Abstract
Mastectomy surgery has been historically judged by its success at preventing local failures after primary invasive breast cancer treatment. Advancements in the use of adjuvant systemic treatments have further reduced the frequency of locoregional recurrences [LRRs], which commonly present as isolated events. Skin-sparing mastectomies, neoadjuvant therapy, and use of sentinel node staging have not increased LRR rates. Chest wall recurrences predominate, and most LRR are clinically detected and operable. The risk of developing a LRR depends of age, tumor size, nodal status, and molecular subtypes as well as prior treatments. Surgical resection and postoperative radiation therapy are therapeutic modalities routinely used to achieve control local disease after LRR. The risk of developing distant metastases and death is elevated. Endocrine and chemotherapy regimens have significantly improved outcomes for this patient population. Specifically, the CALOR trial demonstrated that overall survival was significantly improved with chemotherapy (hazard ratio for death of any cause 0.41; 95% confidence interval 0.0.19–0.89; p = .024), corresponding to a 5-year survival of 88% versus 76%. The recommended drug regimens should be individually tailored based on tumor characteristics and prior treatments.
Keywords
Chest wall recurrence, mastectomy, axillary recurrence, breast cancer
Preventing locoregional failure of breast cancer is still a major objective of treatments today. Since the late 19th century, the effectiveness of mastectomy surgery has been judged by its success at controlling local disease. The use of adjuvant systemic treatments has improved overall outcomes and reduced the frequency of locoregional recurrence(s) (LRRs). According to the US National Cancer Database, breast conserving surgery has now surpassed mastectomy, with or without nodal staging, as the most common operation performed for the treatment of early-stage breast cancer. Advances in breast reconstruction have changed the mastectomy landscape with a shift toward skin- and nipple-areolar–sparing mastectomies and the increased use of contralateral prophylactic mastectomies.
LRRs have been linked to a significantly elevated risk of distant metastases. Because most LRRs precede distant metastases by relatively short time intervals, it is argued that these events are significant indicators of occult disseminated disease. The CALOR (Chemotherapy as Adjuvant for LOcally Recurrent breast cancer) trial results have provided evidence-based rationale for the use of systemic treatments after LRR. It is the first prospective randomized multicenter trial to show significant improvement in disease-free survival (DFS) and overall survival (OS) with physician selected chemotherapy after LRR. Thus essential management strategies for isolated LRR (ILRR) have evolved by addressing the likelihood of distant relapse in addition to refining definitive local interventions. The following sections of this chapter describe the prevalence, modes of presentations, prognosis, and management of LRR after mastectomy.
Definitions
Local failures can occur months to years after mastectomy surgery. In the words of William S. Halsted in 1894, “Local recurrence is the return of the disease in the field of operation.” Although the majority of LRRs occur in patients with a prior invasive cancer, approximately 1% arise in mastectomy-treated ductal carcinomas in situ. Most LRRs present as isolated events and less frequently accompany or follow the diagnosis of distant metastasis. The time interval between recurrences and the appearance of distant metastases can be brief, so it is plausible that these relapses are misclassified as either ILRR or distant metastases.
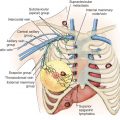
An LRR can involve chest wall skin, subcutaneous tissues, chest wall muscles, or regional nodal areas of the ipsilateral breast ( Figs. 61.1 through 61.3 ). Recurrent cancers are classified according to their anatomic location: skin, mastectomy scar, chest wall, regional lymph nodes (axillary, internal mammary, infraclavicular or Rotter, and supraclavicular), or extranodal recurrences. Contralateral nodal recurrences are excluded and have been defined by the Maastricht Breast Cancer Endpoints Consensus Panel as distant disease rather than a LRR. From a management perspective, LRRs are classified as either operable or inoperable.
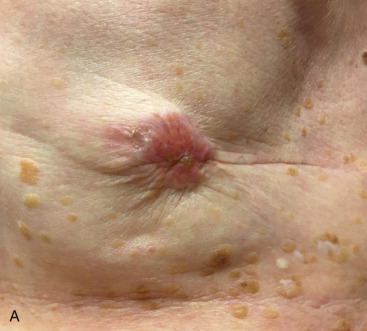
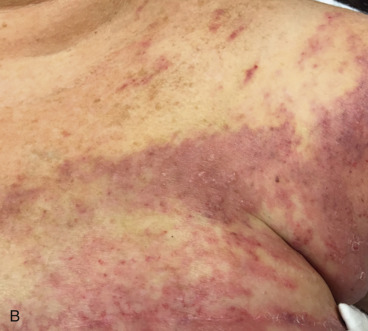

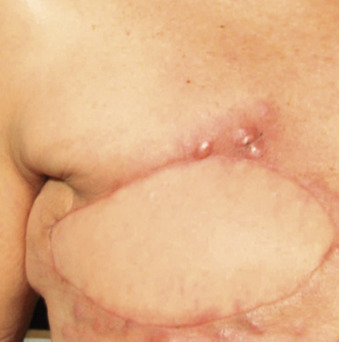
Incidence
The incidence of a LRR is influenced by the molecular characteristics of the primary cancer, stage of disease, extent of surgery, and adjuvant treatments. Systemic regimens have significantly changed in the last few decades, resulting in a lower incidence of locoregional events in some cancer subtypes. Before routine HER2 testing, 3% to 32% of patients with stage I to III breast cancer developed a LRR within 10 years of mastectomy, either as a first event or accompanying distant disease. Approximately 80% of these recurrences are detected within the first 5 years. Node-positive and hormone receptor–negative recurrences tend to present earlier than node-negative and hormone receptor–positive tumors.
The radical mastectomy experience from the National Surgical Adjuvant Breast and Bowel Project (NSABP) provides a frame of reference for examining the incidence of first ILRR treated by surgery alone. In this setting, LRR events were less common than distant metastasis. The 25-year follow-up of this trial found rates of local failure after radical mastectomy to be 5% and 8% and for regional recurrence 4% and 8% in node-negative and node-positive cancers, respectively. In general, skin/chest wall recurrences were the most common, representing 49% to 83% of LRRs. By comparison, nodal recurrences were less frequent constituting 15% to 52% of cases.
Higher disease stage, aggressive tumor biology or tumor subtype, and younger age are some of the prognostic factors associated with higher rates of local recurrence. LRR rates in patients treated with neoadjuvant chemotherapy are similar to those who undergo surgery upfront. Although the literature on the subject is sparse at this time, the incidence of LRR is seemingly related to the degree of tumor response in breast or regional nodes. This topic is discussed in greater detail later in the chapter.
Chest Wall Recurrences
In a pooled analysis of 5352 participants in International Breast Cancer Study Group (IBCSG) trials I through VII, 21.3% experienced a LRR event as a first relapse, and 53% of these were local or chest wall failures. Another series spanning the years 1980 to 2004, reported a 10-year cumulative incidence of LRR for T1 and T2 node-negative cancers of 5.2% with 73% of these recurrences involving the chest wall. However, in the absence of systemic treatments when risk factors such as larger tumor size, lymphovascular invasion, and positive margins are present, the incidence of LRR was 19.7%.
As mastectomy techniques have evolved to less disfiguring skin and nipple-sparing mastectomies, higher rates of LRR could have been expected but have not been found. Specifically, in a study-level meta-analysis of nipple-sparing mastectomies, nipple recurrence were found to be acceptably low at 1.4% after 3 to 5 years. These operative procedures are being extended to BRCA1 and BRCA2 mutation carriers and to patients who have received neoadjuvant therapy. Importantly, there is no indication that LRR rates are measurably higher with these more natural appearing mastectomy procedures.
Invasive locoregional skin or chest wall recurrences are uncommon in mastectomy-treated ductal carcinomas in situ. In a series of 10 patients, recurrences were diagnosed 1 to 16 years after mastectomy. Not surprisingly, residual breast tissue was identified in half of these LRR.
Nodal Recurrences
The extent of axillary nodal surgery has changed greatly since the era of radical mastectomy, when level I to III axillary node dissection was the norm. Regional recurrences ranged from 4% to 8%, with the bulk of these events occurring in the first 5 years. In their pooled analysis of modified radical mastectomy-treated women in seven randomized trials, Wallgren and colleagues found that 26% of LRR involved supraclavicular/infraclavicular nodes compared with 13% in axillary nodes.
Before sentinel node biopsy, recurrence rates were significantly higher when fewer axillary nodes are removed or in the presence of extranodal extension. Weir and colleagues reported that when fewer than 6 nodes were removed during axillary dissection the rate of recurrence was 5% compared with 1%, when more than 10 lymph nodes were excised. LRR are lower in node positive breast cancers as the number of uninvolved lymph nodes resected is higher.
The management of the axilla has shifted toward a selective approach with the implementation of lymphatic mapping and sentinel node biopsy. Local recurrence rates of less than 2% have been reported in clinically node-negative breast cancers after sentinel node biopsy, which indicates that the incidence of recurrences in the axilla is comparable to that observed with axillary node dissections. Lymphatic mapping and sentinel node biopsy after neoadjuvant therapy is gaining acceptance largely based on similar false-negative sentinel node identification rates.
Extraaxillary nodal recurrences refer to supraclavicular, infraclavicular (level III or interpectoral nodes), or internal mammary locations. In newer studies, supraclavicular nodal recurrences are the second most common site after the axilla. Until recent revision of clinical staging by the American Joint Committee on Cancer, supraclavicular nodes were considered synonymous with stage IV disease. Clinical detection of infraclavicular and internal mammary nodal recurrences are rare (see Fig. 61.2 ). However, with the increasing use of new generation computed tomography (CT) scanners and the incorporation of magnetic resonance imaging (MRI) and positron emission tomography (PET)/CT subclinical detection is becoming more prevalent.
Prior Radiation Therapy
Local failure rates vary with the extent of adjuvant treatments administered for the primary cancer. The Early Breast Cancer Trialists’ Collaborative Group studied the effects of comprehensive adjuvant radiotherapy, defined as supraclavicular, axillary, and internal mammary nodal basins, using individual patient level data from 22 randomized trials. There was no statistically significant effect on 10-year cumulative risk of ILRRs after mastectomy and axillary dissection for women with pathologically negative nodes. However, among the 3131 patients with positive nodes, postmastectomy radiation therapy decreased the overall rate of ILRRs from 26.0% (no RT) to 8.1% (RT), log-rank 2 p < .00001. Radiotherapy significantly decreased first LRRs from 20.3% to 3.8% in the group with one to three positive nodes, and from 32.0% to 13.0% in those with four or more. Similarly, radiation improved local control in women who had axillary sampling instead of dissection, reducing the risk of LRR by 0.61.
In a retrospective analysis of 1000 patients from the Danish Breast Cancer Cooperative Group 82 b&c clinical trials, the greatest absolute reduction in LRRs was in the subgroup of patients with unfavorable prognostic characteristics. Notably, in HER2-positive breast cancers, the rate of LRR with trastuzumab and postmastectomy radiation therapy was 0% at 5 years, indicating remarkable synergism between the two treatment modalities ( Table 61.1 ).
| Author/Year/Study Period | N | FU (y) | Receptor | T/N Stage | Systemic Tx | XRT (%) | LRR (%) |
|---|---|---|---|---|---|---|---|
| Peterson 2014 (1998–2009) | 326 | 5 | HER2+ | T1–T2, N0 | Without trastuzumab | 0 | 2.6 |
| Trastuzumab | 0.6 | ||||||
| Lanning 2015 (1998–2007) | 501 | 5 | HER2+ | T1–T4 49% N0 | Without trastuzumab | 25 | 6.5 |
| 5.6 | |||||||
| T1–T4 35% N0 | Trastuzumab | 38 | 0 | ||||
| 2.8 | |||||||
| Tseng 2015 (1997–2012) | 1090 | 5 | HER2+ | T1–3 59.5% N0 | Without trastuzumab | 24.8 | 3.6 |
| T1–3 40.4% N0 | trastuzumab | 41.2 | 0.3 | ||||
| 695 | TNBC | T1–3 55.2% N0 | ChemoTx and/ or TAM | 31.8 | 5.25 | ||
| Zumsteg 2013 (1999–2008) | 198 | 5 | TNBC | T1–T2 N0 | 84% ChemoTx | 0 | 5.4 |
Prior Systemic Therapy
The risk of recurrence is determined by tumor biology and stage at presentation. The natural course of disease can be favorably altered by the use of adjuvant therapies tailored to tumor molecular markers. Chest wall recurrences occurred in 13% of patients who received adjuvant chemotherapy regimens but no radiation. However, the incidence was only 7.5% for those with one to three positive nodes, whereas it was 14% in those with four or more involved nodes. In a combined analysis of 5758 patients with node-positive cancers treated with adjuvant therapies without postmastectomy radiation, 12.2% of patients presented with an ILRR compared with 43%, whose first failure event was distant metastasis. The proportion of skin or chest wall recurrences was 56.9% compared with 22.6% supraclavicular, 11.7% axillary, and less than 1% parasternal and infraclavicular nodal recurrences. Predictive factors for local failure consisted of age less than 50, larger tumors, greater number of positive lymph nodes, and number of resected nodes. In this same study, the 10-year cumulative incidence of isolated regional failures was 3.5% or less, for women with one to three positive nodes, compared with 5.4% to 10.9% for those with four or more nodes, irrespective of tumor size.
Additional risk factors identified in a review of 12 studies involving 12,961 node-negative patients treated by mastectomy alone were less than 40 years of age, lymphovascular invasion, positive or close margins, and tumor size greater than 2 cm. Additionally, tumor biology influences recurrence events. Specifically, higher rates of distant metastasis and LRR are reported for triple-negative breast cancers compared with non–triple negative tumors.
Ma and colleagues reported different time intervals to LRR based on tumor receptors. The shortest median time interval to LRR was 18.2 months for HER2-positive cancers (largely without trastuzumab therapy), followed by 21.8 months for triple-negative and more than twofold longer for luminal A or B cancers. In a pooled analysis of 162 nonirradiated mastectomy-treated T1–2 N0 HER2-positive cancers from the British Columbia Cancer Agency and Massachusetts General Hospital, 5-year LRR-free survival was 99.4% with anti-HER2 therapy compared with 97.4% for those who did not receive trastuzumab. Similarly, patients with HER2 positive tumors experienced higher rates of LRR in the pre-trastuzumab era compared with those who received trastuzumab, wherein chest wall and nodal recurrence rates were cut by half (see Table 61.1 ). Interestingly, the combination of adjuvant trastuzumab with postmastectomy radiation in this nonrandomized retrospective series was associated with 0% LRR at 5 years.
Prior Neoadjuvant Chemotherapy
Patients with palpable breast cancers at presentation are increasingly directed toward neoadjuvant chemotherapy based on tumor molecular characteristics and disease stage. An LRR after neoadjuvant chemotherapy is highly associated with the pathologic response, with lower rate of relapse when a pathologic complete response (pCR) is attained. In a combined analysis of NSABP prospective randomized trials B-18 and B-27, tumor size greater than 5 cm (hazard ratio [HR] 1.58, p = .0095), clinical node-positive status (HR 1.53, p = .017), and pathologically residual nodal disease (HR 4.48, p = .01) were the most significant predictive factors for LRRs among 1071 mastectomy-treated patients without adjuvant radiation therapy. The 10-year cumulative incidence of chest wall recurrences ranged from 0% to 2.2% for patients who had a breast pCR and negative nodes, regardless of their clinical presentation. In clinically node-negative cancers, regional recurrences were slightly higher, ranging from 0% to 6.2%. Among patients who were classified as clinically node-negative and were then found to have residual disease in axillary nodes, the overall rate of LRR ranged from 11.2% to 22.4%.
Genomic Characterization
Another proposed approach to predict locoregional failures among endocrine-responsive cancers is through genomic analysis. Mamounas and coworkers used the 21-gene Recurrence Score (RS) panel to examine LRRs among estrogen receptor–positive, node-negative mastectomy-treated patients on NSABP protocols B-14 and B-20. Of these 505 nonirradiated mastectomy cases, 6.1% had LRRs: 18 chest wall or scar recurrences and 13 nodal or regional recurrences. A high RS was associated with a LRR in more than 10% of this subgroup compared with intermediate or low RS groups. Another study explored the utility of the 21-gene RS for predicting LRRs using the hormone receptor positive cohort of the NSABP B-28 protocol wherein anthracycline-based chemotherapy with or without paclitaxel was randomly assigned. The risk of a LRR for mastectomy-treated subjects with one to three positive nodes was 6% or less in the low RS category versus 9.6% and 23.6% for the intermediate and high RS categories respectively. The predictive value of the RS has been described in other small series. Similarly, EndoPredict, another genomic test, was investigated in the Austrian Breast Cancer Study Group trial 8. This test was prognostic for LRRs, with a 10-year local recurrence–free survival of 97.5% in the EndoPredict low-risk group versus 91% in the high-risk group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree