Malaria
Arlene Dent MD, PhD
Charles H King MD, MS, FACP, FRSTMH
Introduction
Malaria is a life-threatening infectious disease caused by apicomplexan protozoan parasites of the genus Plasmodium. Typically, infection is transmitted from person to person via the bite of an intermediate insect vector, i.e. the female Anopheles spp. mosquito. Globally, there are 300-500 million human cases of malaria each year. One to two million deaths result; the majority are among children under the age of 5 years. More children die of malaria than any other infection. These deaths are unwarranted, as malaria is both preventable and treatable.
The majority of malaria infections and related deaths occur in sub-Saharan Africa, where the resultant economic burden is estimated to be at least US$12 billion per year in lost GDP. Malaria is frequently a disease of the poor, and its presence serves to perpetuate the cycle of poverty. On a global and national level, past programs to reduce transmission have met with some success, but efforts to eradicate malaria completely have proven unsuccessful. In 1998, The Roll Back Malaria (RBM) program was jointly initiated by the World Health Organization (WHO), the World Bank, the United Nations Development Program, UNICEF, and other agencies, with the goal of halving the world’s burden of malaria by 2010. The RBM program focuses on improving access to effective treatment, preventing malaria in pregnancy through intermittent prophylactic therapy, reducing human-mosquito contact through distribution of insecticide-treated bed nets, and improving access and distribution of antimalarial medications. Nonetheless, recent estimates of global malaria burden have shown increasing levels of malaria morbidity and mortality despite these efforts. Development of infrastructure and distribution of preventative and therapeutic interventions remain significant challenges.
Etiology and pathogenesis
Four species of Plasmodium cause human malarial disease. The four species are P. falciparum, P. vivax, P. ovale, and P. malariae. P. falciparum causes the most severe, life-threatening form of malaria. Human infection with P. knowlesi, a recognized pathogen of non-human primates, has also been described.
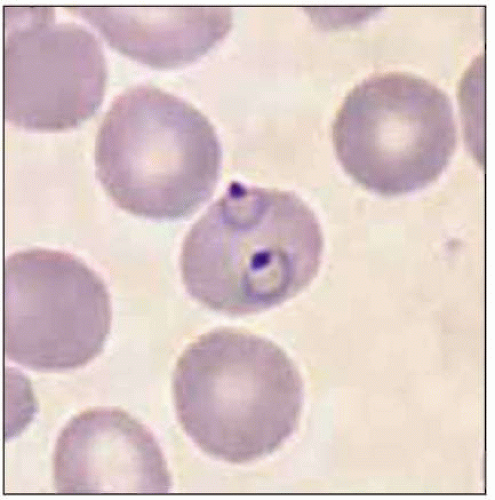
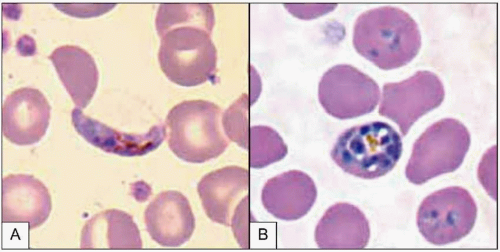
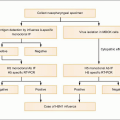
Plasmodium parasites enter the human host when an infected female Anopheles mosquito takes a blood meal, usually during the evening or nighttime. The sporozoites injected in the mosquito’s saliva enter the bloodstream and travel to the liver, where they infect host hepatocytes (Fig. 5.1). Over the next several weeks, the sporozoites mature into schizont forms. With P. vivax and P. ovale infections, schizonts may remain dormant in hepatocytes as hypnozoites for weeks to months before causing clinical relapses. The infected hepatocyte ruptures, releasing merozoites into the circulation. Merozoites then attach to and invade erythrocytes, where they grow as trophozoites (Fig. 5.2). During the next 48-72 hours, these trophozoites grow into schizonts, which, once mature, reproduce asexually by division. The infected red blood cells burst, releasing multiple daughter merozoites, which continue the erythrocytic infection cycle. A small proportion of infecting parasites differentiate into non-replicating sexual forms known as gametocytes. When gametocytes are ingested in a blood meal taken by a mosquito, the life cycle is completed: sexual reproduction occurs via gametocyte fusion in the mosquito midgut to form an ookinete, whence thousands of infective sporozoites result. These then migrate to the mosquito’s salivary glands, ready for transmission via its next bite (Fig. 5.3).
Pathogenesis of the disease has been best described in P. falciparum infection. Malaria symptoms appear 7-14 days
after the infectious mosquito bite. The severity of disease is dependent on several factors: high parasite burdens, combined with the propensity of infected erythrocytes to adhere to host endothelium, lead to microvascular occlusion, cytokine release, metabolic derangement, and acidosis, resulting in the clinical manifestations of severe malaria. Common presentations include respiratory distress syndrome, renal insufficiency, severe anemia, and cerebral malaria. Host factors including sickle cell hemoglobin and glucose-6-phosphate dehydrogenase deficiency genetic polymorphisms can modify the severity of malaria, at the risk of host pathology caused by these traits.
after the infectious mosquito bite. The severity of disease is dependent on several factors: high parasite burdens, combined with the propensity of infected erythrocytes to adhere to host endothelium, lead to microvascular occlusion, cytokine release, metabolic derangement, and acidosis, resulting in the clinical manifestations of severe malaria. Common presentations include respiratory distress syndrome, renal insufficiency, severe anemia, and cerebral malaria. Host factors including sickle cell hemoglobin and glucose-6-phosphate dehydrogenase deficiency genetic polymorphisms can modify the severity of malaria, at the risk of host pathology caused by these traits.
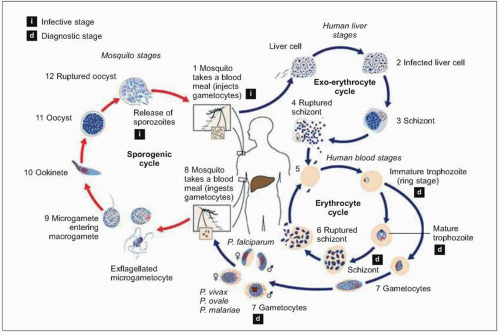 Fig. 5.1 Schematic representation of the malaria life cycle. (Adapted from CDC data.) |
 Fig. 5.2 Electron microscopy image of a merozoite attached to a host erythrocyte. (Courtesy of Dr R Salata.) |
Global epidemiology
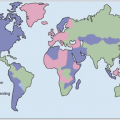
Malaria is one of the most prevalent infections worldwide, with 40% of the world’s population at risk for infection. In addition to children, it is pregnant women (especially primigravidae), and non-immune travelers and foreign workers who are at particular risk for developing severe disease. Political conflicts that cause mass displacement of populations can lead to movement of non-immune people into malaria-endemic regions, which can result in large numbers of cases of acute malaria. The risk of severe malarial disease increases during malaria epidemics, which can occur with periodic climatic changes. Warm climates with high humidity and high rainfall create conditions favorable to mosquito breeding and survival (Fig. 5.4). Man-made environmental changes (such as agricultural projects and mining) can increase the breeding area of vector anopheline mosquitoes.
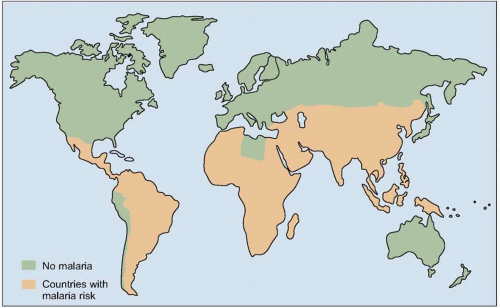 Fig. 5.4 Map of the global distribution of malaria (2003). (Adapted from WHO data.) |
Malaria has been eradicated from the US, Europe, and Australia. However, these areas remain at risk for reintroduction of the parasite. In non-endemic areas, most cases are attributed to persons returning from endemic areas (‘imported’ malaria). There is also the possibility of an imported case resulting in new, locally-transmitted mosquito-borne cases (‘airport transmission’), and persisting asymptomatic infection can result in congenital malaria or in transmission through blood transfusion or organ transplantation.
In the past, chloroquine was a cheap and widely used antimalarial medication, and its prophylactic use was associated with significant declines in regional prevalence. Today, however, resistance to chloroquine is common throughout Africa, Asia, and most of South America. As a result, malaria is re-emerging in many of these areas. Currently, the remaining areas with chloroquine-sensitive malaria are limited to northern parts of Central America.
Resistance to another inexpensive drug, sulfadoxine-pyrimethamine (SP), is also increasing dramatically. P. vivax, which was universally chloroquine-sensitive in the past, is now showing emerging chloroquine resistance in Oceania, Myanmar, Guyana, Colombia, and Brazil. Multidrug resistant malaria is, therefore, an emerging problem that now threatens many countries.
Resistance to another inexpensive drug, sulfadoxine-pyrimethamine (SP), is also increasing dramatically. P. vivax, which was universally chloroquine-sensitive in the past, is now showing emerging chloroquine resistance in Oceania, Myanmar, Guyana, Colombia, and Brazil. Multidrug resistant malaria is, therefore, an emerging problem that now threatens many countries.
Clinical manifestations
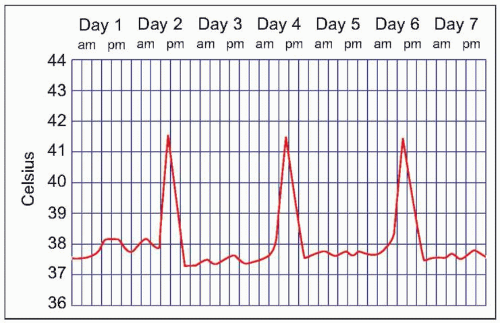
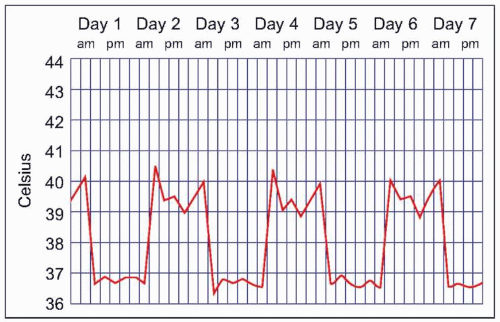
Malaria symptoms appear 7-14 days after an infectious mosquito bite. In non-immune adults, malaria typically produces fever, shaking chills, headache, vomiting, and other flu-like symptoms. Any fever in a traveler who has visited a malaria-endemic area should be considered to be due to malaria until proven otherwise. Delay in diagnosis and treatment can have devastating consequences. At times, malaria may result in classic fever patterns: P. vivax and P. ovale infections are said to cause ‘tertian’ (every 48 hours) fevers (Fig. 5.5), whereas P. malariae causes quartan (every 72 hours) fevers. P. falciparum has been associated with ‘malignant tertian’ fevers where the pattern is hectic rather than consistent (Fig. 5.6). Paroxysms are associated with the fevers and typically occur in three stages. First, an individual will have ‘cold’ shaking chills, followed by fever to 40°C or higher, along with systemic symptoms. As the fever resolves, an individual will experience diaphoresis and fatigue. Fever and splenomegaly are commonly found on physical exam. In addition, pallor from hemolytic anemia is noted along with hepatomegaly and abdominal tenderness. The presence of a rash and lymphadenopathy are not consistent with malaria.
Individuals living in malaria-endemic regions can develop partial immunity to malarial disease. Repeated infections produce cellular and humoral immune responses that limit the severity of disease in subsequent infections. Immunity is not fully protective, however. In an ‘immune’ host, the clinical presentation of malaria symptoms is typically less severe when compared to illness in a non-immune person. However, fever is almost always present and may be associated with headache, chilling, or arthralgias. Oftentimes, partially-immune individuals have lower levels of parasitemia and these are associated with fewer symptoms. Although severe acute disease may not occur, recurrent infection can result in severe and chronic anemia.
In children, manifestations of clinical malaria are similar to those in adults, but they are not typically associated with classic fever patterns (Table 5.1). Malaria in children can be rapidly progressive or fatal if left untreated. Children who survive an episode of severe malaria may have persistent learning impairment or other neurologic damage. Pregnant women and their fetuses are especially vulnerable to malaria. Malaria in pregnancy can cause perinatal mortality, low birth weight, and severe maternal anemia.
Severe malaria is defined by the WHO as malaria parasitemia (usually 5% or higher of circulating red blood cells infected), plus one of the following: prostration (inability to sit up without help), impaired consciousness, respiratory distress or pulmonary edema, seizures, vascular collapse, abnormal bleeding, jaundice, hemoglobinuria, or severe anemia (hemoglobin <50 g/1 or hematocrit <15%).
Mortality from severe malarial disease can exceed 20%, even with optimal management. Individuals with cerebral malaria present with altered consciousness, focal neurologic findings, and seizures, and mortality in these cases can reach 25%; survivors often have residual neurologic deficits.
Mortality from severe malarial disease can exceed 20%, even with optimal management. Individuals with cerebral malaria present with altered consciousness, focal neurologic findings, and seizures, and mortality in these cases can reach 25%; survivors often have residual neurologic deficits.
Table 5.1 Clinical manifestations of malaria in children | ||||||
|---|---|---|---|---|---|---|
|
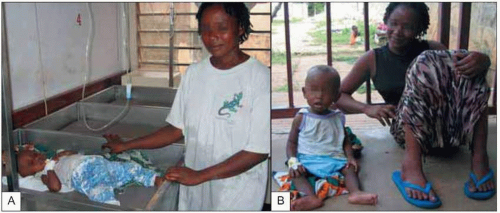 Fig. 5.7 Child being treated with quinine for severe malaria disease (A) and the same child the next day showing her recovery (B). |
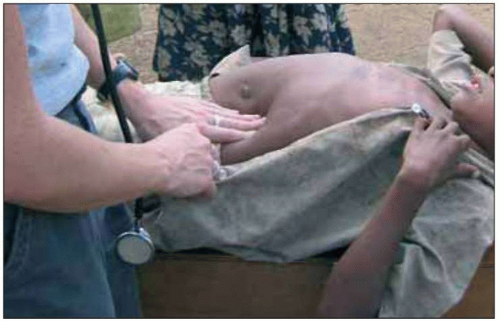
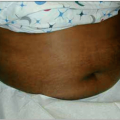
Malaria caused by P. vivax and P. ovale can make children very ill, but these infections rarely cause death in the absence of other disease (Fig. 5.7). Complications that are seen frequently include hepatosplenomegaly (Fig. 5.8), anemia, or late relapse (from liver hypnozoites). P. ovale infection may resolve without treatment. P. malariae infection can remain asymptomatic for much longer than P. falciparum or P. vivax infections, but can cause nephrotic syndrome.
Laboratory findings
Anemia and thrombocytopenia are common in clinically symptomatic malaria. On presentation, the leukocyte count is usually normal or low. Liver function tests may be abnormal, with elevated transaminases and bilirubin. Electrolyte abnormalities including hyponatremia (especially with cerebral malaria) and elevated creatinine may be seen. Hypoglycemia is rare on presentation, but with severe disease and/or therapy with quinine, it may represent a grave complication. Metabolic acidosis is seen with severe disease.