syndrome-associated coronavirus (SARS-CoV), human metapneumovirus (hMPV) and a novel strain of avian influenza A (H5N1).
Table 3.1 Respiratory viral infections and corresponding clinical syndromes in the immunocompetent host | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
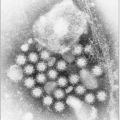
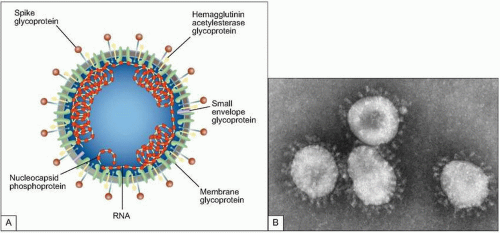 Fig. 3.2 A: Structure of coronavirus virion. (Adapted from Holmes KV. SARS-associated coronavirus. N Engl J Med 2003; 348(20):1948-1951.) B: The negative stain electron micrograph of coronavirus. Coronaviruses have a halo, or crown-like (corona) appearance when viewed under electron microscopy. (Courtesy of CDC.) |
Table 3.2 Spectrum of coronavirus species, hosts, and corresponding clinical syndromes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
likely the representative of a new fourth group of coronaviruses (Fig. 3.2). In fact the sequence analysis suggested that this new virus is an animal virus that has gained the ability to cross the species barrier. The SARS-CoV has four major proteins: the envelope (E), membrane (M), spike (S), and nucleocapsid protein (N). It is the S protein that binds to a species-specific host cell and along with the host’s immune responses determines the virulence of the organism. Several antigenically diverse strains were identified in China; however, only one went on to be responsible for the major outbreak and global spread of SARS.
 Fig. 3.3 Exotic animals regarded as a delicacy in Guangdong, China believed to harbor SARS-CoV. A: Masked palm civets (Paguma larvata). B: Raccoon dog (Nyctereutes procyonoides). |
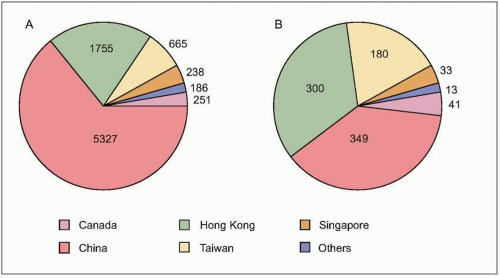
number of cases were in health care workers (greater than 40% of cases in China and Canada). The global average case fatality rate was just over 10% (Fig. 3.5); however, for patients requiring intensive care and/or mechanical ventilation, fatality rates greater than 50% were reached. Predictors of mortality include increasing age, presence of comorbidities, atypical initial symptoms, and some abnormal laboratory findings (Table 3.4).
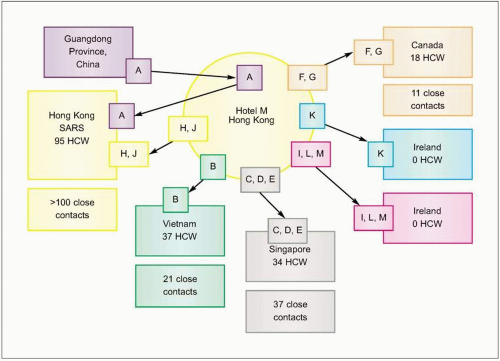 Fig. 3.4 Effect of travel and missed cases on the SARS epidemic. Spread from Hotel M, Hong Kong. Case A from Guangdong Province, China and two hotel guests who became ill, cases H and J, started outbreaks of SARS in three Hong Kong hospitals involving at least 95 health care workers (HCW) and more than 100 contacts. (Adapted from CDC data.) |
Table 3.3 Koch’s postulates as modified by Rivers for viruses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
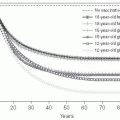
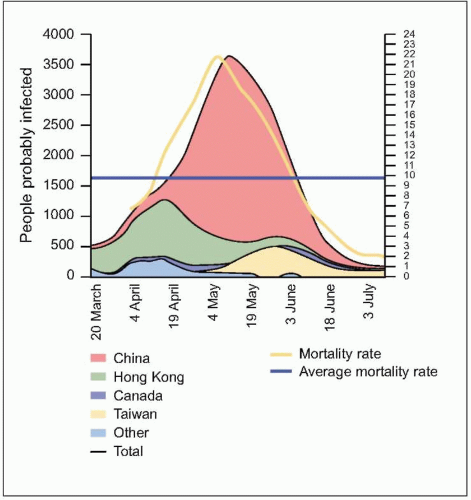 Fig. 3.5 The evolution of the people probably infected, by main countries (moving average of 7 days) and the mortality rates for the last 2 weeks. People probably infected = cumulative case x number of deaths x number of people discharged. Mortality rate = deaths / (deaths + discharged). (Adapted from WHO data.) |
Table 3.4 Risk factors for death or admission to an Intensive Care Unit | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
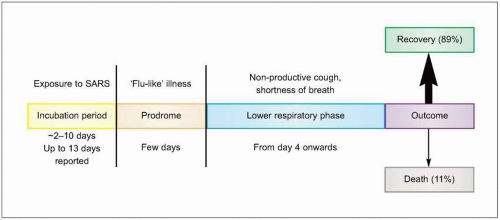
phase (Fig. 3.6). The incubation period varies between 2 and 10 days. Unlike many of the common viral respiratory pathogens, evidence suggests that there are rather few asymptomatic or mild illnesses associated with SARS-CoV, except for children, in whom the disease is uncommon, generally mild, and self limited. Typical adult illness begins with a prodrome of non-specific symptoms including high-grade fever, chills/rigors, myalgias, headache, and diarrhea (Fig. 3.7). Upper respiratory tract symptoms are less common. The clinical course tends to be insidious, and patients frequently improve transiently prior to developing lower respiratory symptoms in the second week of illness, with non-productive cough, dyspnea, and hypoxia. In 10-20% of hospitalized patients, symptoms progress to respiratory failure requiring intubation and mechanical ventilation. It is unclear whether this clinical deterioration is due to ongoing viral replication or uncontrolled immune response mediated by host defenses.
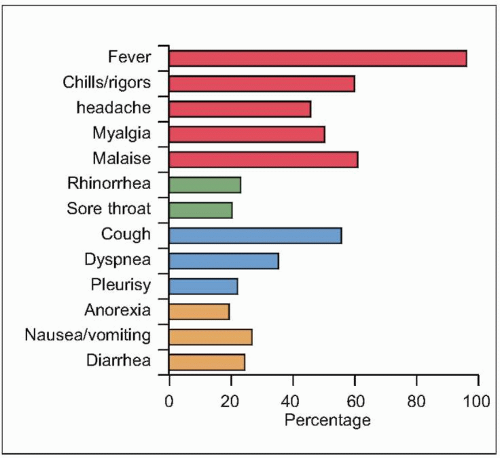 Fig. 3.7 SARS clinical symptoms at presentation. (Data from almost 2000 patients are compiled from several series, including Avendano M, et al., 2003; Booth CM, et al., 2003; Chan PK, et al., 2003; Donnelly CA, et al., 2003; Hsu LY, et al., 2003; Lee N, et al., 2003; Peiris JS, et al., (B), 2003; Poutanen SM, et al., 2003; Rainer TH, et al., 2003; Tsang KW, et al., 2003; Zhong NS, et al., 2003.) |
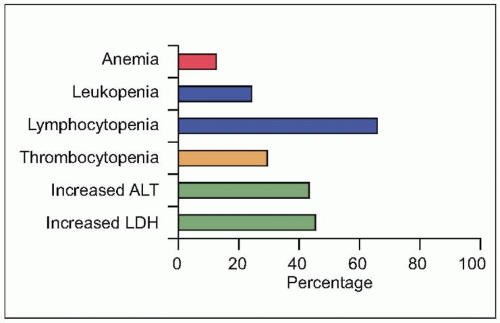 Fig. 3.8 Initial laboratory abnormalities in patients with SARS. (Data from close to 500 patients are compiled from several series, including Booth CM, et al., 2003; Hsu LY, et al., 2003; Lee N, et al., 2003; Peiris JS, et al. (B), 2003; Poutanen SM, et al., 2003; Tsang KW, et al., 2003; Zhao, Z, et al., 2003.) (LDH: serum lactate dehydrogenase level; ALT: serum alanine aminotransferase.) |
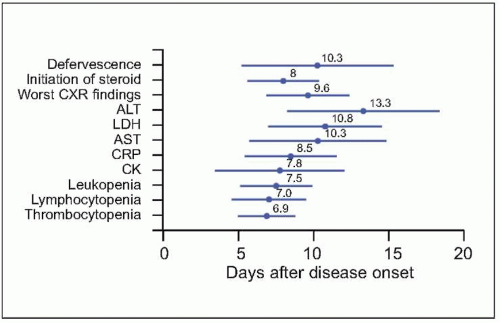 Fig. 3.9 The time relationships between the time points of defervescence, initiation of steroid, and when chest radiographic finding, as well as various laboratory parameters became most severe. Mean and standard deviation (days) are presented. (CXR: chest radiography; ALT: alanine aminotransferase; LDH: lactate dehydrogenase; AST: aspartate aminotransferase; CRP: C-reactive protein; CK: creatine kinase.) (Adapted from CDC data.) |
Table 3.5 Radiographic features of severe acute respiratory syndrome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree