Fig. 3.1
Axial image showing the difference between a high resolution (left) and lower resolution scan in the same patient (right). The polypoidal tumour is much more clearly depicted on the high resolution scan. Similarly the anatomic layers of the bowel wall are also more clearly shown
Summary Points—for MRI Staging Technique
The field of view and matrix parameters should not exceed a pixel size of 0.6 × 0.6 mm, e.g. 200 × 200 mm with 384 × 384 matrix or 160 × 160 mm with a 256 × 256 matrix (note pixel size (mm) is calculated as = field of view/matrix). Voxel size mm3 = pixel size × slice thickness.
The surface phased array coil should be placed correctly over the lower pelvis. For low rectal cancers the distal edge of the coil should lie 10 cm below the symphysis pubis to ensure that the distal rectum is in the centre of the image (Fig. 3.2).
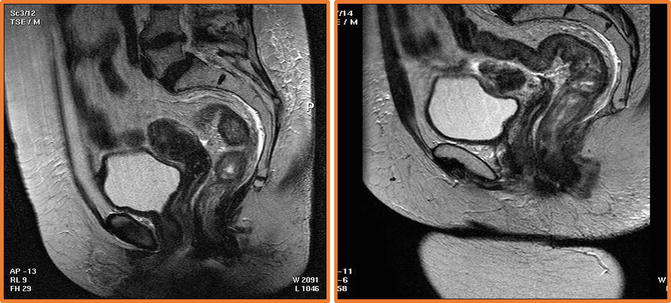
Fig. 3.2
The surface phased array coil should be placed correctly over the lower pelvis. The left hand image shows incorrect placement- the coil has been positioned too high so that there is insufficient signal from the lower third of the rectum. For low and mid rectal cancers the distal edge of the coil should lie 10 cm below the symphysis pubis to ensure that the distal rectum is in the centre of the image
Scans should be obtained perpendicular to the rectal wall; the sagittal MRI scans are used to plan the oblique axial images (Fig. 3.3).
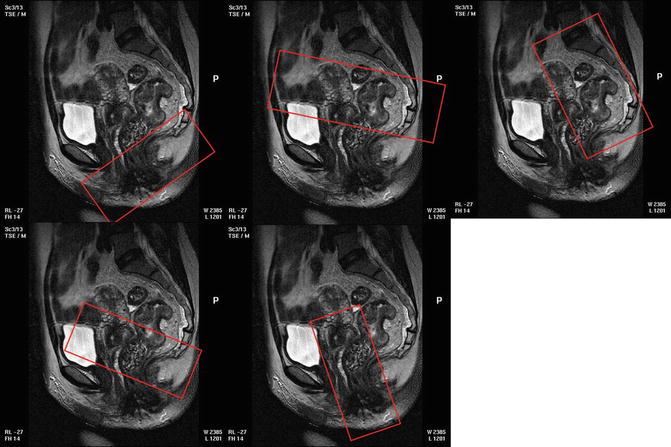
Fig. 3.3
Illlustrates how the sagittal scans can be used to plan the correct scan planes for oblique axial and coronal images that are perpendicular to the rectal wall and coronal to the anal canal
Coronal images should be undertaken parallel to the anal canal to visualise the distal mesorectal plane (Fig. 3.3) and must also be performed using the same high-resolution parameters.
The use of saturation bands reduces image degradation due to abdominal wall motion, and hyoscine butylbromide given as an i.m. injection or oral mebeverine reduces small bowel peristalsis, respectively (Fig. 3.4).
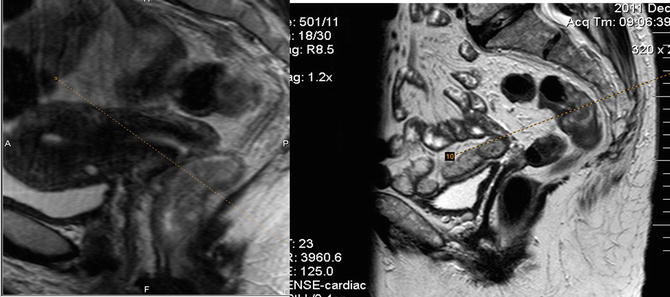
Fig. 3.4
The use of a “saturation bands” to suppress the signal from unwanted areas. For example the anterior abdominal wall produces movement degradation and so placing the saturation band over the abdominal wall (on the right hand image) reduces the image degradation. This is evident on the left hand image where no saturation band has been used
High-resolution coverage should include at least 5 cm above the top of the tumour and to the L5/S1 level for all tumours to ensure that discontinuous tumour deposits are visualised (Fig. 3.3).
T1-weighted imaging, contrast enhanced imaging and fat saturated sequences do not contribute and worsen staging accuracy and should not be used for primary rectal cancer staging.
Caution when using diffusion-weighted imaging for rectal cancer as it does not improve accuracy when compared with high-resolution MRI techniques.
The prolonged examination time caused by additional noncontributory sequences reduces the overall quality of the examination as well as prolongs patient discomfort.
Anatomic Considerations
The major utility of MRI lies in its ability to depict the surgical anatomic planes for preoperative roadmapping, thereby enabling a clear surgical approach that can be defined by the extent of tumour and its relationship to neighbouring structures.
Surgery for rectal cancers can be modified in accordance with the plane required to enable total clearance of the tumour. For the vast majority of patients presenting with rectal cancer, total mesorectal excision (TME) plane surgery enables the primary tumour and all the draining lymph nodes to be removed in an intact package with clear radial, distal and proximal margins. This approach has substantially reduced local recurrence rates from above 40% in non-TME series to 5% in patients where a clear margin is achieved and a good-quality TME specimen is shown. When all patients with rectal cancer are staged by MRI, the prevalence of potential involvement of the TME plane (mrCRM) is 26%, and the use of preoperative therapy enables tumour shrinkage that significantly reduces CRM positivity rates. For those patients that become either mr or pCRM negative as a result of preoperative therapy, the local recurrence rates are 7%, but for those with either persistence of tumour at the TME plane on imaging or pathologic involvement of the CRM, the rates of local recurrence are over 20%. Consequently, in a proportion of patients with persistence of tumour at the mesorectal margins, a beyond TME approach is required to achieve tumour-free resection margins. The major surgical landmarks for rectal cancer surgery are readily visualised using preoperative MRI.
The Mesorectum and Mesorectal Fascia
The rectum is somewhat unique having a mesenchyme that is encircled by a visceral fascial layer that encases the rectum, its draining nodes and neural and vascular structures. On MRI the fascial envelope is shown as a low signal intensity structure that encircles the mesorectum from the level of the distal levator inferiorly to the sacral promontory posteriorly. Anteriorly, the mesorectum ends at the level of the anterior insertion of the peritoneum. Therefore, anteriorly above the peritoneal insertion, the rectum is devoid of a fascia and is covered by the peritoneal serosa which gradually widens until the point of the sigmoid is reached. At this point the mesorectum is no longer anchored to the sacral concavity and is instead posteriorly surrounded by a relatively mobile sigmoid mesentery rooted by the sigmoid vascular branches to their vascular origin/confluence at the IMA and IMV.
Anteriorly Infiltrating Tumours
The peritoneum separating the pelvic and abdominal visceral compartments is seen on high-resolution scans as a low signal intensity layer. Anteriorly the peritoneum can be traced over the surface of the bladder and seminal vesicles or uterus before its attachment to the rectum in the midline. This is well seen on the sagittal images. The peritonealised surface of the rectum does not form part of the circumferential surgical resection margin, and so anterior tumours above this level are not considered as potentially margin involved but can still be infiltrating through the peritoneum. Such tumours have a risk of pelvic recurrence through transperitoneal spread.
The Ureteric Plane/Pelvirectal Space
This space is devoid of lymph node tissue but contains neurovascular structures as they pass forward from the sacrum to the anterior pelvic organs. When tumour is evident in this space, this will either be from direct spread out of mesorectal compartment, from peritoneal spread or from venous invasion.
The Rectovaginal Septum and the Urogenital Compartment
Below the peritoneal insertion anteriorly, a condensation of the rectogenital septum is manifest as a focal low signal intensity band-like thickening of the anterior mesorectum in the anterior midline. In males the fascia forms the plane separating the anterior mesorectum from the prostatic capsule and can be followed inferiorly to the perineal body in the midline (Fig. 3.5).
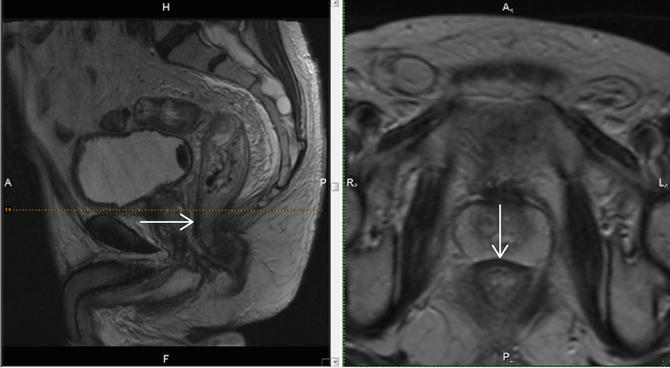

Fig. 3.5
The arrow points to the low signal intensity rectogenital septum which forms a band-like thickening that overlies the anterior mesorectum in the anterior midline
The pelvis can thus be divided into distinct anatomic compartments based upon the boundaries of the peritoneal reflection, the visceral (or mesorectal) fascia, the presacral fascia and the pelvic floor musculature.
The Parietal Fascia and the Pelvic Parietal Compartments (Presacral and Lateral Pelvic Compartments)
Where tumours extend beyond the conventional mesorectal compartment and beyond the mesorectal fascia, patients can then be appropriately referred for a therapeutic strategy that will enable clearance of tumour from undertaking beyond TME plane surgery that could range from adjacent organ removal to total pelvic exenteration depending on the compartmental distribution of tumour as shown on the preoperative MRI.
The Infralevator Compartment (Fig. 3.6)
The levator muscle forms a single sheet of muscle forming the pelvic floor. In the midline, its posterior proximal attachment is seen at the tip of the coccyx , and laterally it forms a ‘hammock-like’ structure on both sides around the mesorectum, with a further point of fixed attachment on both sides at the ischial spines (best seen on the coronal image—Fig. 3.6); laterally the muscle fuses with the obturator fascia, and inferiorly the fibres blend with the puborectalis sling whose anterior fibres attach to the inner surfaces of the upper pubic symphysis on either side of the midline. The lower third of the rectum is defined anatomically from the point of the levator attachment at the tip of the coccyx posteriorly to the levator’s most distal point at the level of the puborectalis sling. This represents a surgically challenging portion of the TME dissection where the mesorectum starts to taper and the proximity of the rectum to the adjacent anterior urogenital compartment limits the space. For tumours arising in this lower third segment of the rectum, even minimal spread beyond the rectal wall, mesorectal fat plane is small and can result in potential CRM involvement.
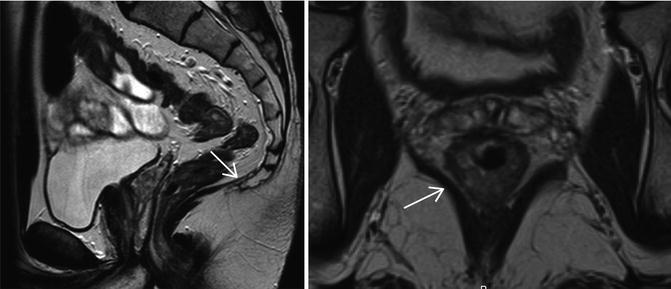

Fig. 3.6
The levator muscle is (arrows) a single sheet of muscle forming the pelvic floor. In the midline, its posterior proximal attachment is seen at the tip of the coccyx, and laterally it forms a ‘hammock-like’ structure on both sides around the mesorectum its most distal point is at the level of the puborectalis sling. The levator origin and its distal insertion effectively defines the anatomic lower third of the rectum where the mesorectum starts to taper resulting in the most challenging portion of surgical dissection in TME rectal cancer surgery
MRI and Local Rectal Cancer Staging
The development of high-resolution MRI and carefully validated image interpretation criteria has created the unique advantage of identifying prognostic factors that predict for the risk of local and distant failure before treatment commences. This precision in preoperative staging was previously not available and can now be used for the benefit of all patients with rectal cancer. For the surgeons and oncologist managing the patient, there is a consequent opportunity to tailor the surgical and preoperative therapeutic approach to reduce the risk of recurrence.
T Classification
For all cancers, the direct relationship between depth of tumour spread and prognosis is a well-established basis for the TNM classification. For patients with rectal cancer, there is a near-linear relationship between outcomes and degree of spread into and beyond the rectal wall. The ability to closely reproduce the low power haematoxylin and eosin stained depiction of tumour of the resection with the high-resolution T2-weighted images can afford millimetre accuracy in stage assessment that enables far more precise prognostication than the broader T categories and stage categories of the traditional AJCC/TNM systems.
Preoperative Assessment of T1 Tumours
Tumour depth into the submucosa can be measured to the nearest millimetre, and the depiction of a preserved submucosal layer can now be used to select patients for a primary local excision approach that avoids the morbidity of major radical surgery. On high-resolution scans , the submucosal layer is of brighter signal than the tumour, and its preservation enables the identification of a patient with a likely T1 cancer (Fig. 3.7).
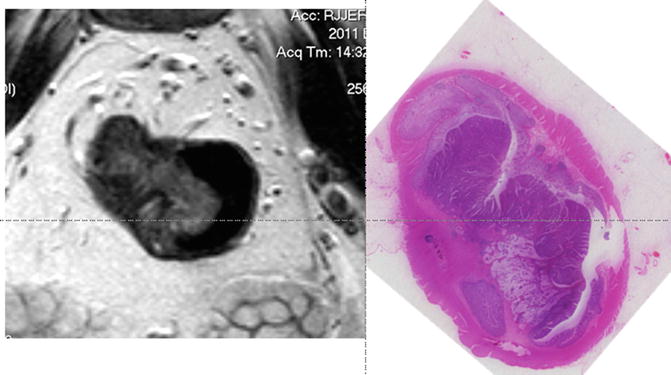

Fig. 3.7
MRI image and corresponding histopathologic section of a T1 tumour
T2 Tumour Spread
The muscularis propria is characterised by the following features: low signal (dark) intensity relative to tumour. It is formed by two layers —the circular and longitudinal muscle coat. The latter is seen as a discontinuous layer with vertically arranged low signal intensity bundles and separated from the inner circular layer by a distinct high signal layer of the myenteric plexus. The degree of muscularis propria preservation enables differentiation between an early invasive T2 and deeper T2 tumour inseparable prognostically from an early T3 tumour (Fig. 3.8).
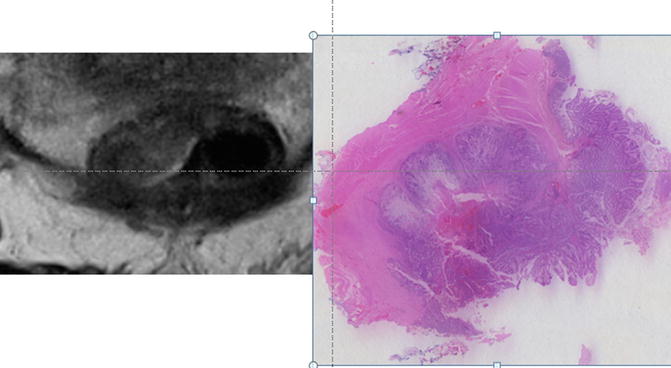

Fig. 3.8
MRI image and corresponding histopathologic section of the T2 tumour
Early T3 Tumours
As tumour advances, the degree of muscularis preservation diminishes until finally tumour is seen to completely replace the muscularis propria layer. Such tumours can be classified as full thickness T2 or T3 <1 mm. These are prognostically identical tumours with a low likelihood of spread to lymph nodes or distant spread and unless bordering the intersphincteric plane readily amenable to cure by primary surgical total mesorectal excision (Fig. 3.9).
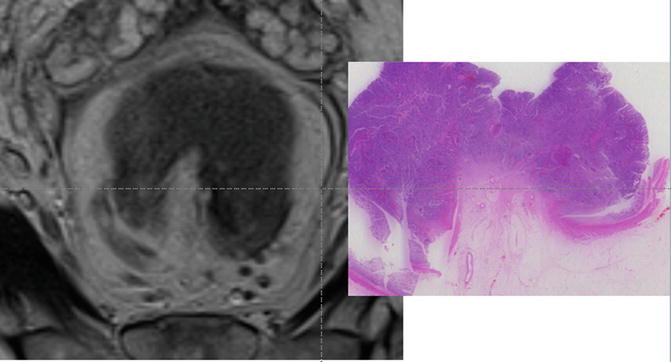

Fig. 3.9
MRI image and corresponding histopathologic section of a T3 tumour
When tumour has clearly spread beyond the muscularis propria, the depth of extramural invasion is an independent prognostic factor [1, 2]. This is defined as the depth in millimetres of tumour spread beyond the outer edge of the muscularis propria. Tumours with less than 1 mm spread have exactly the same prognosis as T2 tumours. Spread between 1 and 5 mm is also associated with cancer-specific survival rates that are similar to T2 tumours regardless of lymph node involvement. For patients with tumour spread beyond 5 mm, there is a consistent reduction in cancer-specific survival, with an increased propensity to distant metastatic disease and local recurrence.
Since the majority of rectal cancers diagnosed comprise T3 tumours, there is merit in addressing the inherent heterogeneity by taking into account the depth of extramural spread. Merkel and others [3] showed that pT3 rectal cancers could be usefully subdivided according to depth of extramural spread as follows: pT3a minimal invasion, <1 mm beyond the border of the muscularis propria; pT3b-slight invasion, 1–5 mm beyond the border of the muscularis propria; pT3c-moderate invasion, >5–15 mm beyond the border of the muscularis propria; and pT3d-extensive invasion, >15 mm beyond the border of the muscularis propria [4]. This prognostic classification based on a study of 850 patients in the Erlangen Cancer Registry was proven to predict survival regardless of nodal stage. This was an important observation as of all parameters assessable on MRI, depth of tumour spread showed the greatest agreement with pathology showing a mean agreement of 0.5 mm when compared with pathology [5]. Since survival and local recurrence outcomes for T2 and early T3 tumours are identical and with increasing use of precision depth of spread measurements afforded by preoperative high-resolution MRI, preoperative decisions regarding chemoradiotherapy (CRT) are increasingly based on the 5 mm depth of extramural spread cut-off rather than the T2/T3 boundary. As further evidence to support this practice, a 5-year follow-up from the MERCURY study, Taylor et al. showed that MRI staged rectal cancers with <5 mm depth of spread 85% 5-year overall survival and 3% local recurrence rates, thus supporting the use of a more rigorous preoperative stratification of rectal cancer patients [6].
Circumferential Resection Margin (CRM)
The importance of tumour spread within 1 mm of the surgical circumferential resection margin (CRM) is well known [7, 8]. The appearance of the predicted CRM on MRI was first described in 1999 and was shown as a low signal intensity structure surrounding the mesorectum on high-resolution imaging. High-resolution MRI is the most accurate imaging modality for consistently identifying the mesorectal fascia and thus the CRM [9]. The first prospective study was conducted in 98 patients and showed that if the distance of tumour was 1 mm or less to the mesorectal fascia, this predicted pathological CRM involvement with 92% agreement (kappa = 0.81) [10]. The high-resolution technique needed to be strictly adhered to but was considered that if the protocol was adhered to and image interpretation criteria established by Brown et al. were followed, then this could be reproduced in a multicentre setting. In 2003, the MERCURY group comprising 11 hospitals in the UK and Europe prospectively tested the hypothesis that tumour depth and distance to CRM could agree with histopathology findings in a multicentre setting. Following TME surgery 327 (94%) patients were found to have clear margins on histopathology [11]. The specificity was 92% (CI 90–95%). The group showed a high risk of pCRM involvement if tumour distance to the mesorectal fascia was 1 mm or less. In a follow-up paper evaluating outcomes of these patients, MRI proved to be as likely to predict the risk of local recurrence as pCRM involvement. When the MRI suggested that the tumour was ≤1 mm from the mesorectal fascia, there was a 20% local recurrence rate compared with 7.1% in the mrCRM ‘clear’ group [6, 12]; the local recurrence rates were identical for patients with pCRM involvement. The group also showed that increasing the threshold for risk of CRM involvement by widening this distance to 2 mm or more did not improve the prediction of likely CRM involvement and would result in substantial overtreatment and toxicity for such patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree