several studies [4, 42, 44, 82, 83]. In a study of 209 men with a variety of haematological malignancies (including 160 with HD) treated with either MVPP or ChlVPP/EVA hybrid, LH levels were higher in patients compared with a cohort of age-matched controls (mean LH 7.9 versus 4.1 IU/l) [4]. Increased LH levels suggest pituitary compensation to a reduction in testosterone production causing reduced hypothalamic-pituitary negative feedback. Testosterone may still remain within the reference range as mild Leydig cell dysfunction may be reflected by raised LH together with low–normal to normal testosterone levels. This combination was found in 67 (32%) following chemotherapy, compared with 2 (4%) of healthy controls. A further 12 (6%) of the chemotherapy-treated patients had a raised LH level alone [4]. These results suggest that a significant proportion of men treated with cytotoxic chemotherapy have biochemical abnormalities indicative of compensated Leydig cell dysfunction, although recent evidence suggests that there is probably no increased risk for frankly subnormal levels of testosterone after treatment with alkylating agents, [40, 43, 50, 52, 67] or cisplatin [47].
Testicular function following high-dose chemotherapy used as preparation for bone marrow transplantation has also been studied. In a study of 155 men treated with cyclophosphamide (200 mg/kg) or busulphan and cyclophosphamide (busulphan 16 mg/kg, cyclophosphamide 200 mg/kg) [42], 67 of 109 who received cyclophosphamide (61%), but only 8 of 46 (17%) patients treated with busulphan and cyclophosphamide, had recovery of testicular function defined by normal LH, FSH and testosterone levels with evidence of sperm production, after an average of 2–3 years post-transplantation. The only prospective study to examine testicular function following high-dose treatment reported data in 13 men who received either BEAM (BCNU, etoposide, Ara-C and melphalan) (n = 11) or melphalan and single-fraction TBI (n = 2) [82]. All had previously received multi-agent chemotherapy and four had abnormal semen parameters before transplantation. All patients were azoospermic 2–3 months post-transplantation with raised FSH levels. LH levels increased and testosterone levels decreased after transplantation indicating Leydig cell dysfunction in addition to germ cell failure. The association between Leydig cell dysfunction and germinal epithelial function was clearly illustrated in a study of 68 patients treated with high-dose chemotherapy (either cyclophosphamide, BCNU and etoposide, busulphan and cyclophosphamide or BCNU, etoposide, doxorubicin and melphalan) as conditioning for bone marrow transplantation. They demonstrated that the majority of those with raised FSH (88% of the patients) also had raised LH level (69% of the patients). In addition, none of the 13 patients with a normal FSH level had an increased LH level. Although this is not surprising given the relative susceptibilities of the Leydig cells and the germinal epithelium to damage, it leaves open the possibility that germinal cell dysfunction plays a role in Leydig cell insufficiency [4]. There was also evidence of recovery of Leydig cell function with a gradual recovery of germinal cell function during the first 10 years after chemotherapy which further indicates that germinal epithelial dysfunction may be etiologically important in the occurrence of Leydig cell insufficiency [4].
In men with testicular cancer, there are several important considerations regarding the effect of treatment on gonadal function. These men are generally young adults in the age range where preservations of reproductive function and fertility are particularly important. In addition to the potential effects of cytotoxic treatment, these men are likely to have significant pre-existing testicular abnormalities (e.g. cryptorchidism, germinal aplasia, contralateral malignant/pre-malignant cells) that may be compounded by treatment. Separating the testicular effects of cancer treatment from pre-existing testicular dysfunction is challenging [44, 69–71, 83]. In an attempt to determine which abnormalities result from cytotoxic chemotherapy, several of these studies also examined pre-treatment testicular function, or compared chemotherapy-treated patients with those who underwent orchidectomy alone. In a study of 170 patients with testicular germ cell cancers treated with cisplatin- or carboplatin-based chemotherapy, compensated Leydig cell dysfunction, as indicated by a raised LH level in the presence of a normal testosterone level, was found in 59–75% of men following chemotherapy, compared with 6–45% in those following unilateral orchidectomy alone [44]. An age-adjusted increase in hypogonadism, defined as LH >12 IU/L or testosterone <8 nmol/L, has also been described in a cohort of 1183 patients with testicular cancer with odds ratios ranging from 3.5 to 7.9 depending on treatment regimen [83].
Radiotherapy and Leydig Cell Function
The testis is one of the most radiosensitive tissues, with very low doses of radiation causing significant impairment of function. Damage may be caused during direct irradiation of the testis or, more commonly, from scattered radiation during treatment directed at adjacent tissues. As previously described, radiotherapy effects on gonadal function depend on the dose, treatment field and fractionation schedule [84]. While relatively low doses may result in damage to the seminiferous epithelium and oligozoospermia [85], much higher doses (>20 Gy) appear to be required to cause Leydig cell dysfunction. However, significant rises in LH have been demonstrated following single-radiation doses above 0.75 Gy [9] and fractionated doses above 2 Gy [77]. No change in testosterone levels was seen at these doses, indicating compensated Leydig cell damage and LH values gradually return to normal levels over 30 months.
Radiotherapy doses delivered directly to the gonad are often in the range of 20–24 Gy which result in eradication of germ cells [86] causing permanent azoospermia [87]. Such radiation doses delivered to the testis have also been shown to result in Leydig cell insufficiency [88], whilst studies involving lower radiation doses have not resulted in testosterone deficiency [9, 75]. In a study of 20 men, previously treated with unilateral orchidectomy for testicular cancer, who received direct testicular irradiation at a dose of 20 Gy in 10 fractions for pre-malignant changes in the remaining testis, there was a significant increase in mean LH levels within the first three months (10.4–15.6 IU/L), with a decrease in mean serum testosterone levels (13.3–10.8 nmol/L) [89]. Similar results were observed in adults treated with high-dose (30 Gy) testicular irradiation following unilateral orchidectomy. Serum testosterone levels were significantly reduced (12.5 versus 16.0 nmol/L), and LH levels were significantly increased (6 versus 16 IU/L) compared with a control group who had undergone unilateral orchidectomy without subsequent radiotherapy [11]. This study also highlighted the potential importance of age in determining the extent of Leydig cell dysfunction. In the five adult men treated with the same testicular dose of irradiation during childhood, the effect on Leydig cell function was more dramatic. The median LH levels was >32 IU/L and the median testosterone level was <2.5 nmol/L, and there was no response to hCG stimulation, suggesting that the prepubertal testis is much more vulnerable to radiation-induced Leydig cell damage [11]. Several other studies have demonstrated Leydig cell dysfunction for male survivors of childhood cancer receiving radiotherapy, including direct radiotherapy to the testis and total body irradiation [40, 67, 68, 90, 91]. Differences in Leydig cell sensitivity between those treated in childhood or in adulthood may be due to important differences in the Leydig cell populations between children and adults. The adult Leydig cell population is not present in prepuberty; however, studies in rats indicate that the stem cell progenitors that give rise to the adult Leydig cell population are present in the prepubertal testis [92]. Sensitivity of these two populations to cytotoxic therapy may decrease from prepuberty to adulthood.
A study using radiotherapy to impair Leydig cell function in 7 men with prostate cancer demonstrated that even with relatively high doses of radiotherapy (17–24 Gy in 2–3 fractions), Leydig cell function is maintained over 3 years of follow-up [93]. However, the results of these studies should be considered in the context of the patient population, namely aged men, with pre-existing pathology/treatment and relatively low pre-radiotherapy testosterone levels.
Clinical Impact of Impaired Leydig Cell Function
While a substantial proportion of men treated for cancer have evidence of mildly impaired Leydig cell function, as indicated by raised LH and low/subnormal testosterone levels, the clinical impact of this effect is not entirely clear. For men with true hypogonadism, there is a clear benefit of androgen replacement on sexual function, bone density, body composition and quality of life [94, 95]; however, there are limited data in patients with cancer. In a cohort of 203 men treated with either MVPP, ChlVPP/EVA hybrid or high-dose chemotherapy for a variety of malignancies, 32% were identified with biochemical evidence of mild Leydig cell insufficiency as defined by a raised LH and a testosterone level in the lower half of the normal range, or frankly subnormal [4]. They had a significantly reduced bone mineral density at the hip compared with a similarly treated cohort with normal hormone levels, with some evidence of altered body composition, reduced sexual activity and alterations in mood [96, 97]. The men were then enrolled into a 12-month randomised, single-blind placebo-controlled trial of testosterone replacement [98]. During the 12-month study period, however, there was no significant improvement in bone density, body composition, sexual function, energy levels or mood in the testosterone-treated group compared with the controls.
Thus, based on the evidence available, the mild biochemical abnormalities (raised LH, low/normal testosterone) observed in many men following cytotoxic chemotherapy are of limited clinical importance in the vast majority of patients, and androgen replacement cannot be routinely recommended for such patients. However, it remains possible that a minority of men with more marked biochemical abnormalities may benefit from androgen therapy. More studies are needed.
Genetic Damage Following Cytotoxic Therapy
In addition to impairment of steroidogenesis and sperm production, there has been concern that cytotoxic chemotherapy may also result in transmissible genetic damage. Animal studies have demonstrated untoward effects in the offspring of animals treated with cytotoxic agents, but no clear evidence for this has been reported in humans. Increased aneuploidy frequency has been observed in human sperm following chemotherapy for HD [99, 100] and an increase in chromosomal abnormalities has been demonstrated several years after treatment for testicular cancer [101], while a study of men who had been treated for cancer in childhood showed that their sperm carried as much healthy DNA as controls [102]. A study of 25 childhood cancer survivors, their healthy partners and 43 offspring also revealed no increased genomic instability in the latter [103]. The majority of epidemiological studies concerning the outcome of pregnancies have not shown any increase in genetically mediated birth defects, altered sex ratios or birthweight effects in the offspring of cancer survivors [104–106]; however, one study involving 8162 children with a paternal history of cancer from a cohort of almost 2 million children, demonstrated a modest increase (Relative Risk −1.17) in the risk of congenital abnormalities [107]. Given the evidence thus far, it is reasonable to conclude that patients treated with cytotoxic therapy who remain fertile are at no/low increased risk of fathering children with genetic abnormalities.
Fertility Preservation for Males Undergoing Cancer Treatment
Semen Cryopreservation and Assisted Reproduction
At present the only established method for preserving reproductive potential in patients undergoing potentially sterilising therapy is cryopreservation of semen for use in ART such as intracytoplasmic sperm injection (ICSI). This should be offered to all men in whom their treatment has a risk of subsequent infertility, and the British Fertility Society recommends that all post-pubertal patients requiring treatment for cancer should be offered sperm banking. A semen sample is most frequently obtained by masturbation, although penile vibratory stimulation or electrostimulation can also be performed under anaesthetic; however, the motility and sperm count may be lower when the latter method is used [108]. Epididymal aspiration and testicular biopsy have also been used to obtain gametes for cryostorage; however, the risk of compromising testicular function questions the suitability of the latter technique. In the UK, semen cryopreservation is governed by the Human Fertilisation and Embryology Act. This legislation stipulates that the individual must understand the implications of what is proposed and that written, informed consent must be obtained prior to semen cryopreservation [109].
Despite recommendations, the option of sperm banking is not always offered. This may be due to issues such as cost, lack of facilities and a poor prognosis from the underlying condition [110]. Typical rates for storage range between 40 and 70% [111], with lack of information often cited as the most common reason for failing to bank [112]. In a study of 902 men with HD, 40% cryopreserved semen before the start of treatment at the median age of 31 years. Three hundred thirty four men wanted to have a child during the median follow-up of 13 years (range 5–36). Ninety nine of 128 (77%) men with no spontaneous success had cryopreserved semen. Altogether, 48 of 78 (62%) men who used the cryopreserved sperm had success with ART and had at least one child. Patients treated with alkylating- or second-line chemotherapy were more likely to subsequently use cryopreserved sperm. The availability of cryopreserved semen doubled the odds of post-treatment fatherhood [113].
Despite the success of semen cryopreservation as an option to males undergoing potentially gonadotoxic therapy, there are important limitations to its utility for certain patient groups. Men with malignancies may have impaired testicular function prior to treatment [114]. There may be difficulty producing a semen sample, and even when a sample can be produced, there may be oligozoospermia and/or reduced sperm motility. Oligozoospermia is found in a third to a half of patients with HD, non-Hodgkin’s lymphoma and testicular cancer pre-treatment, and also in men with leukaemia or soft tissue cancers [17, 115]. Whilst successful fertilisation may be achieved with only a few viable sperm using ICSI, pregnancy rates using this method are lower with abnormal than with normal semen [116]. Another important patient group is children, who despite the relative quiescence of the hypothalamic–pituitary–gonadal axis, are still at significant risk of infertility as a result of cytotoxic therapies [3]. Therefore, alternative strategies to preserve or restore fertility in young patients are currently under investigation, although they remain experimental.
Hormonal Therapy for Fertility Preservation
Hormonal suppression of testicular activity has been postulated as a potential strategy for the preservation of fertility in patients treated for cancer (for detailed review see [3]). This is based on the hypothesis that suppression of the HPG axis prior to cytotoxic therapy would protect the gonad from cytotoxic damage, a concept based largely on a belief that the prepubertal testis is somewhat protected from permanent chemotherapy-induced gonadal damage [10]. In fact, the prepubertal testis is susceptible to damage from cytotoxic therapy and germ cell proliferation has been shown to occur in the prepubertal human and non-human primate testis [117, 118]. On the other hand, numerous studies in rodents (reviewed in [119]) have demonstrated protection of spermatogenesis when hormonal suppression is commenced using agents such as sex steroids, GnRH antagonists or analogues before or during treatment. It has even been shown that recovery of spermatogenesis can be enhanced when hormonal suppression is induced after treatment. However, differences in hormonal and spermatogenetic regulation in rodents and primates may account for the failure to translate this strategy successfully into primates, including humans (see below).
Treatment of adult rats for 6 weeks with testosterone and estradiol, prior to irradiation or procarbazine treatment, resulted in a higher germ cell repopulation index and sperm head count compared to rats receiving irradiation alone [120–122]. Short-term (2 weeks) treatment of rats with a GnRH analogue prior to procarbazine treatment also resulted in a strain-dependant increase in stem cell index and subsequent recovery of sperm counts close to normal values and significantly higher than procarbazine-only treated rats at 90 days [123, 124].
GnRH analogues have also been shown to stimulate recovery of spermatogenesis following irradiation or procarbazine treatment in adult rats [125–128]. The use of a depot formulation of the GnRH analogue, goserelin, in irradiated rats receiving 3.5 Gy, resulted in a 91% repopulation index compared to 31% in the controls at 10-week post-irradiation [126]. Similar results were obtained for treatment with GnRH analogues following procarbazine treatment [127]. GnRH analogues administered to rats up to 15–20 weeks after irradiation can also result in increased numbers of tubules containing differentiating germ cells derived from surviving stem cells 6 weeks later [127, 128]. These results, along with studies demonstrating no cytoprotective effect from chemotherapy or irradiation in a mouse strain with congenital complete gonadotropin deficiency [129], suggest that the mechanism for restoring spermatogenesis is not due to suppressing germ cell activity during cytotoxic treatment. Instead, studies have suggested that the mechanism may involve a reduction in the high levels of intra-testicular testosterone caused by cytotoxic treatment [130] or release of germ cells from the block on differentiation [131]. Transplantation studies have indicated that the block in spermatogonia differentiation following irradiation may be due to damage to somatic cells rather than germ cells [132].
Attempts to reproduce the beneficial effects of GnRH analogues/antagonists in rodents on radiation- induced cytotoxicity in non-human primates have been unsuccessful. For example, the GnRH antagonist cetrorelix failed to increase the proportion of seminiferous tubule cross sections containing germ cells in rhesus monkeys subjected to testicular irradiation [133, 134].
Studies using hormonal manipulations in humans have also failed to demonstrate preservation or restoration of fertility in patients treated for cancer [119]. These studies included the use of GnRH analogues [135, 136], GnRH analogue plus testosterone [137], GnRH analogue plus an antiandrogen [138], testosterone [139] or medroxyprogesterone acetate given before or after treatment [140] in men receiving chemotherapy and/or radiotherapy for HD and testicular cancer. One further study in adult patients treated with cyclophosphamide for nephritis demonstrated that testosterone therapy was associated with recovery by six months in 5/5 patients compared with 1/5 who did not receive testosterone [141]. Overall, no consistent effect of treatment was demonstrated in these studies. However, it must be recognised that in one study there was no control group, and the majority of studies involved 20 patients or less, and are likely to be underpowered to demonstrate any significant effect. Several studies demonstrated that the majority of patients in the control group recovered spermatogenesis, which also reduces the chances of demonstrating a protective effect of hormonal manipulation, whilst the study in which no recovery was seen in either group may indicate that the cytotoxic treatment may result in a complete loss of spermatogonia.
Many of the human studies were carried out before it became clear that hormonal treatments in the rodent studies were demonstrating restoration rather than protection of spermatogenesis, and none of the studies involved the continuation of gonadal-suppressive therapy for a significant period of time after the completion of chemotherapy or radiotherapy. Thus, carefully designed, adequately powered studies are warranted to determine whether suppression of gonadal function with a GnRH agonist or testosterone for a fixed time during and after the completion of irradiation or chemotherapy may prove successful in reducing the impact of these treatments on fertility.
Ex Vivo Techniques for Fertility Preservation
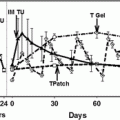
As discussed above, cryopreservation of semen is considered routine clinical practice in many countries. Pubertal boys and men who are able to produce a semen sample should always be offered the possibility to cryopreserve their sperm if they are at risk of infertility due to a gonadotoxic treatment. Unfortunately, prepubertal boys, who are not able to produce sperm at the time their gonadotoxic treatments start, do not have this possibility [142]. In some adults, there may also be other factors, such as ethical or religious beliefs, that exclude cryopreservation of semen as an option for fertility preservation. For these boys and men, testicular biopsy, cryopreservation of the testicular tissue and ex vivo differentiation of male germ cells might be an option, although this approach remains experimental, and currently there are no functional techniques available to generate mature human gametes ex vivo (Fig. 12.4).
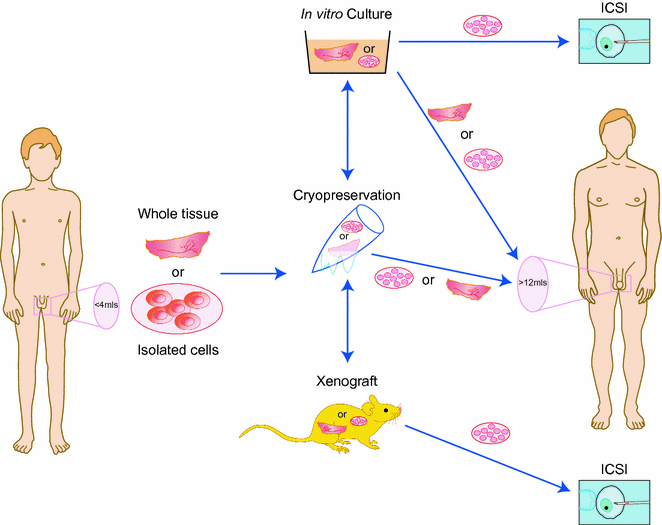

Fig. 12.4
Experimental approaches for fertility preservation in young men prior to cytotoxic therapy. Testicular tissue is removed from the testis prior to cancer treatment. Tissue or isolated cells may be cryopreserved, cultured or xenografted. The material may be replaced in the host testis following the completion of treatment or alternatively differentiated germ cells may be recovered and used for assisted reproduction, e.g. intracytoplasmic sperm injection (ICSI)
Although our understanding of human spermatogenesis has increased dramatically in recent decades, details of the highly complex process of controlling human spermatogenesis are still inadequately understood (see Chap. 3). The limited access to normal human testicular tissue, accounting also for the lack of functional in vitro models, might be one of the main reasons behind this. The use of animal models to elucidate the process of human spermatogenesis is limited as a result of species differences [143, 144]. However, in recent decades, different strategies, including animal studies with a focus on male germ cell or tissue transplantation [145–149], or the use of different types of in vitro cultures [150–156], including various pluripotent stem cells types [157, 158] and the recently described very small embryonic-like stem cells (VSELs) [159, 160], have explored the spermatogenic progress in detail. Prominent experimental conditions for ex vivo differentiation of mammalian male germ cells include (i) in vitro culture systems; (ii) testicular tissue (xeno) grafting; and (iii) SSC transplantation into the seminiferous tubules of the testis [155, 161, 162]. Among these experimental strategies, germ cell or testicular tissue transplantation have demonstrated possibilities to mature SSCs and more advanced germ cells in testicular microenvironments similar to those present in vivo.
Among the three ex vivo differentiation strategies, auto-transplantation of cryopreserved SSCs or testicular tissue into the patient´s testis represents a potential clinical tool for preservation of fertility following gonadotoxic treatment. However, the risk of reintroducing the disease due to re-transplantation of malignant cells back into the patient, particularly in patients with ALL, is one of the major limiting factors for future clinical application of this technique. The establishment of “decontamination” strategies to eliminate malignant cells from testicular tissue fragments is extremely challenging. So far, a specific marker for SSCs has not been identified [163]. Decontamination protocols involving enrichment and isolation of SSCs or the depletion of malignant cells have become a focus for research during recent years [164–166]. However, transplantation of cryopreserved testicular tissue or germ cells back into the patient after treatment is currently unsafe for patients with haematological cancer [142].
100 Years in Search of the Conditions Required for Male Germ Cell Differentiation in Vitro
At the beginning of the last century, organ culture conditions focusing on the differentiation of male germ cells from a variety of different species revealed several important factors necessary for male germ cell differentiation, including in vitro maintenance of the microenvironment provided by the seminiferous epithelium and thereby the intact cell-to-cell communication pathways of somatic and germ cells [155]. These early experiments, followed by many others in the following decades, also demonstrated the importance of maintaining temperature below that of the body [167–169], the need for cell–cell contacts [170–172], the supportive effect on viability of media components including pyruvate, glutamine, vitamins A, C and E, the non-supportive effect of gonadotropins for the success of germ cell cultures [167], as well as the negative influence of high oxygen levels on the viability of cells in vitro [162]. Studies focusing on in vitro differentiation of male germ cells combined with somatic cells, and studies involving enriched germ cell types such as spermatogonia and spermatocytes, clearly demonstrated that a microenvironment resembling the three-dimensional organisation of the seminiferous epithelium in situ (thereby providing cell–cell contact) should be provided [150, 155, 173–175].
The first successful differentiation of human male germ cells with completion of meiosis and the spermiogenic process in vitro was reported in the late 1990s. Tesarik and colleagues obtained human sperm in vitro via the use of seminiferous tubule cultures at 30 °C [153, 176]. The same group also reported the birth of healthy infants as a result of ICSI using in vitro-differentiated sperm from azoospermic patients [153]. In these reports, the differentiation of primary spermatocytes into haploid spermatids, which in vivo takes up to 32 days [176, 177], was reported to occur within 48 h [176] in vitro. Although earlier investigators reported a similar speed for germ cell differentiation in vivo and in vitro, another group reported four years later a similar feature of in vitro maturation. Primary spermatocytes obtained from azoospermic patients with meiotic arrest exhibited features of haploid round spermatids, when cultured for 2–5 days on Vero cells [178].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree