Fig. 18.1
Who should be treated with androgen replacement therapy?
Variations in sex hormone binding globulin (SHBG) gene polymorphisms and SHBG levels directly affect T levels (see Chap. 15). In genome-wide association studies, two single nucleotide polymorphisms were independently associated with serum T levels. One of the polymorphisms affects the ability of SHBG to bind T [19]. Most studies indicate that SHBG levels in men increase with age, which may mask hypogonadism because total T levels may be normalized by higher SHBG with aging [20]. In contrast, in overweight and/or insulin resistant men, total T may be low because the level of SHBG is low [21, 22]. Measuring free and/or bioavailable T in these situations is helpful.
Serum free T should be measured by equilibrium dialysis [16] which is available from most reference laboratories. If the free T measured by equilibrium dialysis is not available, the physician may request a measurement of sex hormone binding globulin (SHBG) and calculate the free T according to an established formula [23] (http://www.issam.ch/freetesto.htm ). However, the applicability of mathematical estimation models to all patients has been questioned as it is based on a predefined affinity of SHBG to bind T, and this affinity may vary [24–26]. Many clinical laboratories continue to measure serum free T using analog displacement assays using automated platforms with chemiluminescent labeled reagents. Such assays of free T do not accurately assess the free T fraction [27, 28], the levels are very low compared to measurements by equilibrium dialysis, and should not be used clinically [29]. Bioavailable T is the fraction of T in the serum that is the sum of free and albumin bound. This can be calculated or measured after ammonium sulfate treatment of the serum. The SHBG-bound hormone is precipitated and the supernatant containing the free and albumin bound fraction is quantitated by a serum T assay [14, 15]. Assays for bioavailable T are not available in many laboratories, and reference ranges have to be established by each laboratory.
If the repeat morning serum T level and/or the serum free T or bioavailable T levels are below the reference range of the laboratory, and the patient presents with symptoms consistent with hypogonadism, he may be considered for T replacement therapy if there are no contraindications; individuals with comorbid conditions that could produce transient stress may have treatment delayed, as treatment for the underlying condition should first be addressed. Next, the levels of LH and FSH should be determined. The finding of an elevated serum LH level is confirmatory for primary hypogonadism in men with low or borderline low serum T levels. Patients with functional or structural hypogonadotropic hypogonadism will have low or inappropriately normal serum LH concentration coupled with low serum T concentrations. While the only therapeutic option for primary hypogonadism is testosterone replacement, patients with hypogonadotropic hypogonadism may have intact testicular function with appropriate gonadotropic stimulation, and they may benefit from alternative treatment modalities that stimulate testosterone secretion and sperm production such as gonadotropins and antiestrogens or aromatase inhibitors. Given the important function of estrogens in bone mass, fat accumulation, and perhaps sexual function in healthy men [30, 31], agents that decrease serum estrogens to low levels are probably not appropriate for the long-term management of hypogonadism [32].
What Are the Contraindications to Androgen Replacement Therapy in Hypogonadal Men?
The absolute contraindications to androgen replacement therapy are untreated or active prostate and breast cancer, as these are androgen-dependent tumors. The other contraindication is an elevated hematocrit or hemoglobin level (e.g., hematocrit >54%). Androgen treatment may cause fluid retention, and in unusual circumstances precipitate or aggravate heart failure. In older patients with symptoms or signs of moderate or of severe congestive heart failure, androgens should not be used until the heart failure has been treated.
While there is minimal evidence to implicate T in the development or aggravation of benign prostate hyperplasia (BPH), if a patient has significant lower urinary tract obstructive symptoms due to BPH then the symptoms should be controlled before instituting T replacement. Obese and older subjects who may be at risk of sleep apnea should be carefully questioned about their ventilatory disturbances during sleep. Patients with moderate or severe obstructive sleep apnea symptoms should be appropriately evaluated or treated before the start of T replacement therapy. On the other hand, recent studies also reveal that testosterone deficiency is associated with obstructive sleep apnea especially in obese subjects [33].
What Safety Tests Should Be Done Before Start of Androgen Replacement?
A digital rectal examination and a serum prostate specific antigen test (PSA) should be performed to assess prostate abnormalities before a middle-aged or older patient is started on androgen treatment, or if the patient presents with a history of lower urinary tract symptoms. If the serum PSA level is elevated, or nodules or irregularities are found, the patient should be referred for Urological assessment. In general, a PSA concentration of >4 ng/ml indicates that the patient should be referred to an Urologist before considering androgen replacement. A complete blood count, liver function tests, HbA1C, and a lipid panel should be performed to ensure that the subject does not have an elevated hemoglobin or hematocrit and to assess the baseline liver enzymes and serum total, LDL and HDL-cholesterol since NAFLD, diabetes and dyslipidemia are common in men seeking treatment for adult onset hypogonadism.
What Are the Benefits and Risks of Androgen Replacement Therapy?
Androgen administration induces pubertal changes in patients who have not undergone puberty. In adult subjects, androgen maintains and restores secondary sex characters including facial, body, and pubic hair, the lower tone voice, external genitalia appearance, and the growth of the prostate. The risks and benefits of testosterone replacement therapy are summarized in Table 18.1 and described in the following sections.
Table 18.1
Benefits and risks of androgen therapy
Benefits | Risks |
|---|---|
• Improves sexual function • Maintains secondary sex characters • ↑ Bone mass, muscle mass, and strength • Decreased fat mass (visceral adiposity) • Improves mood in hypogonadal men? • Cognitive function? • Metabolic benefits? • Coronary vasodilation? • Decrease cardiovascular disease risk? | • Acne, oily skin • Decreased testis volume and suppressed spermatogenesis • ↑ Hematocrit/hemoglobin • Gynecomastia (with some preparations) • Weight gain, fluid retention • ↓ HDL, ↑ LDL: HDL ratio • Sleep apnea? • Increased cardiovascular disease risk? • Prostate dysfunction (benign prostatic hyperplasia, prostate cancer)? |
Sexual Function
Androgen replacement has been shown to improve sexual function more in younger than in older hypogonadal men. These include sexual desire (libido), sexual fantasies, sexual enjoyment and frequency of sexual thoughts, sexual activities, and erectile function in hypogonadal men. In younger hypogonadal men, sexual performance including erectile dysfunction is improved by androgen replacement therapy [34–38]. In older men, erectile dysfunction is frequently multifactorial, other causes such as vascular, neurogenic, psychogenic, medication-induced, and cavernosal problems may coexist. Therefore, improvement in erectile function following T treatment of androgen deficient older men may be less than in younger subjects [39, 40]; however, several controlled trials have shown some beneficial effects [41–43]. While objective data are limited and controversial, it is possible that androgen deficient older men whose erectile dysfunction has been improved by phosphodiesterase V inhibitors (e.g., Sildenafil, Vardenafil, Tadalafil), sexual performance may benefit from cotreatment with T through improvement in libido [42, 44]. When T was administered to older men with low or low normal T levels (<400 ng/dl, 13.9 nmol/l) for three years, there was no improvement in sexual desire, erectile dysfunction, or overall sexual function compared to placebo [45]. In a recent randomized, double-blind, placebo-controlled trial in hypogonadal older men with serum T levels <275 ng/dl (9.5 nmol/l), testosterone replacement resulting in physiological T concentrations moderately improved sexual activity, sexual desire, and erectile function scores as assessed by standardized questionnaires in both men with and without symptoms of sexual dysfunction. This is confirmed by the global impression of increase in libido [43]. It should be noted that androgen replacement to restore serum T to the low normal range appears to induce maximum sexual function improvement in hypogonadal men [35, 36, 38, 46]. Once a threshold level of serum T is achieved with androgen replacement therapy, it has been suggested that further increase in serum T levels may not increase sexual motivation or performance [17, 47].
Mood and Well-Being
While there is no definitive answer whether quality of life improves after androgen replacement in young or older hypogonadal men, improvement in sexual function and mood, working in synchrony may improve quality of life in hypogonadal men. Anecdotal reports have indicated that androgen treatment may cause increased anger and “rage” attacks in men. Moreover, higher salivary T levels were found in athletes and other subjects who were engaged in competitive activities [48]. In a placebo-controlled study in normal men who were administered testosterone at a supra-physiological dose for up to 20 weeks, there was no significant change in aggression and mood parameters [49, 50]. Uncontrolled studies of hypogonadal men who were administered different T preparations have demonstrated enhanced positive mood parameters such as well-being, energy, friendliness, and a reduction in negative mood parameters including anger, fatigue, irritability, and nervousness [49, 51–53]. Thus, in contrast to anecdotal reports, T treatment in placebo-controlled and uncontrolled studies in hypogonadal and eugonadal men either has no effect or appears to improve mood [54]; however, the data remain conflicting. There are no data on beneficial effects in men with established clinical depression [41, 55]. Recently published results of randomized placebo-controlled trials of testosterone replacement (The Testosterone Trials) suggested slight improvement in mood and depressive symptoms in those receiving T replacement compared to placebo- treated older men [43].
Cognitive Function
Studies of cognitive function in hypogonadal younger men are lacking except for uncontrolled studies in men with Klinefelter syndrome in whom T replacement improved verbal skills [56–58]. In younger eugonadal men rendered hypogonadal by exogenous administration of a progestin, verbal memory was reduced, which corrected when T was coadministered with the progestin [59]. In older hypogonadal men, several well-controlled studies with limited numbers of subjects reported better performance on spatial ability, and in some studies, verbal memory also improved [60–63] after androgen replacement. Thus, whether T improves cognitive function remains unanswered.
Bone Mineral Density (BMD)
Androgens are required to achieve peak bone mass in adolescence, and are responsible for the higher BMD in men compared to women. Hypogonadism results in a progressive decrease in bone mass, and is one of the causes of osteoporosis in men. With aging, a progressive loss of BMD is associated with an increased fracture rate. Interestingly, BMD in older men is more strongly positively correlated with serum free estradiol than serum free testosterone levels [64, 65]. The few case reports of estrogen receptor mutations and aromatase deficiency in males all described the presence of severe osteoporosis [66–68]. Thus, the current hypothesis is that estrogens are required for maintaining peak BMD in men [69, 70]. A recent study in induced hypogonadism in which graded doses of T replacement were administered in the presence or absence of an aromatase inhibitor showed a consistent decline in BMD when an aromatase inhibitor was coadministered with T, and this decline was independent of T replacement dose. While it is apparent that estrogen is the primary sex steroid regulator of bone mass in men [71], T also has positive effects on BMD possibly through both estrogen and androgen receptors [72]. The level of estrogen activity in the target tissues required to maintain BMD is not known, but in one study, serum estradiol >10 pg/ml (36.7 pmol/l) and testosterone levels >200 ng/dl (6.9 nmol/l) prevented increases in bone resorption and loss in BMD [71]. In the MrOs study of community dwelling older men, higher SHBG levels were associated with a higher likelihood of prevalent vertebral fracture, and men with serum estradiol levels equal to or less than 17 pg/ml (62.4 pmol/l) had a slightly higher likelihood of prevalent fracture than those with higher levels. Moreover, new or worsening vertebral fracture was associated with higher serum SHBG but not with serum T and estradiol [73].
Androgen replacement by injections, oral, or transdermal preparations have been shown to increase BMD in younger hypogonadal men [38, 74, 75]. The increase in BMD is accompanied by early elevations of bone formation markers and decreases in bone resorption markers [37, 38, 74, 76]. In older men, androgen administration resulted in increased BMD in some studies [77] but not others [78–80]. A randomized controlled trial in hypogonadal men older than age 50, with frailty and osteoporosis, showed significant improvement in lumbar and femoral neck BMD after 12 months of T replacement [81]. The peak increase in bone mineral density has been observed after 12–24 months of androgen replacement [75, 78]. In general, the lower the BMD and serum T level before treatment, the greater the improvement in BMD with T replacement [38, 78]. Though significant changes in BMD have occurred in hypogonadal men after long-term androgen replacement, randomized controlled studies to examine the effect of androgens on bone fractures have not been performed.
Body Composition
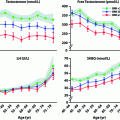
A meta-analysis of changes in body composition after T administration to middle-aged and older men including 29 randomized, controlled trials showed that T decreased fat mass (−6.2%), increased fat-free mass (+2.7%), but caused no change in overall body weight [82]. The conclusion is supported by a more recent meta-analysis of T treatment on body composition in 69 randomized placebo-controlled studies (Fig. 18.2) [83]. It is well established that androgens cause nitrogen retention in the body. Androgen treatment increases lean body mass and muscle mass by increasing muscle protein synthesis [84] to induce muscle fiber hypertrophy [85]. Androgens administered as sublingual tablets, injections or transdermal gels have increased muscle (lean) mass assessed by dual energy X-ray absorptiometry (DEXA) scans in both younger and older hypogonadal men [37, 38, 74, 78, 86, 87]. The increase in muscle mass is associated with increased muscle strength in younger hypogonadal men in both the upper and lower limbs assessed by a number of different techniques [37, 38, 74, 78, 86]. Muscle strength is difficult to quantify in a standardized manner, especially in older men [88], but improvement in strength has recently been demonstrated [40, 77, 87]. Several randomized controlled trials have shown improvements in specific strength measurements after T therapy, such as isometric knee extension, gastrocnemius muscle thickness [89], leg press strength, chest press strength, and climbing power [90] but not in other measures such as grip strength [91, 92], carried load, or gait speed [90, 93]. The increase in muscle mass and strength is directly related to the amount of testosterone administered and to serum T concentrations [46]. However, these increases in muscle mass and strength do not increase walk speed [43] or other daily activities of older men. The only study of T and coordination was in adolescent soccer players in whom serum T levels were positively correlated with soccer-specific performance laboratory tests [94].
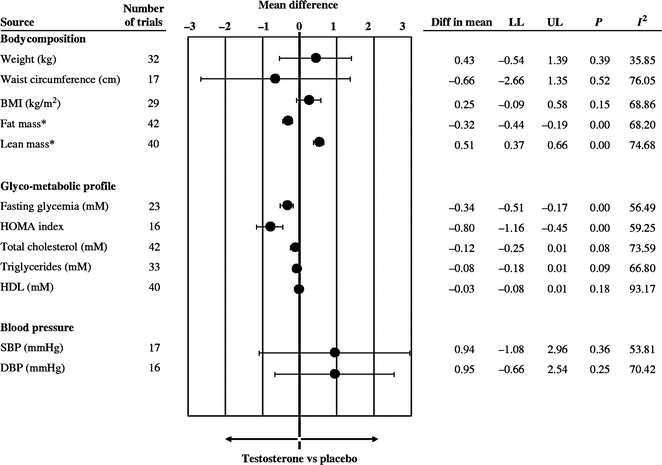

Fig. 18.2
Effect of testosterone therapy on body composition and other metabolic parameters. Weighted mean differences (with 95% CI) of body weight, waist circumference, BMI, fat and lean mass, fasting glycemia, HOMA index, total cholesterol, triglycerides, systolic (SBP), and diastolic (DBP) blood pressure at end point studies used in the meta-analysis. From Corona et al. [83]. Reprinted with permission from Bioscientifica Ltd.
Concomitant with increases in lean mass after androgen substitution in hypogonadal men, are consistent decreases in fat mass and percent body fat measured by DEXA, or visceral fat measured by abdominal CT or MRI scan. Decreases in subcutaneous fat, as well as adiponectin levels were observed in elderly men after six months of testosterone gel treatment [95]. Decrease in fat mass has been demonstrated with injectables and transdermal testosterone but not with sublingual T [37, 38, 74, 78, 86], and the effect of oral TU was less than that of intramuscular T injections [96] which may be related to the amount of T delivered and the serum T levels achieved. A dose-response study in normal men showed that the decrease in fat mass is directly related to the serum T level achieved and the dose of T administered [46]. Decreases in fat mass are also observed in middle-aged or older men receiving T [59, 77, 97]. The decrease in visceral fat observed in some studies has been suggested to result in decreased insulin resistance. A recent meta-analysis showed increases in lean mass and decreased fat mass while using either injectable or oral T formulations [98]. Another study showed a decrease in fat mass and increase in lean mass resulting in no net change in total body mass [99]. Similar positive effects were observed in patients with metabolic syndrome and type 2 diabetes [42, 100, 101].
Metabolic Parameters
A recent meta-analyses of 59 randomized controlled studies showed that T supplementation resulted in a reduction of fasting glucose and decreased insulin resistance (Fig. 18.2) [83]. T replacement has been reported to decrease insulin resistance and improve glycemic control in some studies of hypogonadal men with diabetes [100, 102–104]; however, improvement has not been consistent across all studies [105]. A recent study in hypogonadal men with diabetes and/or metabolic syndrome reported a decrease in insulin resistance after 6 months with no further benefit after 12 months of T replacement using a testosterone gel [42]. While another study of testosterone treatment in patients with type 2 diabetes failed to show significant effects on insulin resistance, a very small but significant reduction in HbA1c was noted, with most improvement in poorly controlled patients [41].
Testosterone replacement also improved serum inflammation markers in patients with type 2 diabetes and metabolic syndrome [100, 106, 107]. The metabolic benefits of T treatment in obesity, type 2 diabetes and metabolic syndrome, and the impact on cardiovascular disease risks are complex [108]. This field remains controversial and the results are inconclusive, and large randomized controlled studies are needed in men with low T levels with close attention to covariates.
Lipid Profile
Supra-physiological doses of T esters administered to healthy men in male contraceptive trials resulted in significant suppression of serum HDL-cholesterol levels and a decrease in HDL to total cholesterol ratios [109]. On the other hand, serum HDL-cholesterol levels were not significantly changed in hypogonadal men administered transdermal T in patches or gels [38, 110–112]. A meta-analysis of 51 testosterone replacement studies has shown a slight decrease in HDL-cholesterol [113] while another meta-analysis of studies in men with total T <346 ng/dl (12 nmol/l) showed reduction of total cholesterol and triglycerides without any effect on HDL-cholesterol [83]. The decrease in serum HDL-cholesterol was dependent on the dose of T administered and the serum T level achieved [114]. Thus, during androgen replacement of hypogonadal men with near physiological doses of testosterone, significant changes in serum lipid levels are uncommon. Moreover, the possible negative effect of a slight lowering of serum HDL-cholesterol levels needs to be considered together with effects of T on other lipoproteins, coagulation, and fibrinolytic factors. The role of HDL in cardiovascular disease has been recently revisited, and the levels of HDL-cholesterol may not be as important as the type and metabolism of HDL [115, 116].
Cardiovascular Disease Risk
Low T levels are associated with established cardiovascular risk factors, such as obesity, metabolic syndrome, and type 2 diabetes. In men undergoing coronary angiogram, men with demonstrable coronary artery disease had a significantly lower serum free androgen index and bioavailable T levels than those who did not have evident coronary artery disease [117, 118]. A number of studies have shown that testosterone replacement may improve risk factors for cardiovascular disease, such as fat mass, insulin resistance, inflammatory markers, and other features of metabolic syndrome (see Section “Metabolic Parameters,” Fig. 18.2). Other large epidemiological studies have shown that men with lower serum T levels have a higher risk of cardiovascular events and all-cause mortality [119–124] (Table 18.2).
Table 18.2
Cardiovascular outcomes in clinical studies
Study | Design | Treatment | No. of subjects | Age | T cutoff | Duration | CVD Outcomes | All-cause mortality |
|---|---|---|---|---|---|---|---|---|
Shores et al. [124] | Retrospective analysis of VA database | None | 858 | >40 | N/A | Avg. 4.3 years | No CVD data | Low T associated with ↑ all-cause mortality (HR 1.88 (1.34–2.63) |
Khaw et al. [120] | Nested case control | None | 825 died to 1489 alive of 11,606 | 40–79 | N/A | Avg. 7 years | Lowest quartile versus highest ↑CV mortality, OR 1.89 (1.16–3.13) | Lowest quartile versus highest ↑ all-cause mortality, OR 1.69 (1.18–2.38); ↑in T by 6 nmol/l associated with OR 0.81 (0.71–0.92) for all-cause mortality |
Laughlin et al. [119] | Prospective observational | None | 794 | 50–91 | N/A | Avg. 11.8 years | Low T↑ CVD mortality, HR 1.38 (1.02–1.85) | Lowest quartile (<241 ng/dl) ↑ all-cause mortality, HR 1.4 (1.14–1.71) |
Shores et al. [224] | Longitudinal cohort study | None | 1032 | 66–97 | N/A | Median 9 years | Low DHT but not T ↑incident CVD when adjusted for other CV risk factors | Low DHT ↑all-cause mortality |
Shores et al. [225] | Retrospective VA database study | Various T formulations (88.6% IM) | 1031 (TRT in 398) | >40 | ≤250 ng/dl (8.7 nmol/l) | Avg. TRT duration 20 months | N/A | TRT ↓ all-cause mortality, HR 0.61 (0.42–0.88). Significant effects in younger (41–59), with diabetes and without CVD |
Muraleedharan et al. [226] | Retrospective cohort (TIMES2 follow up) | Various T formulations (93.8% gel) | 64 on TRT versus 174 no TRT with Type 2 diabetes | Avg. 60.3 ± 11.5 | T < 300 ng/dl (≤10.4 nmol/l) | Avg. TRT duration 41.6 months | N/A | TRT ↓ all-cause mortality, HR 0.43 (0.26–0.77) |
Vigen et al. [132] | Retrospective analysis of VA database | Various T formulations (63.3% patches) | 1223 started TRT, 7486 did not; undergoing coronary angiogram | Avg. 63.8 ± 9 | T < 300 ng/dl | ~10 months of TRT | TRT ↑ CVD, HR 1.29 (1.04, 1.58). Nonsignificant difference in absolute risk | |
Finkle et al. [136] | Health-care prescription database study | Various T formulations (most common—gel) | 55 593 men prescribed TRT | Avg. 54.4 | 1 year pre versus 90 days post prescription | TRT ↑Post/pre-prescription rate ratio for MI—1.36 (1.03–1.81), in >65 years—2.19 (1.27–3.77), in <65 with CV history—2.9 (1.49–5.62) | ||
Baillargeon et al. [135] | Retrospective cohort Medicare database | IM T | 6355 at least 1 T injection versus 19065 matched controls | >66 | N/A | Avg. follow up 49.8 months | TRT not associated with MI, HR 0.84 (0.71–1.05) | Men in highest quartile for MI prognostic score TRT ↓MI risk HR 0.69 (0.53–0.92) |
Basaria et al. [131] | RCT multicenter, 3 centers | Trans-dermal T titrated to 500–1000 ng/dl | 209 | >65 limited mobility | TT 100-350 ng/dl (3.5-12.1 nmol/l) or FT <50 pg/ml (173 pmol/l) | 6 months | TRT ↑ CV related AE, HR 5.8 (2.0–16.8); highest T quartile versus others, HR 2.4 | |
Calof et al. [227] | Meta-analysis | 1084 (651 in TRT and 433 placebo) in 19 trials | Avg. 10 months | No TRT effect on CV events, OR 1.14 (0.59–2.20) | ||||
Haddad et al. [228] | Meta-analysis | CV events reported in 6 trials (161 TRT and 147 placebo) | No TRT effect on CV events, OR 1.82 (0.78–4.23) | |||||
Fernández-Balsells et al. [229] | Meta-analysis | 2679 in 51 trials | 3mo-3 years | No TRT effect on MI, RR 0.91 (0.29–2.82) | No TRT effect on mortality, RR 1.12 (0.7–1.81) | |||
Corona et al. [121] | Meta-analysis | Cross-sectional studies | 5153 patients with and 7513 without CVD in 54 studies | T ↓ in CVD patients. Low T associated with CVD, HR 1.31 (1.28–1.34) for 1 nmol/l ↓ in T | ||||
Longitudinal: | 12,375 subjects in 10 studies | Baseline T ↓ in those with incident CVD; no T difference between case and controls | Baseline T ↓ in those with all-cause mortality | |||||
RCTs in CHD patients | 128 pts with CHD received T and 129 – placebo in 6 RCTs | Avg. 23 weeks | TRT ↑ treadmill test duration and time to ST depression | |||||
Toma et al. [230] | Meta-analysis | RCTs in HF patients | 198 men with HF in 4 studies | 12–52 weeks | TRT ↑ 6-minute walk test, incremental shuttle walk test, and peak oxygen consumption | |||
Xu et al. [137] | Meta-analysis | 2994 in 27 trials | At least 12 weeks | TRT ↑ CV event risk 1.54 (1.09–2.18) overall, in pharma non-funded studies 2.06 (1.34–3.17) but not in pharma funded 0.89 (0.5–1.6) | ||||
Corona et al. [138] | Meta-analysis | 5464 in 74 trials (MACE in 26) | Avg. 34 weeks | TRT not associated with MACE incidence, OR = 1.01 (0.57–1.77); In men with metabolic disease ↓ CVD | ||||
Albert et al. [140] | Meta-analysis | 5328 in 45 trials | 63.3 ± 7.9 | 10.6 ± 8.6 months | TRT not associated with CV risk overall, RR 1.10 (0.86–1.41). ↑ rate during first 12 mos, 1.79 (1.13–2.83); >65 years, 2.9 (1.35–6.21). IM T—0.96 (0.462–1.98), oral 2.28(0.60–8.59), transdermal 2.80 (1.38–5.68) |
In patients with cardiovascular disease, short term administration of T showed variable results. A study showed decreased ST segment depression and anginal symptoms in men with cardiovascular disease treated with T [125, 126]. Acute administration of T to men with exercise-induced myocardial ischemia reduced ST segment depression and increased exercise testing time compared to placebo [127, 128]. Because of this acute action of T, the effect may be ascribed to a direct coronary vasodilatory effect of the steroid. A subsequent study confirmed this hypothesis by showing acute T infusion increased coronary artery diameter and coronary blood flow in men with established coronary artery disease compared to vehicle administration [129]. However, others failed to demonstrate a beneficial effect on acute stress-induced myocardial ischemia [130].
Concerns that T replacement in hypogonadal men may increase adverse cardiovascular events were heightened when a randomized controlled trial in elderly hypogonadal men with mobility limitations and high prevalence of chronic disease was discontinued early due to higher incidence of cardiovascular adverse events in T-treated men compared to the placebo group [131]. These elderly men received 1% transdermal T gel up to 15 g/day. This study, however, was not designed nor powered to evaluate cardiovascular outcomes. The recorded cardiovascular adverse events were not adjudicated by independent committee and were highly variable. Included in the reported major cardiovascular adverse effects were: syncope, elevated blood pressure, tachycardia, and peripheral edema. Despite another randomized controlled study in older intermediate-frail men that did not demonstrate an increase in cardiovascular adverse events [89], the former study engendered a number of reports raising concerns about the cardiovascular safety of T replacement. These more recent reports included case-cohort studies, analyses of prescription data from large databases, and meta-analyses.
A retrospective cohort study in veterans with low T levels (<300 ng/ml) and a high burden of comorbidities who underwent a coronary angiography showed that T use was associated with increased mortality and myocardial infarction and stroke. The absolute difference in cardiovascular event rates was 5.8% higher at 3 year after the coronary angiogram in men who had filled at least one T prescription compared to those who were not treated. In this cohort, 82.4% of the men filled more than one prescription [132]. In another study also of veterans with low T, replacement with T was associated with lower mortality compared with no T treatment [133]. These authors showed in a longitudinal study that serum T and calculated free T were not significantly associated with cardiovascular disease or mortality, but serum dihydrotestosterone (DHT) and free DHT had significant curvilinear association with incident cardiovascular events [134]. In a large case-cohort study of older men, T therapy did not increase the risk of myocardial infarction, and in men at high risk for myocardial infarction, T use was moderately protective [135]. Another cohort study, using a large health-care database, showed that men who received a T prescription have a higher rate of myocardial infarction in the 90 days following the prescription compared to the rate 12 months prior to the prescription. The increase in myocardial infarction rates was observed in men over the age of 65 years. The post/pre-prescription rate difference in myocardial infarction was not observed in men instead prescribed phosphodiesterase 5 inhibitors [136]. It should be noted that neither the patient diagnosis nor the reason for the prescription of T was known.
An older meta-analysis of randomized controlled trials found that T treatment of hypogonadal men was not associated with increased cardiovascular adverse events [113]. A more recent meta-analysis of 27 randomized, controlled trials found increased cardiovascular events risk in T-treated hypogonadal men. Interestingly, increased cardiovascular disease risk was only found in trials that were not funded by the pharmaceutical industry [137]. This was followed by another meta-analysis of T supplementation in men from 75 placebo randomized controlled trials which showed that T treatment was not related to cardiovascular adverse events [138] especially when hypogonadism is properly diagnosed and T replacement correctly performed [139]. Another meta-analysis of 45 trials showed that overall T supplementation was not associated with increased cardiovascular events risk, however, there was a slight increase in risk within the first 12 months of treatment, especially in men >65 years. Intramuscular T appeared to have no effect while oral or transdermal T treatments were associated with increased cardiovascular disease risk [140].
Despite the controversial results, major limitations, and questionable validity of these studies, such reports led the Food and Drug Administration to alert physicians to discuss cardiovascular disease risks with their patients before prescribing androgens (FDA January 2014) and required manufacturers to amend the product label to indicate a possible increased risk of heart attacks and strokes in patients treated with testosterone (FDA March, 2015) [2]. None of the randomized, placebo-controlled testosterone studies to date including The Testosterone Trials were of sufficient power to evaluate cardiovascular adverse outcomes [93]. Thus, the FDA Drug Safety Communication March, 2015 stated “We are also requiring manufacturers of approved testosterone products to conduct a well-designed clinical trial to more clearly address the question of whether an increased risk of heart attack or stroke exists among users of these products.” (http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm)
In summary, there are no reliable data showing that T administration has either an adverse or beneficial effect on coronary artery disease risk, and further research in this area is necessary. There are no data to suggest that T replacement is contraindicated in certain groups of patients; however, when treating older men with multiple comorbidities who are at high cardiovascular risk, extreme care should be exercised. A recent AACE/ACE position statement recommends avoiding T treatment of the frail elderly [141], and risk versus benefits should be discussed with the older patient with testosterone deficiency. A large prospective, randomized, controlled intervention trial in older men with higher risk of major cardiovascular adverse events is being planned by the pharmaceutical companies in response to the request from the FDA to address the question whether T replacement in hypogonadal men changed their risk of cardiovascular disease.
Prostate Disease
Prostate growth and development are known to require the presence of androgens from childhood to adulthood. In younger hypogonadal men in whom prostate diseases are uncommon, androgen replacement results in an increase in the size of the prostate from the smaller volumes at baseline to the range observed in eugonadal men. Progressive increases in prostate volume do not occur with continued T replacement [142, 143]. Serum PSA levels may increase significantly with T replacement, but mostly remain within the normal range [34, 77, 110, 143]. In middle-aged and older men, androgen replacement has resulted in few instances of lower urinary tract obstructive symptoms (LUTS) and increased serum PSA levels resulting in urological referral and ultrasound-guided prostate biopsy [38, 40]. In most of the reports, these prostate-related adverse events occurred early in the course of androgen replacement suggesting that androgen replacement may have unmasked an existing latent or histological cancer by increasing serum PSA levels to above the reference range triggering an early prostate biopsy and diagnosis. More recent studies, however, have not reported worsening of LUTS symptoms in patients with underlying symptoms at baseline [81, 91, 100, 144–146]. A recent meta-analysis of 14 testosterone replacement trials in which LUTS were evaluated by IPSS showed no significant difference between those receiving T and placebo [147]. Most Urologists agree that androgens do not induce BPH. Currently, we recommend that androgens should not be used in subjects with lower urinary tract obstructive symptoms until these symptoms have been assessed and treated.
There is no evidence in hypogonadal young or older men that androgen replacement induces formation of prostate cancer or converts a latent, histological prostate cancer to a clinically significant or metastatic cancer [148]. However, if a hypogonadal patient had a history of, or is at high risk of developing prostate cancer, then androgens should be avoided. Use of calculators to estimate risk of developing prostate cancer (such as http://deb.uthscsa.edu/URORiskCalc/Pages/calcs.jsp) has been recommended [5]. On the other hand, there are instances in which a patient with a distant completely resected intra-prostatic prostate cancer and long standing nearly undetectable PSA levels with severe symptoms and signs of T deficiency seeks T replacement. Treatment of such patients may be justified only with careful surveillance and well documented informed consent [149]. Coadministration of a 5α-reductase inhibitor with testosterone appeared to spare the prostate from androgenic stimulation during T replacement in older, hypogonadal men with symptomatic benign prostatic hyperplasia [150].
Hematocrit, Hemoglobin, and Liver Function Tests
Androgens increase erythropoiesis by multiple mechanisms including: acting directly on bone marrow cells by increasing telomerase activity and extending the length of telomeres [151–153]; an action on the kidney to produce erythropoietin [154]; and by suppressing hepcidin leading to increased iron absorption and incorporation into red blood cells [155, 156]. Androgen therapy in hypogonadal and eugonadal men results in an elevation of hematocrit and hemoglobin. The effect of T to increase red cell indexes has been demonstrated in many androgen replacement studies [38, 86, 110]. The increase in hemoglobin and hematocrit occurs within three months which increase progressively if the dose of T is adjusted upwards. Direct dose-response relationships have been shown between T and hematocrit and hemoglobin concentrations [46]. A greater increase in hemoglobin was seen in older compared to young men with induced hypogonadism [157, 158]. A substantial increase in hemoglobin was found in men with obstructive sleep apnea [92] who are often polycythemic. Thus, androgen therapy must be used with caution in men with baseline hematocrit of 50% or over. When the hematocrit rises above 50%, the subject must be carefully monitored and the T replacement dose adjusted downwards to prevent hyperviscosity and increased risk of thrombosis. The Endocrine Society suggests stopping the therapy if hematocrit is >54% until it normalizes, and reinitiating with a reduced dose [5, 131]. Alternatively, phlebotomy may help decrease hematocrit to safe levels without compromising the treatment plan, and genetic testing for polycythemia vera should be considered.
Abnormal liver function tests have been reported with the use of 17 alkylated androgens [159, 160]. Modifications of the steroid ring were necessary to allow these 17 alkylated androgens to be active after oral administration. Because of the possibility of liver toxicity and the increase in LDL and decrease in HDL-cholesterol with oral 17 alkylated androgens, they are not recommended for use as androgen replacement. It should be noted that native T and T esters administered as replacement therapy do not result in abnormalities of liver function [37, 38, 86, 110].
Other Possible Adverse Effects
Increased oiliness of the skin, and acne, are common complaints after T replacement, especially when high doses are administered. These conditions are treated by topical measures or by either changing the T preparation or reducing the T replacement dose. Because T esters, like native T, are aromatized to estrogens, changes in the androgen to estrogen balance may occur after T replacement which may cause gynecomastia. Testosterone replacement also causes fluid retention during the early weeks of treatment and should be used with caution in older patients with congestive heart failure or poor myocardial function. It has been recommended not to initiate T replacement in patients with poorly controlled heart failure [5]; however, no negative effect has been observed in patients with stable heart failure [161].
Earlier studies reported that sleep-related breathing disorders may be aggravated after T replacement [162]; however, a more recent randomized control study in aged obese men with untreated obstructive sleep apnea showed that while T replacement therapy did increase oxygen desaturation index and hypoxemic sleep time at seven weeks, no differences were observed at the study end point of 18 weeks, and there were no differences in apnea episodes [92]. In addition, no significant worsening of sleep apnea symptoms was observed in relatively healthy hypogonadal patients randomized to T treatment in other studies [144], thus the significance of sleep apnea as an adverse effect of T treatment should be reevaluated. A detailed history for symptoms of sleep apnea before and during T treatment should be obtained. High-risk patients (obese, elderly) should be investigated and treated for sleep apnea before commencement of androgen replacement therapy (see Chap. 19).
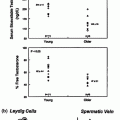
Testosterone administration suppresses gonadotropin secretion, and leads to reduced intratesticular T levels and hypospermatogenesis. These changes are reversible after withdrawal of treatment. The rate of recovery of spermatogenesis depends on the type of androgen used (longer or shorter acting androgen), duration of treatment, sperm concentration at baseline, ethnicity, and age, as shown in healthy men administered androgens in male contraceptive trials (Fig. 18.3) [163]. Because suppression of spermatogenesis may be prolonged with long acting T undecanoate injections, men with hypogonadotropic hypogonadism seeking future fertility should instead be treated with gonadotropins.
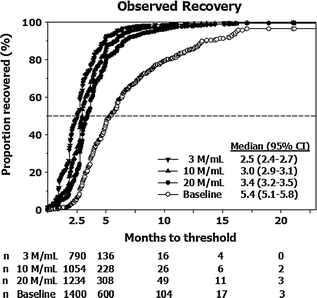

Fig. 18.3
Kaplan-Meier observed plots showing recovery to sperm density thresholds of 3 (inverted triangle), 10 (triangle), 20 (circle) Mil/mL, and back to individual baseline (open circle). Median (95% CI) recovery times for each of the thresholds are shown. The number of subjects (n) remaining at 2.5, 5, 10, 15, and 20 months for each of the above thresholds are shown on the bottom of the graph. From Liu et al. [163] and the Hormonal Male Contraception Summit group. Reprinted with permission from Elsevier
What Are the Available Androgen Preparations?
Table 18.3 shows the currently available androgen preparations and those under development. Numerous new options have been developed and introduced into clinical practice in the past 15 years. The most popular methods are long acting intramuscular injections and transdermal gel preparations. Traditional intramuscular formulations are low cost; however, gels present a more convenient option for many patients. The long acting T undecanoate injection with one injection every 10–12 weeks is preferred by many patients and physicians. Gel tablets for buccal and nasal administration of testosterone have also been developed, but these have to be administered two to three times a day. Future goals of the pharmaceutical industry are to develop modified androgen and synthetic androgen receptor modulators to avoid potential adverse effects (for example, stimulatory effects of androgens on prostate growth) while maintaining the beneficial effects on bone, muscle mass, sexual function, and mood.
Table 18.3
Androgen preparations and delivery systems
Currently available | Under development | |
|---|---|---|
Injectable | T enanthate T cypionate T undecanoate | T microspheres |
Oral | T undecanoate (not available in the US) | T undecanoate (other formulations) Dimethandrolone Selective androgen receptor modulators (SARMS) |
Transdermal | T nonscrotal patch T gels/lotions | Other T gels, creams DHT gel |
Transmucosal | Trans-buccal T Intranasal T | |
Implants | T pellets (available as 75 mg in US; 100 or 200 mg in Europe or Australia) | 7α methyl 19 nor-T (MENT) |
Injectables
Injectable intramuscular solutions have been the mainstay of T replacement therapy since the 1940s. Injectable T is usually esterified, which makes the molecule more oil soluble to extend the rate of resorption of T from the injection site so that a longer duration of action is achieved. Because testosterone is released from the injection site, the metabolism of T is not affected. Therefore, different T esters result in different durations of action. Testosterone enanthate and cypionate are T esters usually administered as a deep intramuscular injection. The usual recommended dose is 200 mg in one mL oil administered every two weeks. The pharmacokinetics (PK) of TE is graphically shown in Fig. 18.4. Serum T levels peaked within 1–3 days after administration, and reached a trough after two weeks. In many subjects, the level of serum T achieved may reach a concentration which is higher than the normal adult male reference range for the first few days after an injection. The PK of T injections has been well studied [164, 165]. Most patients can be taught to self-administer injections. In some patients, the high peaks and low troughs of serum T levels may result in mood swings and acne. In such patients, the dose may be decreased and the frequency of the injections increased, for example, T enanthate or cypionate may be administered at 100 mg every 7–10 days.
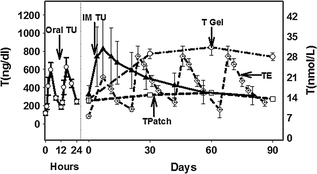

Fig. 18.4
Graphical presentation of pharmacokinetic profiles of different testosterone preparations. TE Testosterone enanthate 200 mg IM once every three weeks; TU IM Testosterone undecanoate 750 mg IM once in ten weeks after 3 or 4 injections; T patch Trough levels after application of 5 mg T patch; T gel Trough levels after application of 10 g T gel; and Oral TU Levels after oral administration of TU 200 mg twice a day [170, 183, 222, 223]
Testosterone undecanoate (TU) was developed and marketed initially in China for the treatment of hypogonadism [166]. The depot preparation (TU 250 mg/mL, 1000 mg per injection) is widely used in Europe, Middle East, South America, and Australia. The recommended regular dosing interval is 10–14 weeks; a booster dose at four to six weeks after the first injection helps to achieve steady state levels. After administration of 1000 mg TU, the serum T level peaked in the first or second week, and remained within the normal range for as long as 12 weeks [167, 168]. A lower dose formulation is available in the United States (250 mg/mL, 750 mg per 3 ml injection), is dosed approximately every 10 weeks, with the second booster injection administered 4 weeks after the first injection [169, 170].
The injectable preparations may pose a greater risk of polycythemia than with transdermal preparations, as the T concentration peak is higher, especially with shorter acting formulations [171]. There have been reports of bouts of coughing after IM injections, which has been ascribed to pulmonary oil microembolism, especially with depot TU, as well as rare possible anaphylactic reactions. These observations have led to a requirement in the United States that TU must be administered in a clinic or physician’s office, and patients must be observed for 30 min after the injection. With the use of long acting T, it is recommended that serum T levels should be measured prior to an upcoming injection (trough level)—with a goal of serum T being within the lower normal range. In older men, it may be prudent to start with a lower T dose and use short acting preparations before transitioning to longer acting injectables in case adverse events occur [172].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree