The Vector
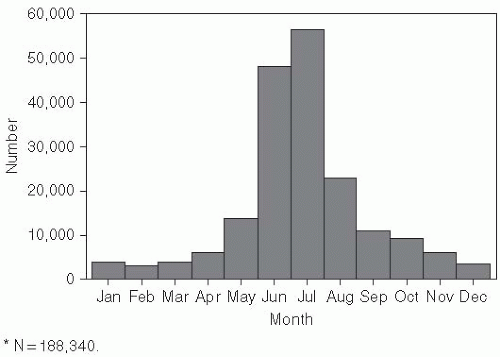
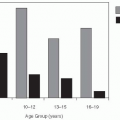
Cases of Lyme disease occur primarily in the summer (
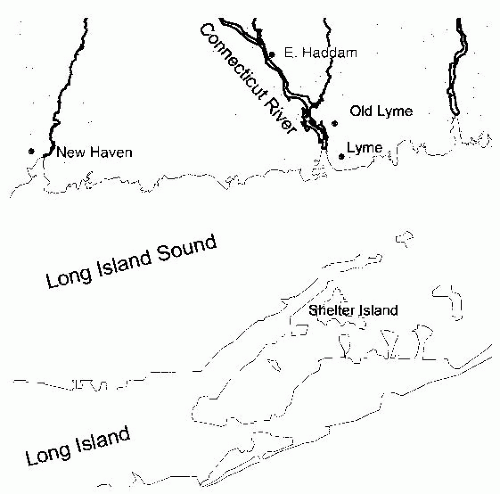
Figure 26-2), indicating that the disease is seasonal and consistent with the possibility suggested by one of the original patients that it was tick borne. Therefore, in the summers of 1976 and 1977, ticks and patients in the areas around the Connecticut River were studied.
3,
4 and
5 A surveillance system was established using healthcare providers in the area around Old Lyme, Connecticut, including both sides of the river. To enhance surveillance, introductory lectures were conducted at three southern Connecticut hospitals, and specialists in dermatology, rheumatology, pediatrics, and internal medicine were contacted.
The broad scope of the surveillance program was intended to reduce bias in the collection of cases. A case was defined as a recent episode of EM or a diagnosis of Lyme arthritis. Forty-three new cases of Lyme disease were identified, mostly on the east side of the Connecticut River, where the incidence was 2.8 cases/1000 residents. The incidence was 0.1 case/1000 residents on the west side of the river—a difference of almost 30-fold. Nine of these 43 individuals (21%) remembered a tick bite at the site of the initial skin lesion within the 3 previous weeks. One individual had saved the tick, which was identified as the deer tick Ixodes scapularis.
For each case, 2 control participants were chosen from neighbors who lived within 150 feet of the case. In addition to matching on local environmental conditions, cases and controls were matched by age and sex. Epidemiologic investigation (
Table 26-1) revealed that the patients with Lyme disease had more cats and farm animals and had more often noted ticks on their pets and themselves than their neighbors without disease.
4Analysis of the ticks collected showed that
Ixodes scapularis (occasionally referred to as
I. dammini) was much more abundant on the east than
on the west side of the Connecticut River. Immature
I. scapularis were 13 times more abundant on whitefooted mice
(Peromyscus leucopus) and adult
I. scapularis were 16 times more abundant on whitetailed deer
(Odocoileus virginianus) in communities on the east side compared to the west side of the river (
Table 26-2). Although no pathogen was isolated from the ticks or the people with Lyme disease, these data provided strong epidemiologic evidence for a tick-transmitted agent as the cause of Lyme disease.
5Extensive searches for the etiologic agent of Lyme disease using a wide variety of culture techniques proved unsuccessful. However, the agent was identified in the summer of 1981 when Willy Burgdorfer, a medical entomologist specializing in the study of ticks as vectors of infectious agents, was analyzing ticks from various parts of Long Island, New York. As part of his studies, he noted the presence of spirochetes in the midguts of most
Ixodes ticks collected on Shelter Island, which is located directly across Long Island Sound from the mouth of the Connecticut River (
Figure 26-1). In an indirect immunofluorescent assay, these spirochetes were stained by sera from patients with Lyme disease, but not by sera from individuals without a history of Lyme disease,
6 suggesting an etiologic link to the disease. In retrospect, organisms morphologically characteristic of spirochetes had been associated with EM in Europe in 1948,
7 but had not become accepted as the EM infectious agent. Subsequently, the Lyme disease spirochete was isolated from the blood and tissues of patients with Lyme disease
8,
9 and was identified as a new species of
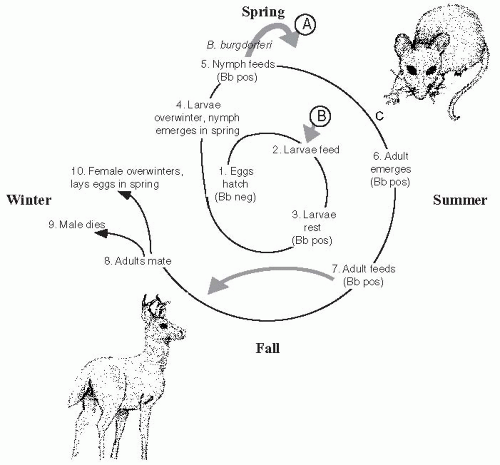
Borrelia, Borrelia burgdorferi.10I. scapularis, like most hard ticks, has a complicated life cycle that requires 2 years to complete and includes progression through the stages of egg, six-legged larvae, eight-legged immature nymph and the eight-legged reproductively mature adult tick (
Figure 26-3). At each stage, a blood meal is required for morphogenesis and progression to the next stage. Adult ticks lay eggs in the early spring that hatch to become larvae.
Ixodes, like all tick species, walk to the ends of grasses and tree leaves, where they “quest,” front legs waving in the air, until a suitable host brushes past them (
Figure 26-4). Ticks do not fly, hop, drop, or jump to their next meal. The larvae feed once and then rest for the remainder of the year. The following spring, nymphs emerge and feed once.
The white-footed mouse is a primary host for immature
I. scapularis and develops persistent infection with
B. burgdorferi,11 but other mammals,
reptiles, and birds, which are variably susceptible to persistent infection with
B. burgdorferi, are also fed upon by
I. scapularis ticks. Both nymphs and adults will feed on humans and can transmit Lyme disease.
12 After the nymph feeds, the adult emerges and feeds once in the summer/fall. Usually, adults feed on large mammals such as domestic pets, humans, and deer. Adults mate preferably on white-tailed deer. The male tick dies, while the female overwinters and lays eggs the following spring.
Interstage and vertical transmission of
B. burgdorferi is rare (less than 0.1%), so eggs are not commonly infected.
13 The larvae may acquire the spirochete at the first feeding, and tick saliva contains proteins that facilitate acquisition of
Borrelia from an infected vertebrate host.
13 The chance of the first host being infected depends on its susceptibility to
B. burgdorferi infection and on being previously bitten by an infected tick. The white-footed mouse, for example, has many litters of pups each year. Because vertical transmission is rare, mice infected in the previous year will not pass their infection on to the next generation. Instead, nymphal ticks, which emerge in the spring, are responsible for transmitting
B. burgdorferi to the next generation of mice. If nymphal forms of the tick emerge and feed before the larvae hatch, as is the case in the northern United States and Canada, host populations have a higher prevalence than in regions where larvae may hatch and feed before the nymphs, which is the case in the southern United States. This order of feeding, combined with feeding on
B. burgdorferi-incompetent hosts in the southeast United States, explains the high prevalence of
B. burgdorferi in
Ixodes ticks from the northeastern United States (50%) as compared to the southeastern United States (1%).
14The distribution of
I. scapularis is probably determined by the need for high humidity and availability of host species, particularly deer.
15 Populations of this tick are abundant in the United States in the northeast and upper Midwest and in southern Canada.
15,
16 Related ticks—
I. pacificus, the western black-legged tick;
I. ricinus, the sheep tick; and
I. persulcatus—are the primary vectors for Lyme disease along the Pacific Coast of North America, in Europe, and in Asia.
14,
15,
17 The enzootic cycles of
B. burgdorferi on the Pacific coast and in the southeastern United States are maintained primarily between reservoir rodents and species of
Ixodes ticks (e.g.,
I. spinipalpis, I. affinis, I. minor) that rarely bite humans.
18 The immature forms of bridge vector ticks that do bite humans (e.g.,
I. scapularis, I. pacificus) have a variety of suitable hosts including lizards, which are not susceptible to
B. burgdorferi,19 resulting in a low penetrance of
B. burgdorferi in these regions.
14
The Infectious Agent
Borrelia are motile, helical, gram-negative spirochetal bacteria that are maintained in zoonotic cycles involving a variety of wild mammals and birds as reservoirs. By definition, reservoir species are hosts that are commonly infected with an organism and remain infectious for the vector for prolonged periods of time.
11,
20 For
B. burgdorferi, this is determined largely by ability of the host complement system to inactivate the spirochetes.
21,
22 and
23 B. burgdorferi has been isolated from the blood of white-footed mice
(Peromyscus leucopus), which are abundant, highly susceptible to persistent infection, and a preferred host of
I. scapularis at early stages of the life cycle (
Figure 26-5), and do not become resistant to repeated tick feeding.
11,
18,
24 Vector competence describes the inherent ability of an
arthropod to become infected with the organism and subsequently to transmit the infectious agent to a new vertebrate host. Larval ticks acquire
B. burgdorferi when they feed on infected mice, persistent infection is established in the tick, and all subsequent stages of the vector remain infected unless
Borrelia in the midgut are inactivated through subsequent feeding on an incompetent host.
20,
22,
25 Infected nymphal ticks then transmit
B. burgdorferi to uninfected mice. Data indicate that mice are the most important reservoir species for maintaining the invertebrate cycle of infection and that the abundance of this host in endemic areas correlates with the risk of infection.
26 Deer, although important for the tick life cycle, have blood that can inactivate
Borrelia and are dead-end hosts.
15,
22
The genome of
B. burgdorferi is small (fewer than 1.5 megabases) and consists of an unusual linear chromosome and more than 20 linear and circular plasmids that encode a variety of virulence factors, including lipoproteins.
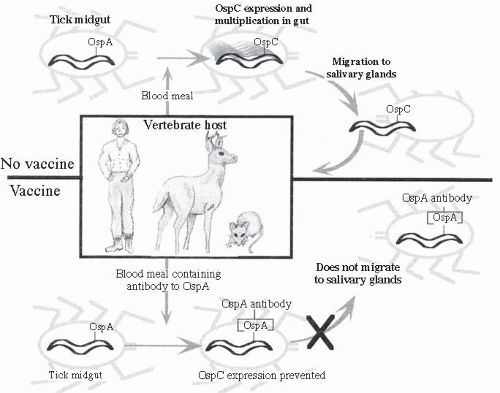
27 The structure includes an inner membrane, an outer membrane, and periplasmic flagella. The primary outer surface lipoproteins (Osp) vary antigenically between strains and can undergo phase shifts as an important means of organism adaptation to growth in vertebrate and invertebrate hosts.
28,
29 and
30 OspA, which has multiple distinct antigenic variants, and OspB are encoded on a bicistronic operon and expressed on the surface of spirochetes within the midgut of unfed ticks. OspA binds to a tick receptor TROPSA and, along with OspB, promotes adherence and survival in the tick midgut that is essential for
B. burgdorferi colonization.
31,
32,
33 and
34 When infected nymphs take a blood meal, the
Borrelia cease expression of OspA, thus releasing them from the gut, and begin to express OspC (
Figure 26-6). This switch is induced in part by the increase in temperature and decrease in pH in the tick midgut associated with taking a blood meal.
35Infected ticks have several hundred
B. burgdorferi in the gut lumen. When a blood meal is taken, the organisms begin to proliferate and increase their numbers more than a hundred-fold. The members of this expanded population of
Borrelia cross the midgut epithelium into the hemolymph, and then enter the salivary glands. It takes approximately 60 hours after tick attachment for sufficient numbers of organisms
to be present in salivary glands for infection of a new host.
36,
37,
38 and
39 OspC is the primary surface antigen expressed by
B. burgdorferi in vertebrate hosts and is important for successful infection and subsequent dissemination.
40,
41,
42,
43 and
44 This lipoprotein is polymorphic, with specific versions of OspC being associated with infection of different vertebrate hosts and potentially with severity of human disease.
29,
45,
46,
47 and
48Other lipoproteins expressed by
B. burgdorferi that are important during tick feeding and host infection include proteins that bind complement regulatory factors in host plasma.
Borrelia are susceptible to immobilization and lysis through the alternative complement pathway.
21 Resistance to complementdependent lysis depends on the ability of
Borrelia to bind factor H and/or factor H-like proteins in the blood meal through complement regulatoryacquiring surface proteins (CRASPs). Complement regulatory factors in host plasma control the alternative pathway of complement activation at the level of C3b and prevent lysis by complement bound to a surface.
49 B. burgdorferi produces three families of CRASPs that confer resistance to complement present in plasma.
50,
51 and
52 This circumvention of innate immunity allows persistent infection in the vertebrate host and prevents lysis in the tick’s gut.
Eighteen genospecies of
B. burgdorferi sensu lato are now recognized, of which three—
B. burgdorferi sensu stricto, B. afzelii, and
B. garinii—are associated with human disease.
53 Each of these genospecies has a different reservoir host and is associated with different clinical manifestations of infection.
54,
55 An important determinant of vertebrate host and tick infection is susceptibility of the
Borrelia genospecies to complement-dependent lysis.
22 For instance, sera from lizards and deer can lyse
B. burgdorferi s.s. and
B. afzelii, while sera from mice and humans cannot. In contrast,
B. garinii is lysed by serum from mice, but not by serum from birds, the reservoir host for this genospecies.
23