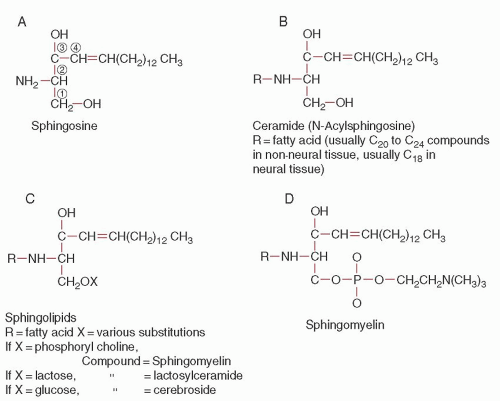
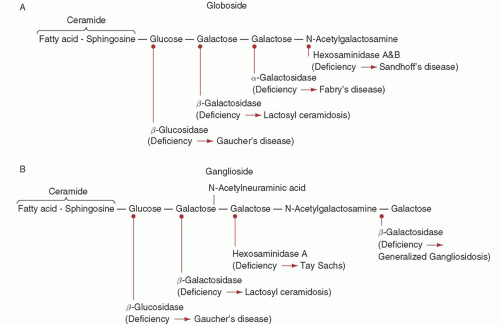
storage disease and one of the most prevalent genetic disorders among Ashkenazi Jewish individuals, with an incidence of about 1 in 1,000 and a carrier frequency of about 1 in 15.3
onset and a milder course than patients with one N370S allele and another mutant allele. However, the wide variability in clinical presentation among Gaucher disease patients cannot be fully explained by the underlying acid β-glucosidase mutations, and presumably other “modifier” genes can influence disease severity.
TABLE 59.1 BIOCHEMICAL AND PHENOTYPIC CHARACTERISTICS OF GAUCHER AND NIEMANN-PICK DISEASES | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
TABLE 59.2 MOLECULAR BASIS OF GAUCHER AND NIEMANN-PICK DISEASES | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree